Preparation of Graphene Oxide-Maghemite-Chitosan Composites for the Adsorption of Europium Ions from Aqueous Solutions
Abstract
1. Introduction
2. Results and Discussion
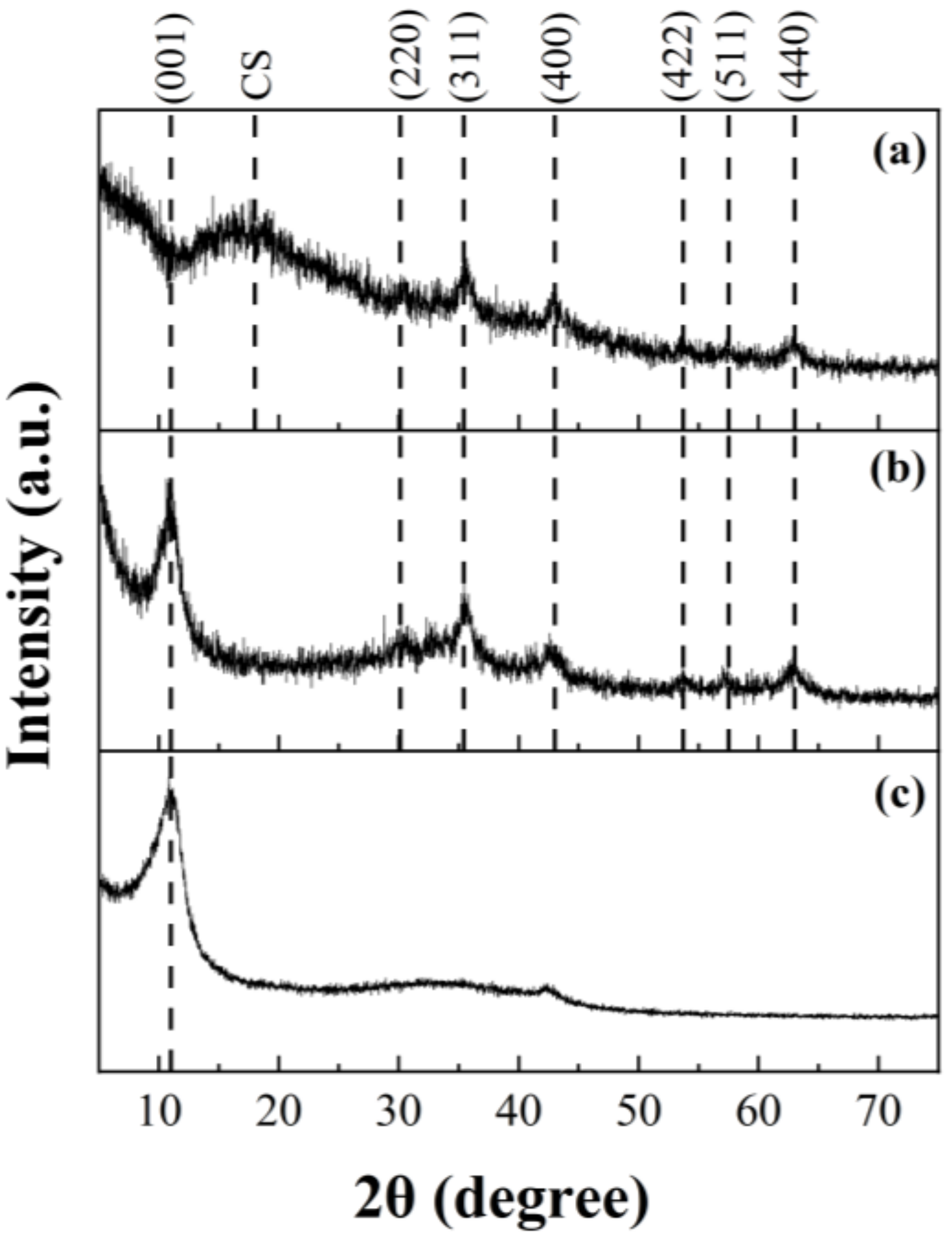
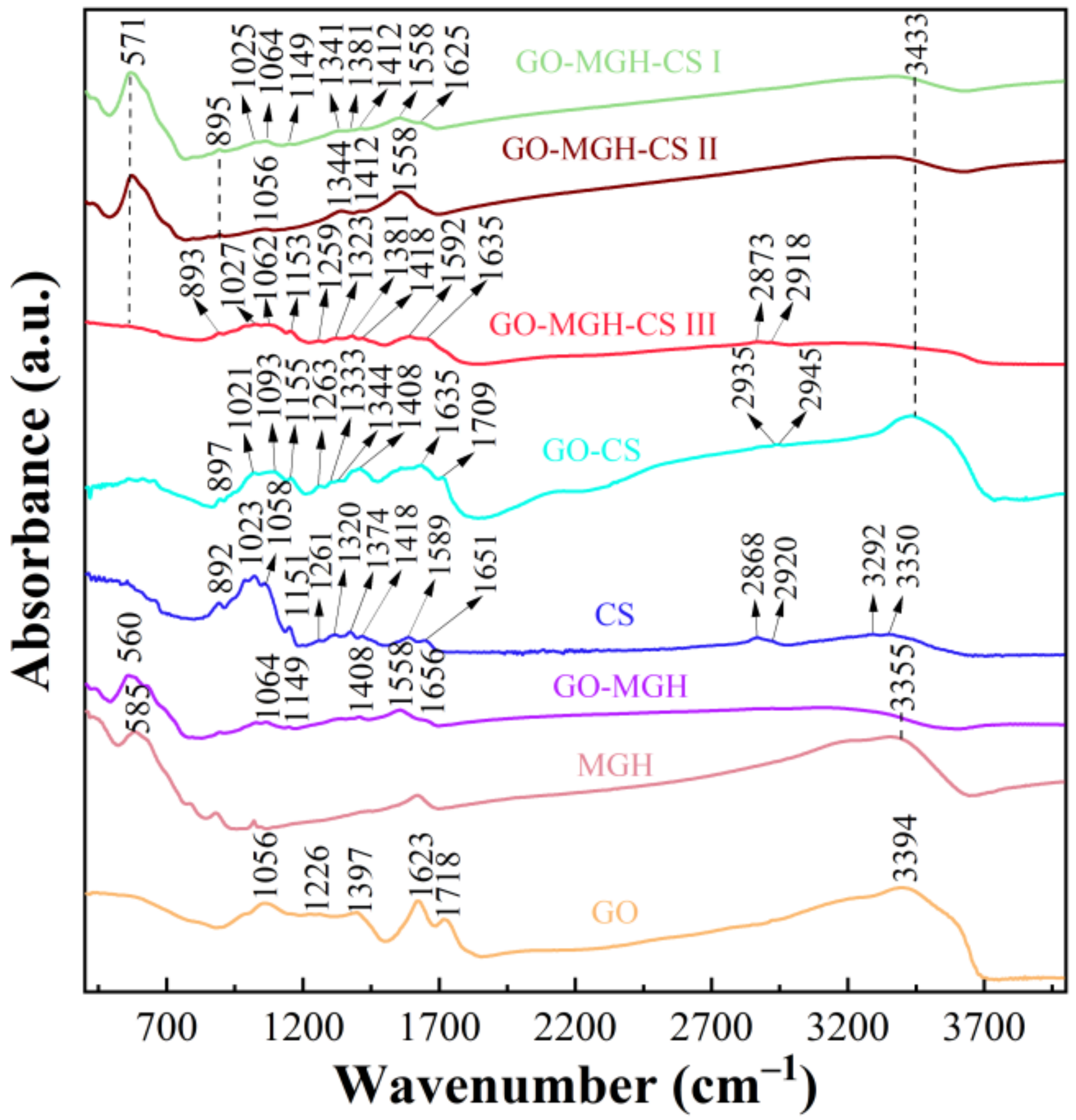
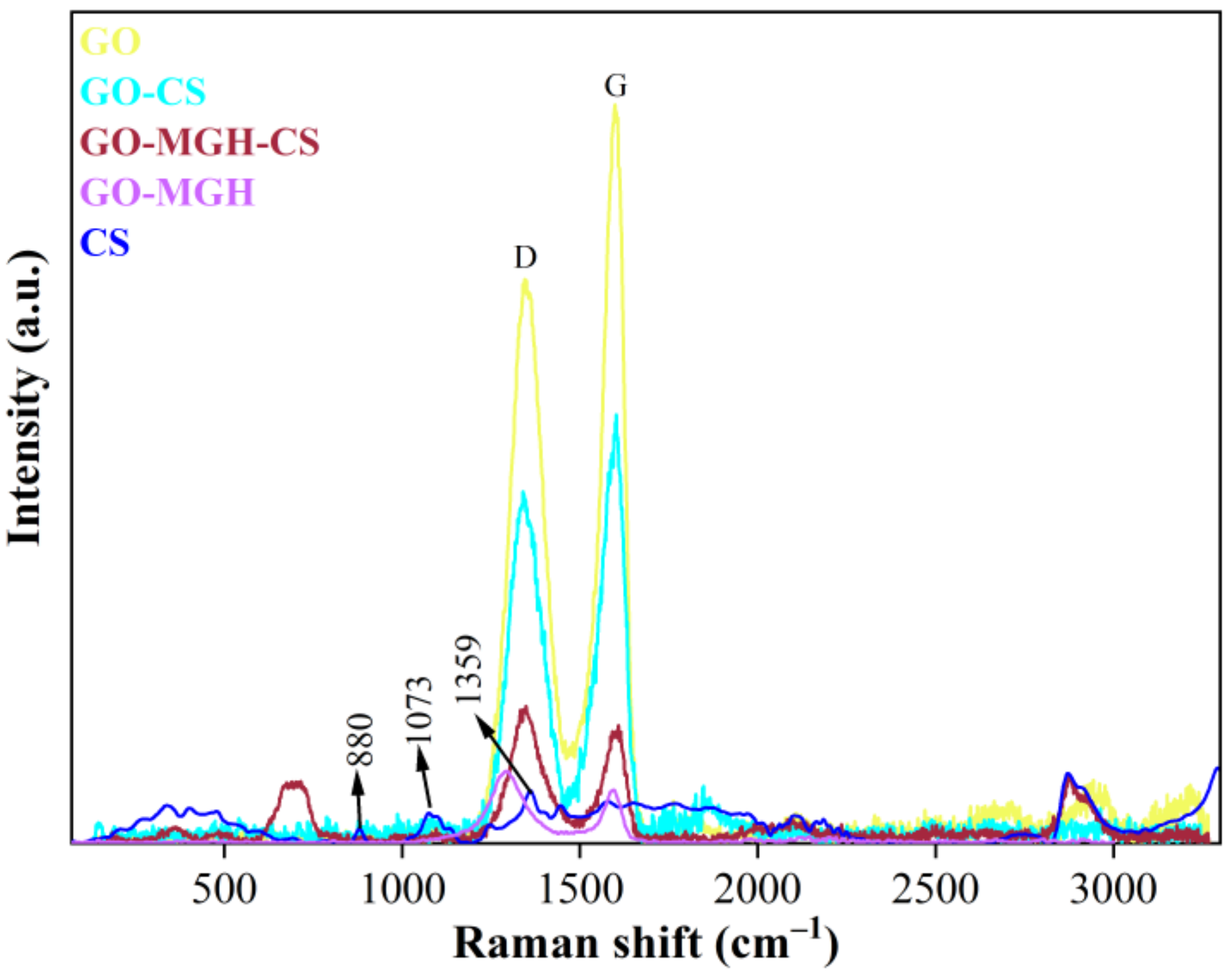
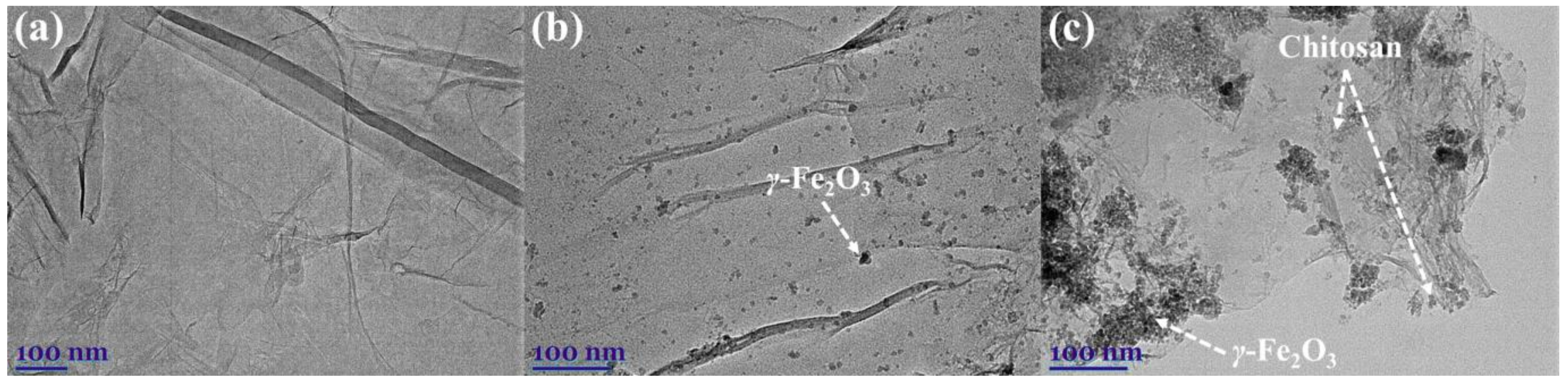
2.1. Material Characterisation
2.2. Adsorption Studies
2.2.1. Effect of the Initial Concentration of Eu(III) and the Adsorption Isotherms
2.2.2. Effect of pH
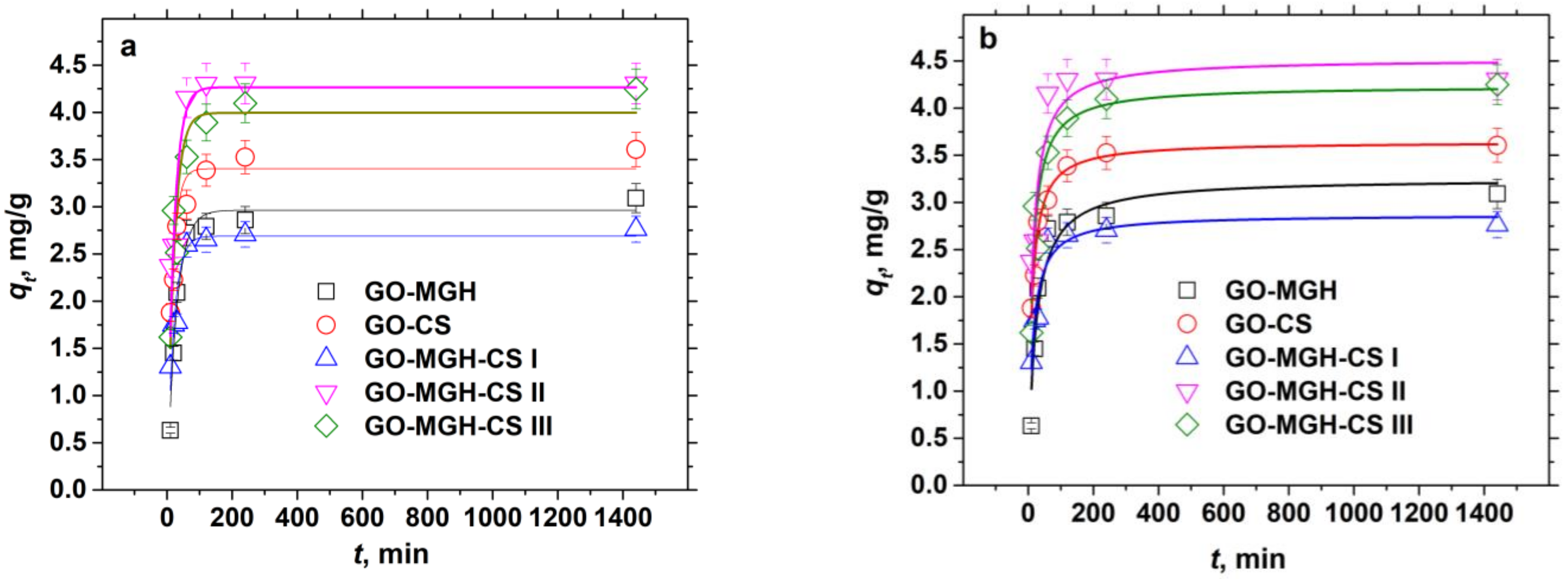
2.2.3. Kinetic Studies
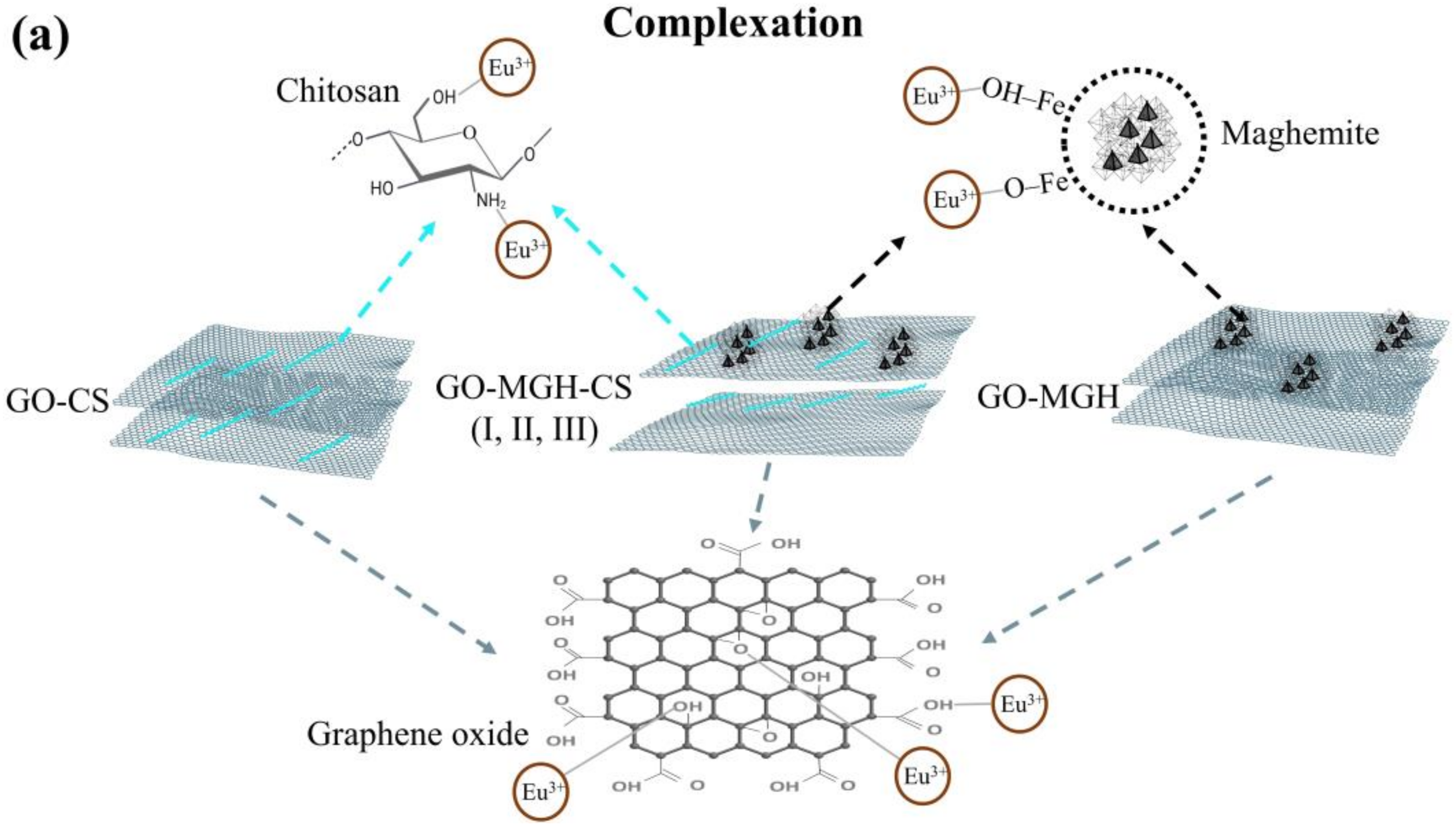
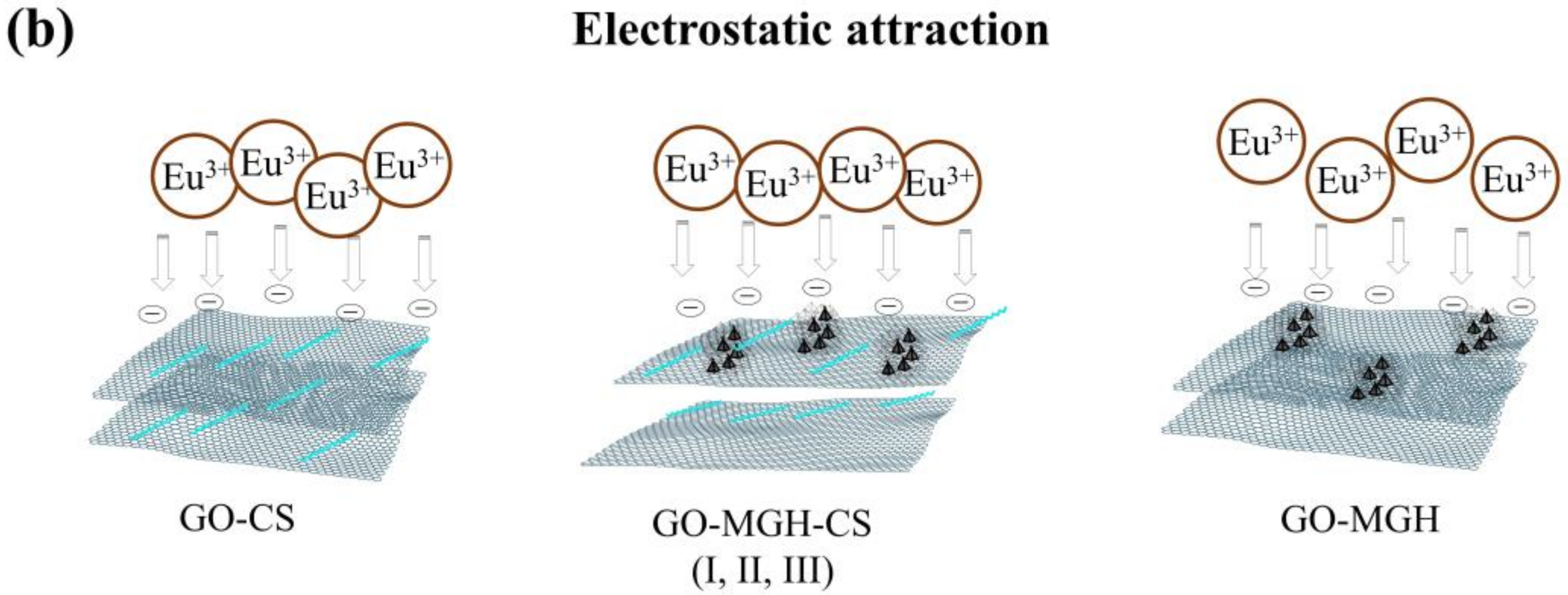
2.3. Adsorption Mechanism
2.4. Removal of Eu(III), Pu(IV), and Am(III) from Natural Waters
2.5. Adaptive Neuro-Fuzzy Interference System
3. Materials and Methods
3.1. Preparation of Composites
3.2. Characterisation
3.3. Batch Experiments
3.4. Determination of the Point of Zero Charge
3.5. Removal of Europium, Americium, and Plutonium Ions from Natural Waters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Yang, S.; Cheng, G.; Gu, P. The Adsorption of Europium and Uranium on the Sodium Dodecyl Sulfate Modified Molybdenum Disulfide Composites. J. Chem. Eng. Data 2020, 65, 2178–2185. [Google Scholar] [CrossRef]
- Liu, F.; Huang, W.; Wang, S.; Hu, B. Investigation of Adsorption Properties and Mechanism of Uranium (VI) and Europium (III) on Magnetic Amidoxime-Functionalized MCM-41. Appl. Surf. Sci. 2022, 594, 153376. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, M.; Lan, J.; Yuan, L.; Yu, J.; Li, J.; Peng, J.; Chai, Z.; Gibson, J.K.; Zhai, M.; et al. Radiation Controllable Synthesis of Robust Covalent Organic Framework Conjugates for Efficient Dynamic Column Extraction of 99TcO4−. Chem 2020, 6, 2796–2809. [Google Scholar] [CrossRef]
- Santos, E.A.; Ladeira, A.C.Q. Recovery of Uranium from Mine Waste by Leaching with Carbonate-Based Reagents. Environ. Sci. Technol. 2011, 45, 3591–3597. [Google Scholar] [CrossRef]
