Strictinin: A Key Ingredient of Tea
Abstract
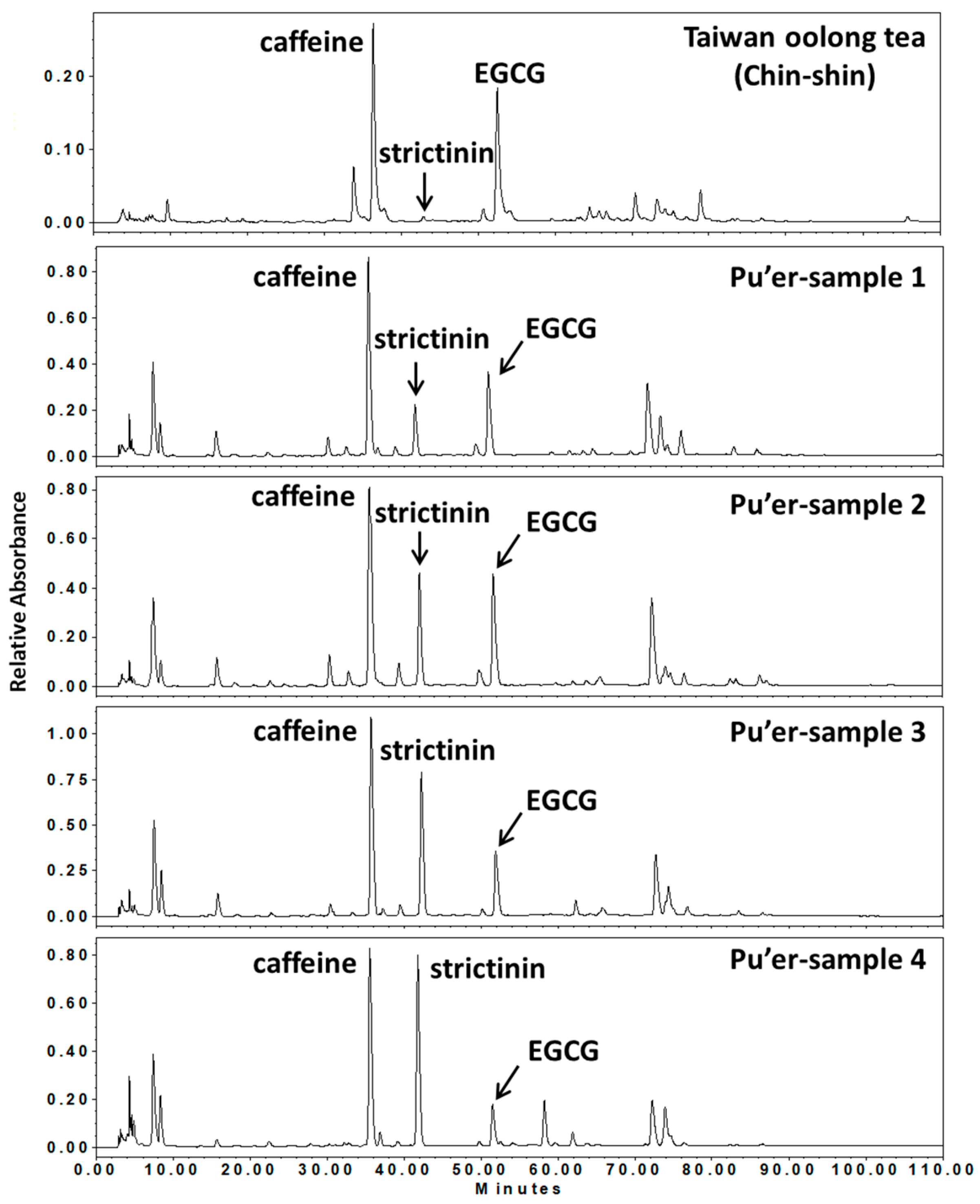
1. Introduction
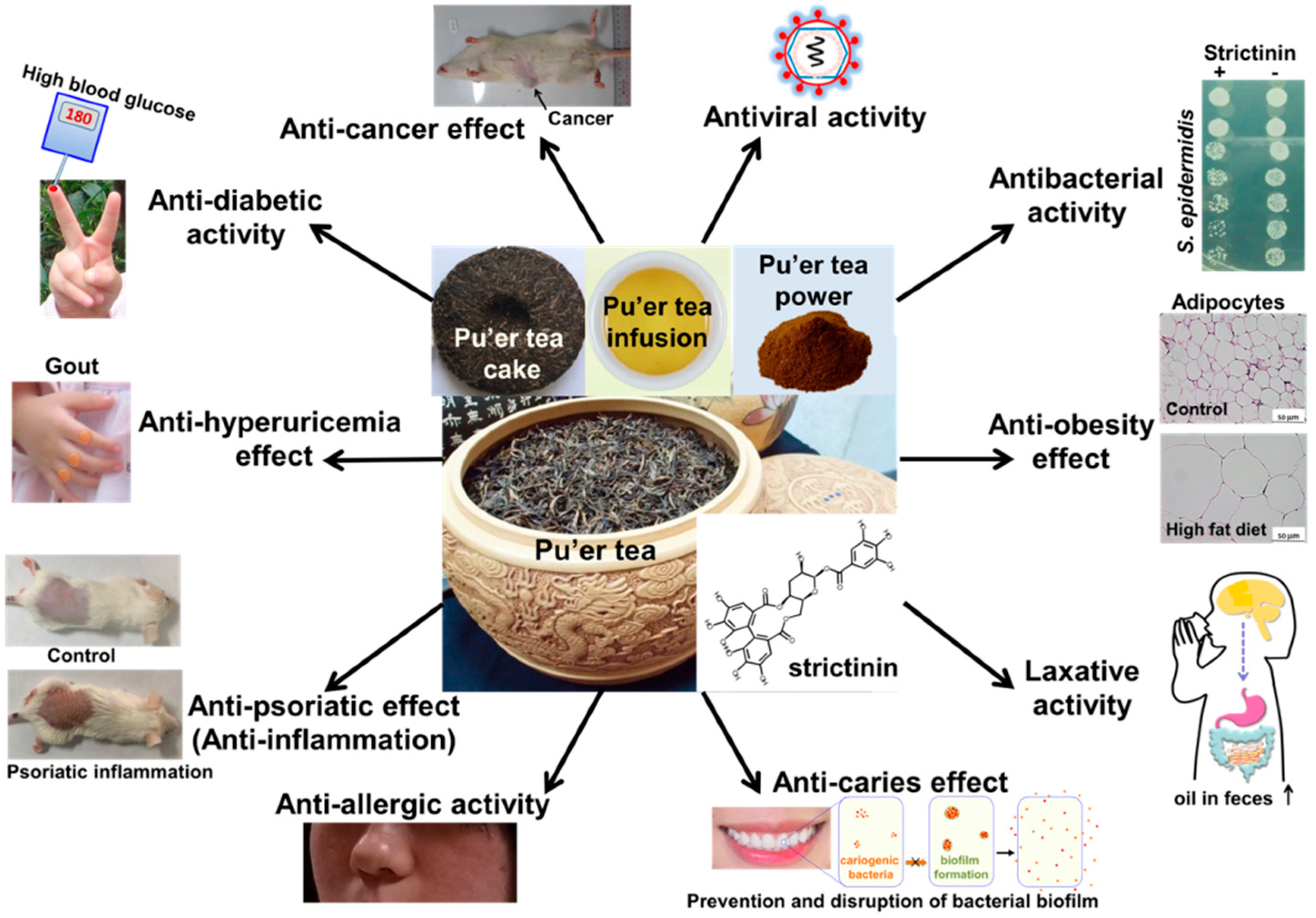
2. Functional Activities of Strictinin
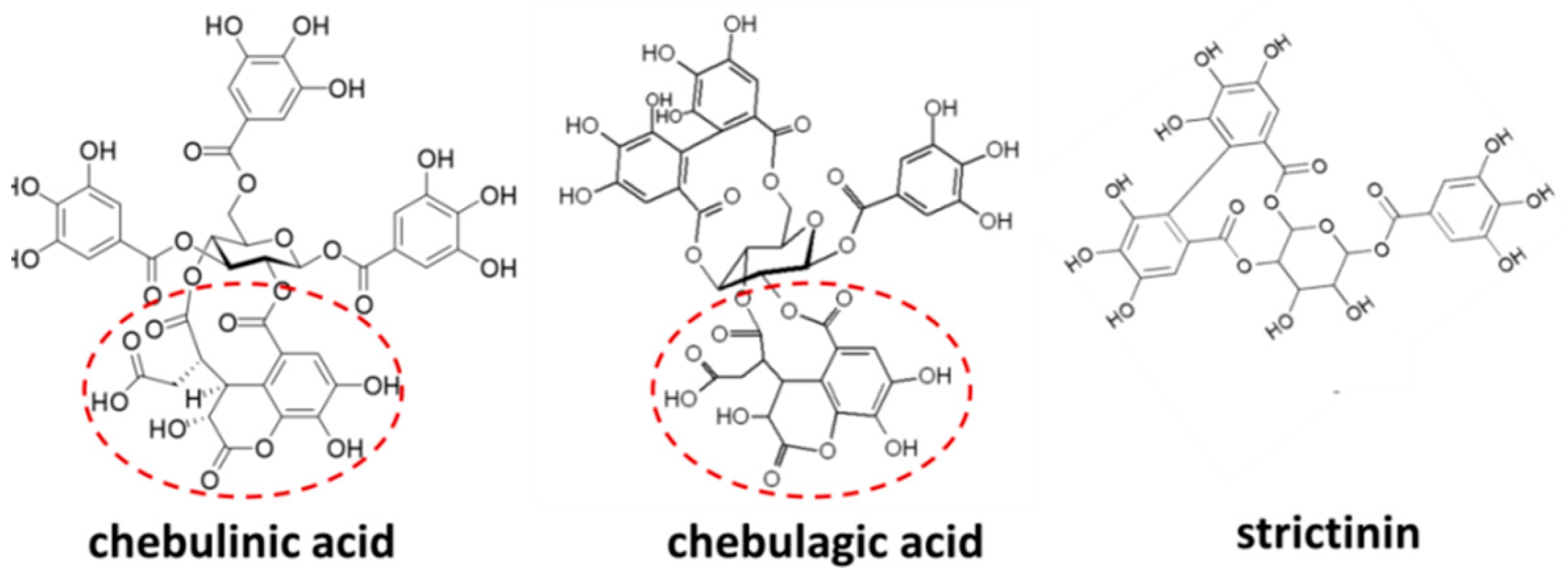
2.1. Antiviral Activity
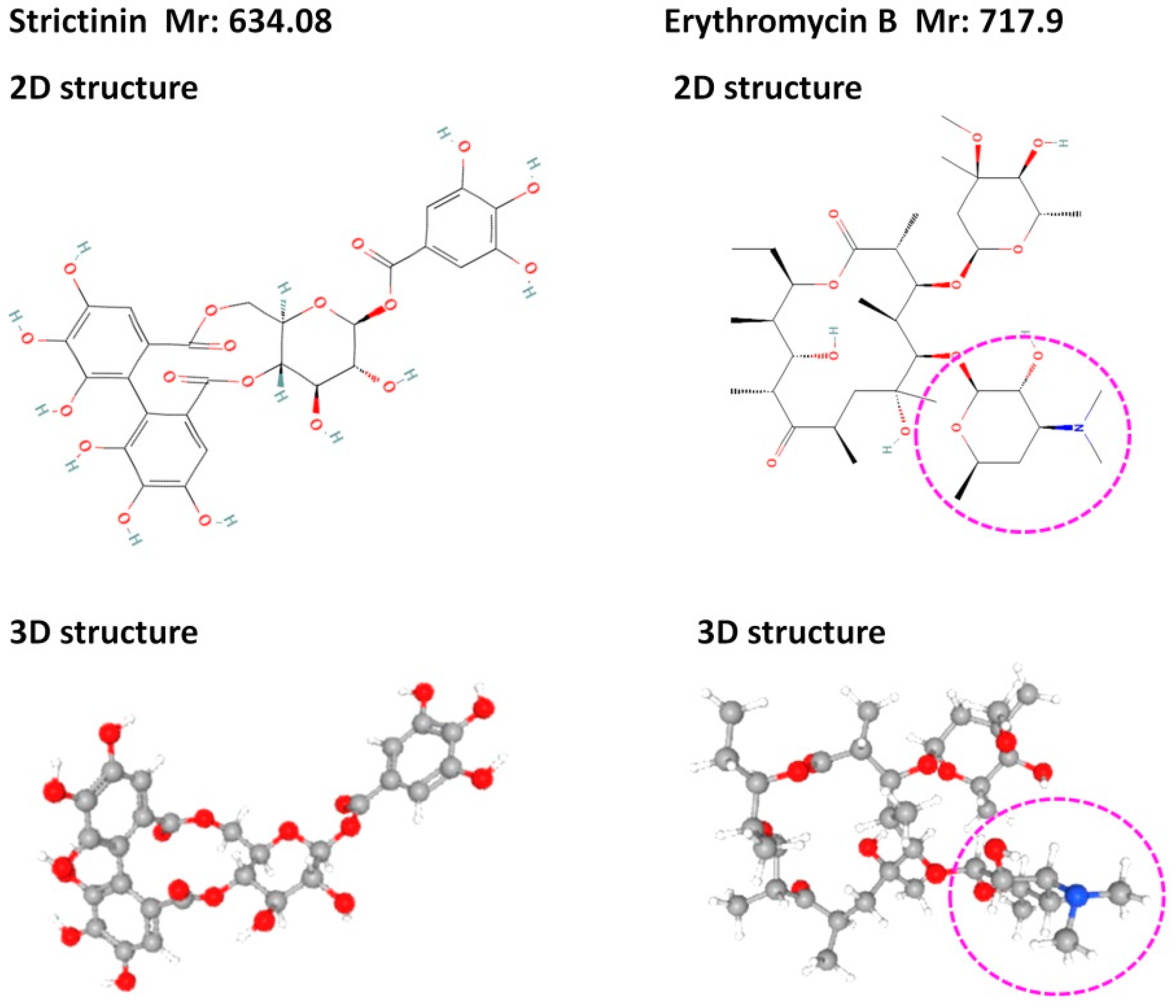
2.2. Antibacterial Activity
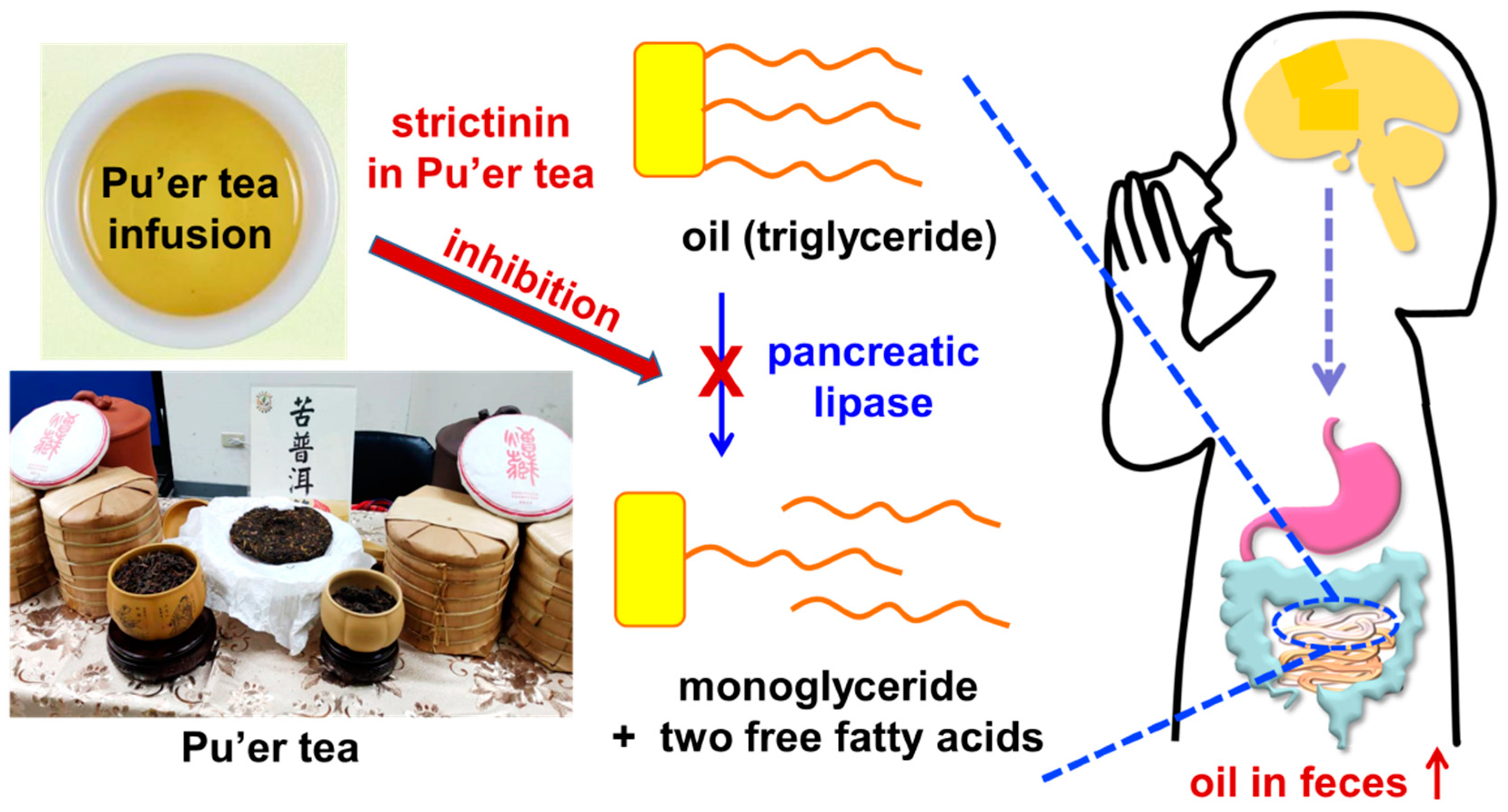
2.3. Anti-Obesity Effect
2.4. Laxative Activity
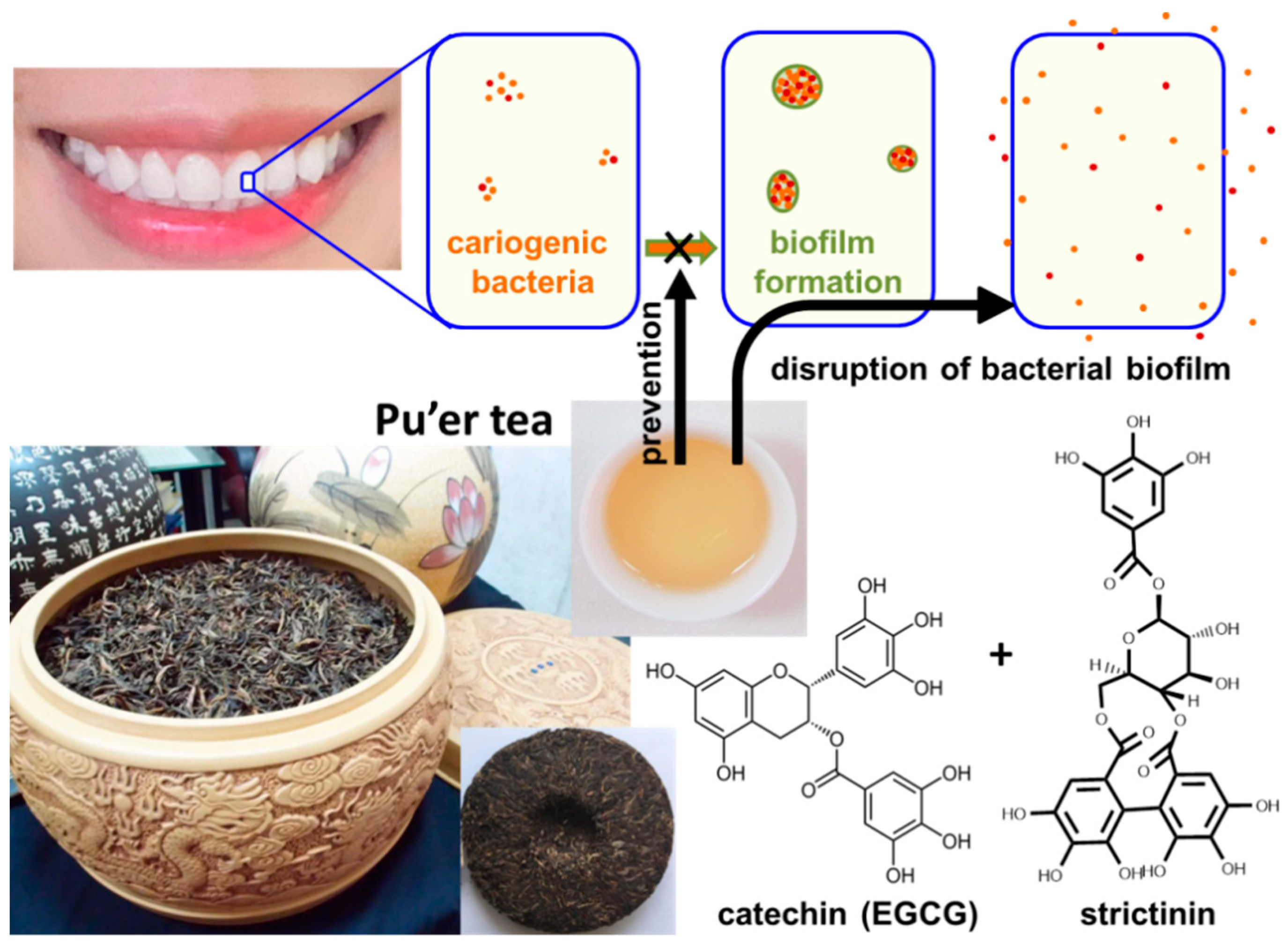
2.5. Anticaries Effect
2.6. Anti-Allergic Activity
2.7. Antipsoriatic Effect
2.8. Antihyperuricemia Effect
2.9. Antidiabetic Activity
2.10. Anticancer Effect
2.11. Summary of Functional Activities of Strictinin
3. Application of Striction
3.1. Powder of Pu’er Tea Infusion
3.2. Bitter Citrus Tzen Tea
3.3. Edible Oral Care Products
4. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Casuariin, stachyurin and strictinin, new ellagitannins from Casuarina stricta and Stachyurus praecox. Chem. Pharm. Bull. 1982, 30, 766–769. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Tannins of casuarina and stachyurus species. Part 1. Structures of pendunculagin, casuarictin, strictinin, casuarinin, casuariin, and stachyurin. J. Chem. Soc. Perkin Trans. I 1983, 1765–1772. [Google Scholar] [CrossRef]
- Mady, M.S.; Elsayed, H.E.; El-Sayed, E.K.; Hussein, A.A.; Ebrahim, H.Y.; Moharram, F.A. Polyphenolic profile and ethno pharmacological activities of Callistemon subulatus (Cheel) Craven leaves cultivated in Egypt. J. Ethnopharmacol. 2022, 284, 114698. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H. Unique distribution of ellagitannins in ripe strawberry fruit revealed by mass spectrometry imaging. Curr. Res. Nutr. Food Sci. 2021, 4, 821–828. [Google Scholar] [CrossRef]
- Takayoshi, J.; Huang, Y.L.; Matsuo, Y.; Saito, Y.; Li, D.P.; Tanaka, T. Ellagitannin digestion in moth larvae and a new dimeric ellagitannin from the leaves of Platycarya strobilacea. Molecules 2021, 26, 4134. [Google Scholar] [CrossRef]
- Kim, Y.H.; Fujimura, Y.; Sasaki, M.; Yang, X.; Yukihira, D.; Miura, D.; Unno, Y.; Ogata, K.; Nakajima, H.; Yamashita, S.; et al. In situ label-free visualization of orally dosed strictinin within mouse kidney by MALDI-MS imaging. J. Agric. Food Chem. 2014, 62, 9279–9285. [Google Scholar] [CrossRef]
