Phytochemical Analysis and Evaluation of the Antioxidant, Antiproliferative, Antibacterial, and Antibiofilm Effects of Globularia alypum (L.) Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. Yield of Different Extraction Methods
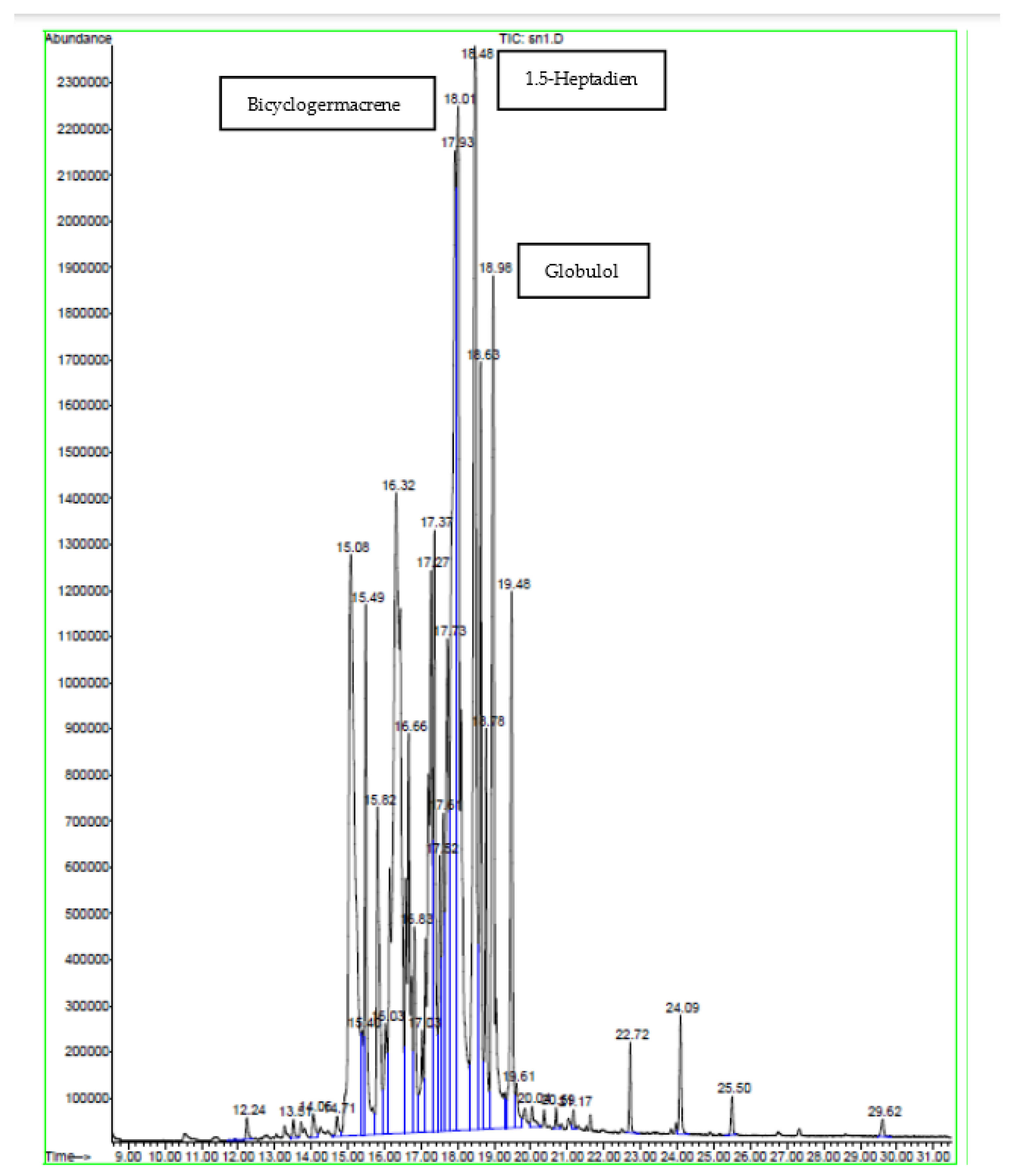
2.2. GC-MS Analysis
2.3. Determination of Minerals
2.4. Antioxidant Activity
2.5. Antiproliferative Effect
2.6. Evaluation of Antibacterial Activity
2.7. Antibiofilm Activity
3. Materials and Methods
3.1. Plant Material
3.2. The GC-MS Analysis
3.3. Determination of Minerals
3.3.1. Determination of Potassium and Calcium
3.3.2. Determination of Nitrogen
3.4. Antioxidant Activity
3.4.1. ABTS Scavenging Assay
3.4.2. β-Carotene
3.5. Antiproliferative Effect
3.6. Antibacterial Activity
3.7. Antibiofilm Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqui, A.J.; Danciu, C.; Ashraf, S.A.; Moin, A.; Singh, R.; Alreshidi, M.; Patel, M.; Jahan, S.; Kumar, S.; Alkhinjar, M.I.M.; et al. Plants-derived Biomolecules as potent antiviral phytomedicines: New insights on ethnobotanical evidences against coronaviruses. Plants 2020, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Barteková, M.; Adameová, A.; Görbe, A.; Ferenczyová, K.; Pecháňová, O.; Lazou, A.; Dhalla, N.S.; Ferdinandy, P.; Giricz, Z. Natural and synthetic antioxidants targeting cardiac oxidative stress and redox signaling in cardiometabolic diseases. Free Radical Biol. Med. 2021, 169, 446–477. [Google Scholar] [CrossRef] [PubMed]
- Ciampi, F.; Sordillo, L.M.; Gandy, J.C.; Caroprese, M.; Sevi, A.; Albenzio, M.; Santillo, A. Evaluation of natural plant extracts as antioxidants in a bovine in vitro model of oxidative stress. J. Dairy Sci. 2020, 103, 8938–8947. [Google Scholar] [CrossRef] [PubMed]
- El Hachlafi, N.; Chebat, A.; Bencheikh, R.S.; Fikri, B.K. Ethnopharmacological study of medicinal plants used for chronic diseases treatment in Rabat-Sale-Kenitra region (Morocco). Ethnobot. Res. Appl. 2020, 20, e02191. [Google Scholar] [CrossRef]
- Zekeya, N.; Ibrahim, M.; Mamiro, B.; Ndossi, H.; Kilonzo, M.; Mkangara, M.; Chacha, M.; Chilongola, J.; Kideghesho, J. Potential of natural phenolic antioxidant compounds from Bersamaabyssinica (Meliathacea) for treatment of chronic diseases. Saudi J. Biol. Sci. 2022, 29, 103273. [Google Scholar] [CrossRef]
- Tiss, M.; Souiy, Z.; Achour, L.; Hamden, K. Anti-obesity, anti-hyperglycaemic, anti-antipyretic and analgesic activities of Globulariaalypum extracts. Arch. Physiol. Biochem. 2020, 128, 1453–1460. [Google Scholar]
- Friščić, M.; Petlevski, R.; Kosalec, I.; Madunić, J.; Matulić, M.; Bucar, F.; Hazler Pilepić, K.; Maleš, Ž. Globulariaalypum L. and Related Species: LC-MS Profiles and Antidiabetic, Antioxidant, Anti-Inflammatory, Antibacterial and Anticancer Potential. Pharmaceuticals 2022, 15, 506. [Google Scholar] [CrossRef]