- Maloubier, M.; Shuh, D.K.; Minasian, S.G.; Pacold, J.I.; Solari, P.L.; Michel, H.; Oberhaensli, F.R.; Bottein, Y.; Monfort, M.; Moulin, C.; et al. How Do Radionuclides Accumulate in Marine Organisms? A Case Study of Europium with Aplysina Cavernicola. Environ. Sci. Technol. 2016, 50, 10730–10738. [Google Scholar] [CrossRef]
- Yu, J.; Yuan, L.; Wang, S.; Lan, J.; Zheng, L.; Xu, C.; Chen, J.; Wang, L.; Huang, Z.; Tao, W.; et al. Phosphonate-Decorated Covalent Organic Frameworks for Actinide Extraction: A Breakthrough under Highly Acidic Conditions. CCS Chem. 2019, 1, 286–295. [Google Scholar]
- Newsome, L.; Morris, K.; Trivedi, D.; Bewsher, A.; Lloyd, J.R. Biostimulation by Glycerol Phosphate to Precipitate Recalcitrant Uranium(IV) Phosphate. Environ. Sci. Technol. 2015, 49, 11070–11078. [Google Scholar] [CrossRef]
- Candela, A.M.; Benatti, V.; Palet, C. Pre-Concentration of Uranium (VI) Using Bulk Liquid and Supported Liquid Membrane Systems Optimized Containing Bis (2-Ethylhexyl) Phosphoric Acid as Carrier in Low Concentrations. Sep. Purif. Technol. 2013, 120, 172–179. [Google Scholar] [CrossRef]
- Bruggeman, C.; Maes, N. Uptake of Uranium (VI) by Pyrite under Boom Clay Conditions: Influence of Dissolved Organic Carbon. Environ. Sci. Technol. 2010, 44, 4210–4216. [Google Scholar] [CrossRef]
- Li, Y.; Sheng, G.; Sheng, J. Magnetite Decorated Graphene Oxide for the Highly Efficient Immobilization of Eu(III) from Aqueous Solution. J. Mol. Liq. 2014, 199, 474–480. [Google Scholar] [CrossRef]
- Lujanienė, G.; Beneš, P.; Štamberg, K.; Šapolaitė, J.; Vopalka, D.; Radžiūtė, E.; Ščiglo, T. Effect of Natural Clay Components on Sorption of Cs, Pu and Am by the Clay. J. Radioanal. Nucl. Chem. 2010, 286, 353–359. [Google Scholar] [CrossRef]