- Yang, X.; Tomás-Barberán, F.A. Tea is a significant dietary source of ellagitannins and ellagic acid. J. Agric. Food Chem. 2019, 67, 5394–5404. [Google Scholar] [CrossRef]
- Mizukami, Y.; Sawai, Y.; Yamaguchi, Y. Simultaneous analysis of catechins, gallic acid, strictinin, and purine alkaloids in green tea by using catechol as an internal standard. J. Agric. Food Chem. 2007, 55, 4957–4964. [Google Scholar] [CrossRef]
- Dou, J.; Lee, V.S.Y.; Tzen, J.T.C.; Lee, M.R. Identification and comparison of phenolic compounds in the preparation of oolong tea manufactured by semifermentation and drying processes. J. Agric. Food Chem. 2007, 55, 7462–7468. [Google Scholar] [CrossRef]
- Chen, G.H.; Lin, Y.L.; Hsu, W.L.; Hsieh, S.K.; Tzen, J.T.C. Significant elevation of antiviral activity of strictinin from Pu’er tea after thermal degradation to ellagic acid and gallic acid. J. Food Drug Anal. 2015, 23, 116–123. [Google Scholar] [CrossRef]
- Chen, G.H.; Lin, Y.L.; Xu, J.R.; Tzen, J.T.C. Tea from wild Pu’er tree is rich in strictnin, a phenolic compound possessing inhibitory potency on influenza virus. J. Agric. For. 2014, 63, 129–137. [Google Scholar]
- Saha, R.K.; Takahashi, T.; Kurebayashi, Y.; Fukushima, K.; Minami, A.; Kinbara, N.; Ichitani, M.; Sagesaka, Y.M.; Suzuki, T. Antiviral effect of strictinin on influenza virus replication. Antivir. Res. 2010, 88, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.Y.; Xiang, J.; Wang, Z.S.; Jin, J.; Wang, Y.Q.; Li, Q.S.; Li, D.; Fang, Z.T.; Lu, J.L.; Ye, J.H.; et al. Theacrine From Camellia kucha and Its Health Beneficial Effects. Front. Nutr. 2020, 7, 596823. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Ye, C.X.; Kato, M.; Crozier, A.; Ashihara, H. Theacrine (1,3,7,9-tetramethyluric acid) synthesis in leaves of a Chinese tea, kucha (Camellia assamica var. kucha). Phytochemistry 2002, 60, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.R.; Kuo, P.C.; Li, Y.C.; Jhuo, C.F.; Hsu, W.L.; Tzen, J.T.C. Theacrine and strictinin, two major ingredients for the anti-influenza activity of Yunnan Kucha tea. J. Ethnopharmacol. 2020, 262, 113190. [Google Scholar] [CrossRef] [PubMed]
- Jhuo, C.F.; Hsu, Y.Y.; Chen, W.Y.; Tzen, J.T.C. Attenuation of tumor development in mammary carcinoma rats by theacrine, an antagonist of adenosine 2A receptor. Molecules 2021, 26, 7455. [Google Scholar] [CrossRef]