- Rahman, M.; Rahaman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Abubakr, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied. Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Chibuye, B.; Singh, S.I.; Chimuka, L.; Maseka, K.K. A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Abedrabba, M.; Bouix, M.; Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercuscoccifera L. and Juniperusphoenica L. fruit extracts. Food Chem. 2007, 105, 1126–1134. [Google Scholar] [CrossRef]
- Lameirao, F.; Pinto, D.F.; Vieira, E.F.; Peixoto, A.; Freire, C.; Sut, S.; Rodrigues, F. Green-sustainable recovery of phenolic and antioxidant compounds from industrial chestnut shells using ultrasound-assisted extraction: Optimization and evaluation of biological activities in vitro. Antioxidants 2020, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Quispe Candori, S.; Foglio, M.A.; Rosa, P.T.V.; Meireles, M.A.A. Obtaining b-caryophyllene from Cordiaverbenacea de Candolle by super crtitical fluid extraction. J. Supercrit. Fluids. 2008, 46, 27–32. [Google Scholar] [CrossRef]
- Khlifi, D.; Hamdi, M.; El Hayouni, A.; Cazaux, S.; Souchard, J.P.; Couderc, F.; Bouajila, J. Global Chemical Composition and Antioxidant and Anti-Tuberculosis Activities of Various Extracts of Globulariaalypum L. (Globulariaceae) Leaves. Molcules 2011, 16, 10592–10603. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.C.; Luque-de-Castro, M.D. Ultrasound-assisted extraction for the analysis of phenolic compounds in strawberries. Anal. Bioanal. Chem. 2004, 379, 1106–1112. [Google Scholar] [CrossRef]
- Héthelyi, E.; Tétényi, P.; Dabi, E.; Dános, B. The role of mass spectrometry in medicinal plant research. Biomed. Environ. Mass Spectrom. 1987, 14, 627–632. [Google Scholar] [CrossRef]
- Al-Qahtani, W.H.; Dinakarkumar, Y.; Arokiyaraj, S.; Saravanakumar, V.; Rajabathar, J.R.; Arjun, K.; Gayathri, P.K.; Appaturi, J.N. Phyto-chemical and biological activity of Myristicafragrans, an ayurvedic medicinal plant in Southern India and its ingredient analysis. Saudi J. Biol. Sci. 2022, 5, 3815–3821. [Google Scholar] [CrossRef]
- Tan, G.; Wang, Z.; Che, L.; Yin, S. Immunotherapeutic effects on murine pancreatic carcinoma by beta-elemene combined with dendritic cells modified with genes encoding interleukin-23. Front. Med. China 2007, 1, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Li-fei, M.; Wei-Min, H.; Jun, L.; Jing, S. Study on the antioxidant effects of β-elemene. Chin. J. Clin. Pharmacol. Therap. 2012, 7, 727–731. [Google Scholar]
- Asraoui, F.; Kounnoun, A.; El Cadi, H.; Cacciola, F.; El Majdoub, Y.O.; Alibrando, F.; Mandolfino, F.; Dugo, P.; Luigi Mondello, L.; Louajri, A.A. Phytochemical investigation and antioxidant activity of Globulariaalypum L. Molecules 2021, 26, 759. [Google Scholar] [CrossRef]