- Zheng, M.; Ji, H.; Duan, J.; Dang, C.; Chen, X.; Liu, W. Efficient Adsorption of Europium (III) and Uranium (VI) by Titanate Nanorings: Insights into Radioactive Metal Species. Environ. Sci. Ecotechnol. 2020, 2, 100031. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.H.; Kuo, C.H.; Huang, L.H.; Su, C.C. Carbon Footprint Assessment of Recycling Technologies for Rare Earth Elements: A Case Study of Recycling Yttrium and Europium from Phosphor. Waste Manag. 2017, 60, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Taylor, S.D.; Powell, B.A.; Becker, U. An Ab Initio Study of the Adsorption of Eu3+, Pu3+, Am3+, and Cm3+ Hydroxide Complexes on Hematite (001) Surface: Role of Magnetism on Adsorption. Surf. Sci. 2017, 664, 120–128. [Google Scholar] [CrossRef]
- Chen, C.L.; Wang, X.K.; Nagatsu, M. Europium Adsorption on Multiwall Carbon Nanotube/Iron Oxide Magnetic Composite in the Presence of Polyacrylic Acid. Environ. Sci. Technol. 2009, 43, 2362–2367. [Google Scholar] [CrossRef]
- Modi, M.K.; Pattanaik, P.; Dash, N.; Subramanian, S. Sorption of Radionuclides. Int. J. Pharm. Sci. Rev. Res. 2015, 34, 122–130. [Google Scholar]
- Yong, S.K.; Shrivastava, M.; Srivastava, P.; Kunhikrishnan, A.; Bolan, N. Environmental Applications of Chitosan and Its Derivatives. Rev. Environ. Contam. Toxicol. 2014, 233, 1–43. [Google Scholar]
- Wang, J.; Han, Q.; Wang, K.; Li, S.; Luo, W.; Liang, Q.; Zhong, J.; Ding, M. Recent Advances in Development of Functional Magnetic Adsorbents for Selective Separation of Proteins/Peptides. Talanta 2022, 253, 123919. [Google Scholar] [CrossRef]
- Ahmad Wani, A.; Shahadat, M.; Wazed Ali, S.; Ziauddin Ahammad, S.; Kashif Uddin, M. Recent Advances and Future Perspectives of Polymer-Based Magnetic Nanomaterials for Detection and Removal of Radionuclides: A Review. J. Mol. Liq. 2022, 365, 119976. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and Application of Tetrabromobisphenol A Imprinted Electrochemical Sensor Based on Graphene/Carbon Nanotubes Three-Dimensional Nanocomposites Modified Carbon Electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wu, X.; Wu, P. Multi-Mode Surface Plasmon Resonance Absorber Based on Dart-Type Single-Layer Grapheme. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Ren, X.; Gao, X.; Tan, X.; Li, J.; Chen, C.; Huang, Y.; Wang, X. Removal of Pb (II) Ions from Aqueous Solutions on Few-Layered Graphene Oxide Nanosheets. Dalton Trans. 2011, 40, 10945–10952. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G.; Li, Y.; Yang, X.; Ren, X.; Yang, S.; Hu, J.; Wang, X. Efficient Removal of Arsenate by Versatile Magnetic Graphene Oxide Composites. RSC Adv. 2012, 2, 12400–12407. [Google Scholar] [CrossRef]
- Chen, H.; Li, J.X.; Zhang, S.W.; Ren, X.M.; Sun, Y.B.; Wen, T.; Wang, X.K. Study on the Acid-Base Surface Property of the Magnetite Graphene Oxide and Its Usage for the Removal of Radiostrontium from Aqueous Solution. Radiochim. Acta 2013, 101, 785–794. [Google Scholar] [CrossRef]
- Liu, M.; Chen, C.; Hu, J.; Wu, X.; Wang, X. Synthesis of Magnetite/Graphene Oxide Composite and Application for Cobalt (II) Removal. J. Phys. Chem. C 2011, 115, 25234–25240. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-Step Fabrication of Graphene Oxide Enhanced Magnetic Composite Gel for Highly Efficient Dye Adsorption and Catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar]
- Wei, J.; Aly Aboud, M.F.; Shakir, I.; Tong, Z.; Xu, Y. Graphene Oxide-Supported Organo-Montmorillonite Composites for the Removal of Pb (II), Cd (II), and As (V) Contaminants from Water. ACS Appl. Nano Mater. 2020, 3, 806–813. [Google Scholar] [CrossRef]
- Romanchuk, A.Y.; Slesarev, A.S.; Kalmykov, S.N.; Kosynkin, D.V.; Tour, J.M. Graphene Oxide for Effective Radionuclide Removal. Phys. Chem. Chem. Phys. 2013, 15, 2321–2327. [Google Scholar] [CrossRef]
- Kuzenkova, A.S.; Romanchuk, A.Y.; Trigub, A.L.; Maslakov, K.I.; Egorov, A.V.; Amidani, L.; Kittrell, C.; Kvashnina, K.O.; Tour, J.M.; Talyzin, A.V.; et al. New Insights into the Mechanism of Graphene Oxide and Radionuclide Interaction. Carbon 2020, 158, 291–302. [Google Scholar] [CrossRef]