- Parashar, U.D.; Anderson, L.J. Severe acute respiratory syndrome: Review and lessons of the 2003 outbreak. Int. J. Epidemiol. 2004, 33, 628–634. [Google Scholar] [CrossRef]
- Wu, D.; Wu, T.; Liu, Q.; Yang, Z. The SARS-CoV-2 outbreak: What we know. Int. J. Infect. Dis. 2020, 94, 44–48. [Google Scholar] [CrossRef]
- Tu, E.C.; Hsu, W.L.; Tzen, J.T.C. Strictinin, a major ingredient in Yunnan kucha tea possessing inhibitory activity on the infection of mouse hepatitis virus to mouse L cells. Molecules 2023, 28, 1080. [Google Scholar] [CrossRef]
- Kadioglu, O.; Saeed, M.; Greten, H.J.; Efferth, T. Identification of novel compounds against three targets of SARS CoV-2 coronavirus by combined virtual screening and supervised machine learning. Comput. Biol. Med. 2021, 133, 104359. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Chen, T.Y.; Chung, C.Y.; Noyce, R.S.; Grindley, T.B.; McCormick, C.; Lin, T.C.; Wang, G.H.; Lin, C.C.; Richardson, C.D. Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J. Virol. 2011, 85, 4386–4398. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.T.; Chen, T.Y.; Lin, S.C.; Chung, C.Y.; Lin, T.C.; Wang, G.H.; Anderson, R.; Lin, C.C.; Richardson, C.D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Cooper, L.; Chen, Z.; Lee, H.; Rong, L.; Cui, Q. Discovery of chebulagic acid and punicalagin as novel allosteric inhibitors of SARS-CoV-2 3CLpro. Antivir. Res. 2021, 190, 105075. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, A.; Polachira, S.K.; Nair, R.; Agarwal, A.; Mishra, N.N.; Gupta, S.K. Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids. BMC Complement. Altern. Med. 2017, 17, 110. [Google Scholar] [CrossRef]
- Li, P.; Du, R.; Wang, Y.; Hou, X.; Wang, L.; Zhao, X.; Zhan, P.; Liu, X.; Rong, L.; Cui, Q. Identification of chebulinic acid and chebulagic acid as novel influenza viral neuraminidase inhibitors. Front. Microbiol. 2020, 11, 182. [Google Scholar] [CrossRef]
- Brittain, D.C. Erythromycin. Med. Clin. N. Am. 1987, 71, 1147–1154. [Google Scholar] [CrossRef]
- Hsieh, S.K.; Xu, J.R.; Lin, N.H.; Li, Y.C.; Chen, G.H.; Kuo, P.C.; Chen, W.Y.; Tzen, J.T.C. Antibacterial and laxative activities of strictinin isolated from Pu’er tea (Camellia sinensis). J. Food Drug Anal. 2016, 24, 722–729. [Google Scholar] [CrossRef]