- Khantouche, L.; Guesmi, F.; Motri, S.; Mejri, M.; Abderabba, M. Nutritional Composition, Analysis of Secondary Metabolites and Antioxidative Effects of the Leaves of Globulariaalypum L. Indian J. Pharm. Sci. 2018, 80, 274–281. [Google Scholar] [CrossRef]
- Ndomou, S.C.H.; Djikeng, F.T.; Teboukeu, G.B.; Doungue, H.T. Hermann Arantes Kohole Foffe, Cristelle Tsapla Tiwo, Hilaire Macaire Womeni. J. Agric. Food Res. 2021, 3, 100105. [Google Scholar]
- Jrah Harzallah, H.; Neffati, A.; Skandrani, I.; Maaloul, E.; Chekir Ghedira, L.; Mahjoub, T. Antioxidant and antigenotoxic activities of Globulariaalypum leaves extracts. J. Med. Plant Res. 2010, 4, 2048–2053. [Google Scholar]
- Rossi, P.; Bao, L.; Luciani, A.; Panighi, J.; Desjobert, J.M.; Costa, J.; Casanova, J.; Jean-Bolla, M.; Berti, L. (E)-Methylisoeugenol and Elemicin: Antibacterial Components of Daucuscarota L. Essential Oil against Campylobacter jejuni. J. Agric. Food Chem. 2007, 55, 7332–7336. [Google Scholar] [CrossRef] [PubMed]
- Surveswaran, S.; Cai, Y.Z.; Corke, H.; Sun, M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem. 2007, 102, 938–953. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Wang, T.; Ding, Q.; Khalil, A.; Xu, S.; Lin, A.; Yao, H.; Xie, W.; Zhu, Z.; et al. Antioxidant Properties of Novel Dimers Derived from Natural β-Elemene through Inhibiting H2O2-Induced Apoptosis. ACS Med. Chem. Lett. 2017, 8, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Trevizan, L.N.F.; Nascimento, K.F.; Santos, J.A.; Kassuya, C.A.L.; Cardoso, C.A.L.; Vieira, M.D.C.; Moreira, F.M.F.; Croda, J.; Formagio, A.S.N. Anti-inflammatory, antioxidant and anti-Mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylusedulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016, 4, 510–515. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants—Geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. Vitr. 2009, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.S.; Moreiras, A.M.S.; Abel, C.; Sohrabi, R.; Lee, S.; Gershenzon, J.; Tholl, D. The major volatile organic compound emitted from Arabidopsis thaliana flowers, the sesquiterpene (E)-β-caryophyllene, is a defense against a bacterial pathogen. New Phytol. 2012, 193, 997–1008. [Google Scholar] [CrossRef]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.P.; Calixto, B.J. β-caryophyllene inhibits dextran sulfate sodium-induced colitis in mice through CB2 receptor activation and PPARγ pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef]