- Boulanger, N.; Kuzenkova, A.S.; Iakunkov, A.; Romanchuk, A.Y.; Trigub, A.L.; Egorov, A.V.; Bauters, S.; Amidani, L.; Retegan, M.; Kvashnina, K.O.; et al. Enhanced Sorption of Radionuclides by Defect-Rich Graphene Oxide. ACS Appl. Mater. Interfaces 2020, 12, 45122–45135. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, N.; Kuzenkova, A.S.; Iakunkov, A.; Nordenström, A.; Romanchuk, A.Y.; Trigub, A.L.; Zasimov, P.V.; Prodana, M.; Enachescu, M.; Bauters, S.; et al. High Surface Area “3D Graphene Oxide” for Enhanced Sorption of Radionuclides. Adv. Mater. Interfaces 2022, 9, 2200510. [Google Scholar] [CrossRef]
- Tripathy, M.; Hota, G. Maghemite and Graphene Oxide Embedded Polyacrylonitrile Electrospun Nanofiber Matrix for Remediation of Arsenate Ions. ACS Appl. Polym. Mater. 2020, 2, 604–617. [Google Scholar] [CrossRef]
- Hu, Z.; Qin, S.; Huang, Z.; Zhu, Y.; Xi, L.; Li, Z. Recyclable Graphene Oxide-Covalently Encapsulated Magnetic Composite for Highly Efficient Pb(II) Removal. J. Environ. Chem. Eng. 2017, 5, 4630–4638. [Google Scholar] [CrossRef]
- Samuel, M.S.; Shah, S.S.; Subramaniyan, V.; Qureshi, T.; Bhattacharya, J.; Pradeep Singh, N.D. Preparation of Graphene Oxide/Chitosan/Ferrite Nanocomposite for Chromium (VI) Removal from Aqueous Solution. Int. J. Biol. Macromol. 2018, 119, 540–547. [Google Scholar] [CrossRef]
- Tran, H.V.; Bui, L.T.; Dinh, T.T.; Le, D.H.; Huynh, C.D.; Trinh, A.X. Graphene Oxide/Fe3O4/Chitosan Nanocomposite: A Recoverable and Recyclable Adsorbent for Organic Dyes Removal. Application to Methylene Blue. Mater. Res. Express 2017, 4, 035701. [Google Scholar] [CrossRef]
- Taher, F.A.; Kamal, F.H.; Badawy, N.A.; Shrshr, A.E. Hierarchical Magnetic/Chitosan/Graphene Oxide 3D Nanostructure as Highly Effective Adsorbent. Mater. Res. Bull. 2018, 97, 361–368. [Google Scholar] [CrossRef]
- Khan, S.; Anjum, R.; Bilal, M. Revealing Chemical Speciation Behaviors in Aqueous Solutions for Uranium (VI) and Europium (III) Adsorption on Zeolite. Environ. Technol. Innov. 2021, 22, 101503. [Google Scholar] [CrossRef]