- Wu, S.C.; Yen, G.C.; Wang, B.S.; Chiu, C.K.; Yen, W.J.; Chang, L.W.; Duh, P.D. Antimutagenic and antimicrobial activities of pu-erh tea. LWT-Food Sci. Technol. 2007, 40, 506–512. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Zhang, H.; Sun, W.; Li, Z.; Zhang, F.; Zhang, H.; Chen, F.; Zhang, H.; An, J.; et al. Antimicrobial mechanism of strictinin isomers extracted from the root of Rosa roxburghii Tratt (Ci Li Gen). J. Ethnopharmacol. 2020, 250, 112498. [Google Scholar] [CrossRef]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Lee, L.K.; Foo, K.Y. Recent advances on the beneficial use and health implications of Pu-Erh tea. Food Res. Int. 2013, 53, 619–628. [Google Scholar] [CrossRef]
- Chen, T.Y.; Wang, M.M.C.; Hsieh, S.K.; Hsieh, M.H.; Chen, W.Y.; Tzen, J.T.C. Pancreatic lipase inhibition of strictinin isolated from Pu’er tea (Cammelia sinensis) and its anti-obesity effects in C57BL6 mice. J. Funct. Foods 2018, 48, 1–8. [Google Scholar] [CrossRef]
- Zhi, J.; Melia, A.T.; Eggers, H.; Joly, R.; Patel, I.H. Review of limited systemic absorption of orlistat, a lipase inhibitor, in healthy human volunteers. J. Clin. Pharmacol. 1995, 35, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Derdemezis, C.S.; Gazi, I.F.; Nakou, E.S.; Mikhailidis, D.P.; Elisaf, M.S. Orlistat-associated adverse effects and drug interactions: A critical review. Drug Saf. 2008, 31, 53–65. [Google Scholar] [CrossRef]
- Sall, D.; Wang, J.; Rashkin, M.; Welch, M.; Droege, C.; Schauer, D. Orlistat induced fulminant hepatic failure. Clin. Obes. 2014, 4, 342–347. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Gan, R.Y.; Zhu, F. Chemical constituents and biological properties of Pu-erh tea. Food Res. Int. 2022, 154, 110899. [Google Scholar] [CrossRef]
- Loesche, W.J. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef]
- Chen, X.; Daliri, E.B.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.H. Microbial etiology and prevention of dental caries: Exploiting natural products to inhibit cariogenic biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Liao, M.H.; Wang, X.R.; Hsu, W.L.; Tzen, J.T.C. Pu’er tea rich in strictinin and catechins prevents biofilm formation of two cariogenic bacteria, Streptococcus mutans and Streptococcus sobrinus. J. Dent. Sci. 2021, 16, 1331–1334. [Google Scholar] [CrossRef]