- Amiel, E.; Ofir, R.; Dudai, N.; Soloway, E.; Rabinsky, T.; Rachmilevitch, S. β-caryophyllene, a compound isolated from the biblical balm of gilead (Commiphoragileadensis), is a selective apoptosis inducer for tumor cell lines. Evid.-Based Complement. Altern. Med. 2012, 2012, 872394. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Pichette, A. Potentiating effect of beta-caryophyllene on anticancer activity of alpha-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.I.B.; Teixeira, M.F.S.; De Oliveira, V.M.A. Screening of Amazonian plants from the Adolpho Ducke forest reserve, Manaus, state of Amazonas, Brazil, for antimicrobial activity. Mem. Inst. Oswaldo Cruz 2008, 103, 31–38. [Google Scholar] [CrossRef]
- Lozienne, K.; Ausra, S.; Juozas, L. Scavenging and antimicrobial properties of extracts. Food Chem. 2007, 103, 546–559. [Google Scholar] [CrossRef]
- Hailu, T.; Endris, M.; Kaleab, A.; Tsige, G.M. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J. Ethnopharmacol. 2005, 100, 168–175. [Google Scholar] [CrossRef]
- Tegos, G.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef]
- Tim Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Hayakawa, S.; Kawamura, M.; Sato, T.; Hirano, T.; Fujimura, S. An α-lipoic acid derivative, and anti-ros agent, prevents the acquisition of multi-drug resistance in clinical isolates of Pseudomonas aeruginosa. J. Infect. 2018, 25, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Zhou, L.; Huang, Y.; Wang, Y.; Hao, X.; Wang, J. Antimicrobial activity of globulol isolated from the fruits of Eucalyptus globulus Labill. Nat. Prod. Res. 2008, 22, 569–575. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.M.M.; Brugnera, D.F. Disinfectant action of Cymbopogon sp. essential oils in different phases of biofilm formation by Listeria monocytogenes on stainless steel surface. Food Control. 2010, 21, 549–553. [Google Scholar] [CrossRef]
- Anwar, H.; Dasgupta, M. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob. Agents Chemother. 1990, 34, 2043–2046. [Google Scholar] [CrossRef] [PubMed]
- Watnick, P.; Kolter, R. Minireview: Biofilm, city of microbes. J. Bacteriol. 2000, 182, 2675–2679. [Google Scholar] [CrossRef] [PubMed]
- Melo, L. Biofilm formation and its role in fixed film processes. In The Handbook of Water and Wastewater Microbiology; Academic Press: London, UK, 2003; pp. 337–349. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Martin-Prevel, P.; Gagnard, J.; Gautier, P. L’analyse Végétale dans le Contrôle de L’alimentation des Plantes Tempérées et Tropicales; Lavoisier Tec et Doc: Paris, France, 1984; p. 810. [Google Scholar]
- El Arem, A.; Saafi, A.; Mechri, B.; Lahouar, L.; Issaoui, M.; Hammami, M.; Achour, L. Effects of the Ripening Stage on Phenolic Profile, Phytochemical Composition and Antioxidant Activity of Date Palm Fruit. J. Agric. Food Chem. 2012, 60, 10896–10902. [Google Scholar] [CrossRef]