- Carvalho, R.S.; Daniel-Da-Silva, A.L.; Trindade, T. Uptake of Europium (III) from Water Using Magnetite Nanoparticles. Part. Part. Syst. Charact. 2016, 33, 150–157. [Google Scholar] [CrossRef]
- Ding, C.; Cheng, W.; Sun, Y.; Wang, X. Retracted Article: Determination of Chemical Affinity of Graphene Oxide Nanosheets with Radionuclides Investigated by Macroscopic, Spectroscopic and Modeling Techniques. Dalton Trans. 2014, 43, 3888–3896. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, Y.; Liu, H.; Jin, Z.; Chen, T. The Kinetic and Thermodynamic Adsorption of Eu (III) on Synthetic Maghemite. J. Mol. Liq. 2016, 221, 171–178. [Google Scholar] [CrossRef]
- Cadogan, E.I.; Lee, C.H.; Popuri, S.R.; Lin, H.Y. Efficiencies of Chitosan Nanoparticles and Crab Shell Particles in Europium Uptake from Aqueous Solutions through Biosorption: Synthesis and Characterization. Int. Biodeterior. Biodegrad. 2014, 95, 232–240. [Google Scholar] [CrossRef]
- Arunraj, B.; Talasila, S.; Rajesh, V.; Rajesh, N. Removal of Europium from Aqueous Solution Using Saccharomyces cerevisiae Immobilized in Glutaraldehyde Cross-Linked Chitosan. Sep. Sci. Technol. 2019, 54, 1620–1631. [Google Scholar]
- Souza, P.R.; Dotto, G.L.; Salau, N.P.G. Artificial Neural Network (ANN) and Adaptive Neuro-Fuzzy Interference System (ANFIS) Modelling for Nickel Adsorption onto Agro-Wastes and Commercial Activated Carbon. J. Environ. Chem. Eng. 2018, 6, 7152–7160. [Google Scholar] [CrossRef]
- Onu, C.E.; Nwabanne, J.T.; Ohale, P.E.; Asadu, C.O. Comparative Analysis of RSM, ANN and ANFIS and the Mechanistic Modeling in Eriochrome Black-T Dye Adsorption Using Modified Clay. S. Afr. J. Chem. Eng. 2021, 36, 24–42. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Mehrabpour, M.; Esfandyari, M.; Alidadi, H.; Davoudi, M. Modeling of Simultaneous Adsorption of Dye and Metal Ion by Sawdust from Aqueous Solution Using of ANN and ANFIS. Chemom. Intell. Lab. Syst. 2018, 181, 72–78. [Google Scholar] [CrossRef]