- Holgate, S.T. The epidemic of allergy and asthma. Nature 1999, 402 (Suppl. 6760), B2–B4. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Kubo, T.; Miyase, T.; Tanino, S.; Yoshimoto, M.; Sano, M.; Yamamoto-Maeda, M.; Yamada, K. Identification of an inhibitor for interleukin 4-induced epsilon germline transcription and antigen-specific IgE production in vivo. Biochem. Biophys. Res. Commun. 2001, 280, 53–60. [Google Scholar] [CrossRef]
- Kim, Y.H.; Ninomiya, Y.; Yamashita, S.; Kumazoe, M.; Huang, Y.; Nakahara, K.; Won, Y.S.; Murata, M.; Fujimura, Y.; Yamada, K.; et al. IL-4 receptor α in non-lipid rafts is the target molecule of strictinin in inhibiting STAT6 activation. Biochem. Biophys. Res. Commun. 2014, 450, 824–830. [Google Scholar] [CrossRef]
- Liu, L.; Cai, X.C.; Sun, X.Y.; Zhou, Y.Q.; Jin, M.Z.; Wang, J.; Ma, T.; Li, B.; Li, X. Global prevalence of metabolic syndrome in patients with psoriasis in the past two decades: Current evidence. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1969–1979. [Google Scholar] [CrossRef]
- Zhong, L.; Luo, N.; Zhong, X.; Xu, T.; Hao, P. The immunoregulatory effects of natural products on psoriasis via its action on Th17 cells versus regulatory T cells balance. Int. Immunopharmacol. 2022, 110, 109032. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhu, Q.; Wang, S.; Li, X.; Sun, Z.; Li, N.; Feng, J.; Ding, H.; Dong, S.; Wang, H. Clinical efficacy and safety of using calcipotriol-betamethasone compounding agent for psoriasis treatment: A systematic review and meta-analysis. Arch. Dermatol. Res. 2022, 314, 633–641. [Google Scholar] [CrossRef]
- Hari, G.; Kishore, A.; Karkala, S.R.P. Treatments for psoriasis: A journey from classical to advanced therapies. How far have we reached? Eur. J. Pharmacol. 2022, 929, 175147. [Google Scholar] [CrossRef]
- Lin, P.Y.; Jhuo, C.F.; Lin, N.H.; Chen, W.Y.; Tzen, J.T.C. Assessing anti-psoriatic effects of bitter Pu’er tea and its three major compounds, strictinin, theacrine and epigallocatechin gallate in Iimiquimod-treated mice. Compounds 2022, 2, 293–306. [Google Scholar] [CrossRef]
- Takayama, S.; Kawanishi, M.; Yamauchi, K.; Tokumitsu, D.; Kojima, H.; Masutani, T.; Iddamalgoda, A.; Mitsunaga, T.; Tanaka, H. Ellagitannins from Rosa roxburghii suppress poly(I:C)-induced IL-8 production in human keratinocytes. J. Nat. Med. 2021, 75, 623–632. [Google Scholar] [CrossRef]