- Barros, L.; Ferreira, M.J.; Queiros, B.; Ferreira, I.C.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 2, 413–419. [Google Scholar] [CrossRef]
- Dbeibia, A.; Ben Taher, F.; Altamar, K.; Haddaji, N.; Mahdi, A.; Amri, Z.; Mzoughi, R.; Jabeur, C. Control of Staphylococcus aureus methicillin resistant isolated from auricular infections using aqueous and methanolic extracts of Ephedra alata. Saudi J. Biol. Sci. 2022, 29, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, K.; Kouidhi, B.; Jrah, H.; Mahdouani, K.; Bakhrouf, A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011, 11, 29. [Google Scholar] [CrossRef]



| Extraction Method | Yield % |
|---|---|
| Soxhlet | 63.2 ± 0.07 b |
| Sonication | 64.8 ± 0.45 a |
| Maceration | 48.8 ± 0.04 c |
| Peak | Compound | Similarity % | RT | Content % |
|---|---|---|---|---|
| 1 | Bicyclogermacrene | 95 | 15.08 | 9.65 |
| 2 | β-Elemene | 99 | 15.49 | 2.81 |
| 3 | Pentadecane | 60 | 15.81 | 2.43 |
| 4 | 1.5-Heptadiene | 91 | 16.32 | 13.18 |
| 5 | Elemicin | 99 | 16.66 | 3.75 |
| 6 | Dendrolasin | 90 | 16.83 | 1.18 |
| 7 | Globulol | 97 | 17.28 | 5.47 |
| 8 | Viridiflorol | 97 | 17.37 | 3.57 |
| 9 | γ-Gurjunene | 90 | 17.52 | 1.48 |
| 10 | γ-1-cadinene | 70 | 17.62 | 1.90 |
| 11 | 2-Pentadecanone | 93 | 18.78 | 1.84 |
| Peak | Compound | Similarity % | RT | Content % |
|---|---|---|---|---|
| 1 | β-elemene | 91 | 15.39 | 0.79 |
| 2 | β-Caryophyllene | 95 | 15.76 | 1.10 |
| 3 | Camphene | 92 | 16.06 | 1.13 |
| 4 | Valencene | 87 | 16.18 | 1.91 |
| 5 | Zingiberene | 90 | 16.30 | 3.14 |
| 6 | Elemicin | 98 | 16.52 | 0.72 |
| 7 | Elemol | 89 | 16.88 | 0.82 |
| 8 | Calarene | 94 | 17.23 | 5.65 |
| 9 | Hexadecanoic acid | 99 | 20.25 | 3.37 |
| 10 | Carbonic acid | 95 | 21.55 | 2.64 |
| Peak | Compound | Similarity % | RT | Content % |
|---|---|---|---|---|
| 1 | α- Farnesene | 76 | 16.42 | 0.92 |
| 2 | Elemicin | 99 | 16.70 | 12.54 |
| 3 | Elemol | 91 | 16.87 | 1.87 |
| 4 | Spathulenol | 99 | 17.24 | 3.28 |
| 5 | Isospathulenol | 94 | 17.66 | 1.20 |
| 6 | Italicene | 72 | 17.87 | 5.09 |
| Minerals | N (%) | P (%) | K (%) | Ca (%) | Na (%) | Fe (%) |
|---|---|---|---|---|---|---|
| Content | 1.41 | 0.04 | 0.61 | 1.38 | 0.05 | 0.017 |
| IC50 (mg/mL) | Ascorbic Acid | Soxhlet | Sonication | Maceration |
|---|---|---|---|---|
| ABTS scavenging assay | 0.06 ± 0.02 b | 0.25 ± 0.01 d | 0.18 ± 0.00 c | 0.04 ± 0.01 a |
| β-carotene | 0.59 ± 0.00 c | 0.65 ± 0.05 d | 0.28 ± 0.09 b | 0.15 ± 0.00 a |
| Zone of Inhibition (mm) | ||||
|---|---|---|---|---|
| Strains | Tetracycline | Soxhlet | Sonication | Maceration |
| E.coli | 29.10 ± 0.00 | N | N | N |
| P. aeruginosa | 27.20 ± 0.53 | N | N | N |
| S. typhimurium | 25.01 ± 1.02 | N | N | N |
| S. aureus | 21.00 ± 1.82 a | 11.2 ± 0.30 d | 14.50 ± 0.30 b | 12.00 ± 0.06 c |