- Noorani Khomeyrani, S.F.; Azqhandi Azqhandi, M.H.; Ghalami-Choobar, B. Rapid and Efficient Ultrasonic Assisted Adsorption of PNP onto LDH-GO-CNTs: ANFIS, GRNN and RSM Modeling, Optimization, Isotherm, Kinetic, and Thermodynamic Study. J. Mol. Liq. 2021, 333, 115917. [Google Scholar] [CrossRef]
- Mendoza-Castillo, D.I.; Reynel-Ávila, H.E.; Sánchez-Ruiz, F.J.; Trejo-Valencia, R.; Jaime-Leal, J.E.; Bonilla-Petriciolet, A. Insights and Pitfalls of Artificial Neural Network Modeling of Competitive Multi-Metallic Adsorption Data. J. Mol. Liq. 2018, 251, 15–27. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.W.; Jung, W.G. Facile and Safe Graphene Preparation on Solution Based Platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Zhang, X.; He, M.; Zhan, Y.; Yang, W.; Wu, K. Microstructure, Mechanical and Electrical Properties of Hybrid Copper Matrix Composites with Fe Microspheres and RGO Nanosheets. Molecules 2022, 27, 6518. [Google Scholar] [CrossRef]
- Jiang, Y.; Gong, J.L.; Zeng, G.M.; Ou, X.M.; Chang, Y.N.; Deng, C.H.; Zhang, J.; Liu, H.Y.; Huang, S.Y. Magnetic Chitosan–Graphene Oxide Composite for Anti-Microbial and Dye Removal Applications. Int. J. Biol. Macromol. 2016, 82, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Khan, R.; Zulfiqar; Khan, T.Z.; Khan, G.; Khattak, S.; Rahman, M.U.; Ali, S.; Iqbal, Z.; Burhanullah; et al. Dielectric and Magnetic Properties of Cobalt Doped γ-Fe2O3 nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 13698–13707. [Google Scholar] [CrossRef]
- Yadav, B.S.; Singh, R.; Vishwakarma, A.K.; Kumar, N. Facile Synthesis of Substantially Magnetic Hollow Nanospheres of Maghemite (γ-Fe2O3) Originated from Magnetite (Fe3O4) via Solvothermal Method. J. Supercond. Nov. Magn. 2020, 33, 2199–2208. [Google Scholar] [CrossRef]