- Babio, N.; Martínez-González, M.A.; Estruch, R.; Wärnberg, J.; Recondo, J.; Ortega-Calvo, M.; Serra-Majem, L.; Corella, D.; Fitó, M.; Ros, E.; et al. Associations between serum uric acid concentrations and metabolic syndrome and its components in the PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Nagata, N.; Shimakami, T.; Shirakura, T.; Matsui, C.; Ni, Y.; Zhuge, F.; Xu, L.; Chen, G.; Nagashimada, M.; et al. Xanthine oxidase inhibition attenuates insulin resistance and diet-induced steatohepatitis in mice. Sci. Rep. 2020, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Thottam, G.E.; Krasnokutsky, S.; Pillinger, M.H. Gout and metabolic syndrome: A tangled web. Curr. Rheumatol. Rep. 2017, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.C.; Chang, Y.T.; Pranata, R.; Cheng, Y.H.; Chen, Y.C.; Kuo, P.C.; Huang, Y.H.; Tzen, J.T.C.; Chen, R.J. Alleviation of hyperuricemia by strictinin in aml12 mouse hepatocytes treated with xanthine and in mice treated with potassium oxonate. Biology 2023, 12, 329. [Google Scholar] [CrossRef]
- Sun, J.; Ren, J.; Hu, X.; Hou, Y.; Yang, Y. Therapeutic effects of Chinese herbal medicines and their extracts on diabetes. Biomed. Pharmacother. 2021, 142, 11197. [Google Scholar] [CrossRef] [PubMed]
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef]
- Barkaoui, M.; Katiri, A.; Boubaker, H.; Msanda, F. Ethnobotanical survey of medicinal plants used in the traditional treatment of diabetes in Chtouka Ait Baha and Tiznit (Western Anti-Atlas), Morocco. J. Ethnopharmacol. 2017, 198, 338–350. [Google Scholar] [CrossRef]
- Tolmie, M.; Bester, M.J.; Serem, J.C.; Nell, M.; Apostolides, Z. The potential antidiabetic properties of green and purple tea [Camellia sinensis (L.) O Kuntze], purple tea ellagitannins, and urolithins. J. Ethnopharmacol. 2023, 309, 116377. [Google Scholar] [CrossRef]
- Lin, H.C.; Lee, C.T.; Yen, Y.Y.; Chu, C.L.; Hsieh, Y.P.; Yang, C.S.; Lan, S.J. Systematic review and meta-analysis of anti-hyperglycaemic effects of Pu-erh tea. Int. J. Food Sci. Technol. 2019, 54, 516–525. [Google Scholar] [CrossRef]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Boyle, P. Triple-negative breast cancer: Epidemiological considerations and recommendations. Ann. Oncol. 2012, 23 (Suppl. 6), vi7–vi12. [Google Scholar] [CrossRef]
- Brar, J.; Fultang, N.; Askey, K.; Tettamanzi, M.C.; Peethambaran, B. A novel anti-triple negative breast cancer compound isolated from medicinal herb Myrothamnus flabellifolius. J. Med. Plant Res. J. Med. Plant Res. 2018, 12, 7–14. [Google Scholar]