| Strains | E. Coli | P. aeruginosa | S. typhimurium | S. aureus |
|---|---|---|---|---|
| MIC (mg/mL) | ||||
| Soxhlet | N | N | N | 6.25 |
| Sonication | N | N | N | 6.25 |
| Maceration | N | N | N | 6.25 |
| MBC (mg/mL) | ||||
| Soxhlet | N | N | N | >25 |
| Sonication | N | N | N | >25 |
| Maceration | N | N | N | >25 |
| Concentration (mg/mL) | 25 | 12.5 | 6.25 | 3.12 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methods | So | S | M | So | S | M | So | S | M | So | S | M |
| E.coli | N | N | N | N | N | N | N | N | N | N | N | N |
| S. typhimurium | N | N | N | N | N | N | N | N | N | N | N | N |
| S. aureus | 20.49 | 35.78 | 27.19 | 15.06 | 28.15 | 20.01 | 10.59 | 20.3 | 17.10 | 8.50 | 16.01 | 10.24 |
| P.aeruginosa | N | N | N | N | N | N | N | N | N | N | N | N |
| Collection Site | Geographical Location | ||
|---|---|---|---|
| Longitude (E) | Latitude (N) | Altitude (m) | |
| Ouardanin | 10°40′35″ | 35°42′35″ | 75 |
| Extraction Methods | Soxhlet | Sonication | Maceration |
|---|---|---|---|
| Solvent | Ethanol | Ethanol | Ethanol |
| Temperature (°C) | 40 | 25 | 25 |
| Time (min) | 60 | 10 | 240 |
| Plant: solvent proportion | 5:100 | 5:100 | 5:100 |
| Centrifugation | Not applicable | 5 min at 5000 rpm and 4 °C | Not applicable |
| Equipment | Not applicable | Ultrasound bath (130 kHz) | Not applicable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouir, S.; Dbeibia, A.; Bouhajeb, R.; Haddad, H.; Khélifa, A.; Achour, L.; Ghardallou, M.; Zaïri, A. Phytochemical Analysis and Evaluation of the Antioxidant, Antiproliferative, Antibacterial, and Antibiofilm Effects of Globularia alypum (L.) Leaves. Molecules 2023, 28, 4019. https://doi.org/10.3390/molecules28104019
Nouir S, Dbeibia A, Bouhajeb R, Haddad H, Khélifa A, Achour L, Ghardallou M, Zaïri A. Phytochemical Analysis and Evaluation of the Antioxidant, Antiproliferative, Antibacterial, and Antibiofilm Effects of Globularia alypum (L.) Leaves. Molecules. 2023; 28(10):4019. https://doi.org/10.3390/molecules28104019
Chicago/Turabian StyleNouir, Sahar, Amal Dbeibia, Rim Bouhajeb, Houda Haddad, Amani Khélifa, Lotfi Achour, Mariem Ghardallou, and Amira Zaïri. 2023. "Phytochemical Analysis and Evaluation of the Antioxidant, Antiproliferative, Antibacterial, and Antibiofilm Effects of Globularia alypum (L.) Leaves" Molecules 28, no. 10: 4019. https://doi.org/10.3390/molecules28104019
APA StyleNouir, S., Dbeibia, A., Bouhajeb, R., Haddad, H., Khélifa, A., Achour, L., Ghardallou, M., & Zaïri, A. (2023). Phytochemical Analysis and Evaluation of the Antioxidant, Antiproliferative, Antibacterial, and Antibiofilm Effects of Globularia alypum (L.) Leaves. Molecules, 28(10), 4019. https://doi.org/10.3390/molecules28104019