- Fultang, N.; Illendula, A.; Chen, B.; Wu, C.; Jonnalagadda, S.; Baird, N.; Klase, Z.; Peethambaran, B. Strictinin, a novel ROR1-inhibitor, represses triple negative breast cancer survival and migration via modulation of PI3K/AKT/GSK3ß activity. PLoS ONE 2019, 14, e0217789. [Google Scholar] [CrossRef] [PubMed]
- Tzen, J.T.C. “Bitter Citrus Tzen Tea”—Introducing a new trend in healthy drinks for modern people. NCHU ARCH 2021, 1, 6–7. [Google Scholar]
- Kao, J.Y.; Li, Y.C.; Wang, M.M.C.; Kuo, P.C.; Tzen, J.T.C. Analysis of major flavonoid glycosylates of Citrus aurantium L. cv. Hutou Gan. J. Agric. For. 2020, 67, 261–272. [Google Scholar]
- Nie, Y.C.; Wu, H.; Li, P.B.; Xie, L.M.; Luo, Y.L.; Shen, J.G.; Su, W.W. Naringin attenuates EGF-induced MUC5AC secretion in A549 cells by suppressing the cooperative activities of MAPKs-AP-1 and IKKs-IκB-NF-κB signaling pathways. Eur. J. Pharmacol. 2012, 690, 207–213. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Nie, Y.C.; Li, P.B.; Shen, J.G.; Su, W.W. Mucoactive effects of naringin in lipopolysaccharide-induced acute lung injury mice and beagle dogs. Environ. Toxicol. Pharmacol. 2014, 38, 279–287. [Google Scholar] [CrossRef]
- Gao, S.; Li, P.; Yang, H.; Fang, S.; Su, W. Antitussive effect of naringin on experimentally induced cough in Guinea pigs. Planta Med. 2011, 77, 16–21. [Google Scholar] [CrossRef]
- Dong, W.; Wei, X.; Zhang, F.; Hao, J.; Huang, F.; Zhang, C.; Liang, W. A dual character of flavonoids in influenza A virus replication and spread through modulating cell-autonomous immunity by MAPK signaling pathways. Sci. Rep. 2014, 4, 7237. [Google Scholar] [CrossRef]
- Wang, M.M.C.; Chen, Y.J.; Tzen, J.T.C. Tzen oolong tea converted from oolong tea by baking and aging periodically. J. Agric. For. 2014, 63, 89–96. [Google Scholar]
- Lo, Y.H.; Chen, Y.J.; Chang, C.I.; Lin, Y.W.; Chen, C.Y.; Lee, M.R.; Lee, V.S.; Tzen, J.T. Teaghrelins, unique acylated flavonoid tetraglycosides in Chin-shin oolong tea, are putative oral agonists of the ghrelin receptor. J. Agric. Food Chem. 2014, 62, 5085–5091. [Google Scholar] [CrossRef]
- Li, Y.C.; Wu, C.J.; Lin, Y.C.; Wu, R.H.; Chen, W.Y.; Kuo, P.C.; Tzen, J.T.C. Identification of two teaghrelins in Shy-jih-chuen oolong tea. J. Food Biochem. 2019, 43, e12810. [Google Scholar] [CrossRef] [PubMed]
- Jhuo, C.F.; Hsieh, S.K.; Chen, C.J.; Chen, W.Y.; Tzen, J.T.C. Teaghrelin Protects SH-SY5Y cells against MPP(+)-induced neurotoxicity through activation of AMPK/Sirt1/PGC-1α and ERK1/2 pathways. Nutrients 2020, 12, 3665. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.K.; Lin, H.Y.; Chen, C.J.; Jhuo, C.F.; Liao, K.Y.; Chen, W.Y.; Tzen, J.T.C. Promotion of myotube differentiation and attenuation of muscle atrophy in murine C2C12 myoblast cells treated with teaghrelin. Chem.-Biol. Interact. 2020, 315, 108893. [Google Scholar] [CrossRef]
- Jhuo, C.F.; Hsieh, S.K.; Chen, W.Y.; Tzen, J.T.C. Attenuation of skeletal muscle atrophy induced by dexamethasone in rats by teaghrelin supplementation. Molecules 2023, 28, 688. [Google Scholar] [CrossRef] [PubMed]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Goel, D.; Goel, G.K.; Chaudhary, S.; Jain, D. Antibiotic prescriptions in pediatric dentistry: A review. J. Fam. Med. Prim. Care 2020, 9, 473–480. [Google Scholar] [CrossRef]
- Simmer, J.P.; Hardy, N.C.; Chinoy, A.F.; Bartlett, J.D.; Hu, J.C. How fluoride protects dental enamel from demineralization. J. Int. Soc. Prev. Community Dent. 2020, 10, 134–141. [Google Scholar] [CrossRef]
- Rose, M.A.; Garcez, T.; Savic, S.; Garvey, L.H. Chlorhexidine allergy in the perioperative setting: A narrative review. Br. J. Anaesth. 2019, 123, e95–e103. [Google Scholar] [CrossRef]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 110. [Google Scholar] [CrossRef]
- Bansal, R.; Jain, A.; Goyal, M.; Singh, T.; Sood, H.; Malviya, H.S. Antibiotic abuse during endodontic treatment: A contributing factor to antibiotic resistance. J. Fam. Med. Prim. Care 2019, 8, 3518–3524. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzen, J.T.C. Strictinin: A Key Ingredient of Tea. Molecules 2023, 28, 3961. https://doi.org/10.3390/molecules28093961
Tzen JTC. Strictinin: A Key Ingredient of Tea. Molecules. 2023; 28(9):3961. https://doi.org/10.3390/molecules28093961
Chicago/Turabian StyleTzen, Jason T. C. 2023. "Strictinin: A Key Ingredient of Tea" Molecules 28, no. 9: 3961. https://doi.org/10.3390/molecules28093961
APA StyleTzen, J. T. C. (2023). Strictinin: A Key Ingredient of Tea. Molecules, 28(9), 3961. https://doi.org/10.3390/molecules28093961