- Karthika, V.; AlSalhi, M.S.; Devanesan, S.; Gopinath, K.; Arumugam, A.; Govindarajan, M. Chitosan Overlaid Fe3O4/rGO Nanocomposite for Targeted Drug Delivery, Imaging, and Biomedical Applications. Sci. Rep. 2020, 10, 18912. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Characterization of Iron Oxides Commonly Formed as Corrosion Products on Steel. Hyperfine Interact. 1998, 112, 59–66. [Google Scholar] [CrossRef]
- Dominic, P.E.; Dickson, F.J.B. Mössbauer Spectroscopy; Cambridge University Press: Cambridge, UK, 1986; p. 193. [Google Scholar]
- Van Lierop, J.; Ryan, D.H. Mössbauer Spectra of Single-Domain Fine Particle Systems Described Using a Multiple-Level Relaxation Model for Superparamagnets. Phys. Rev. B 2001, 63, 064406. [Google Scholar] [CrossRef]
- Mørup, S.; Tronc, E. Superparamagnetic Relaxation of Weakly Interacting Particles. Phys. Rev. Lett. 1994, 72, 3278. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, S.A.; Aladresi, A.A.M.; Alharbi, S.A.; Chinnathambi, A. Ovalbumin-Mediated Synthesis and Simultaneous Functionalization of Graphene with Increased Protein Stability. Green Chem. Lett. Rev. 2020, 13, 60–67. [Google Scholar] [CrossRef]
- Sohrabijam, Z.; Zamanian, A.; Saidifar, M.; Nouri, A. Prepartion and Characterization of Superparamagnetic Chitosan Coated Maghemite (γ-Fe2O3) for Gene Delivery. Procedia Mater. Sci. 2015, 11, 282–286. [Google Scholar] [CrossRef][Green Version]
- Chandrasekaran, S.; Hur, S.H.; Kim, E.J.; Rajagopalan, B.; Babu, K.F.; Senthilkumar, V.; Chung, J.S.; Choi, W.M.; Kim, Y.S. Highly-Ordered Maghemite/Reduced Graphene Oxide Nanocomposites for High-Performance Photoelectrochemical Water Splitting. RSC Adv. 2015, 5, 29159–29166. [Google Scholar] [CrossRef]
- Ferreira, F.N.; Benevides, A.P.; Cesar, D.V.; Luna, A.S.; de Gois, J.S. Magnetic Solid-Phase Extraction and Pre-Concentration of 17β-Estradiol and 17α-Ethinylestradiol in Tap Water Using Maghemite-Graphene Oxide Nanoparticles and Determination via HPLC with a Fluorescence Detector. Microchem. J. 2020, 157, 104947. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, R.; Sun, D.; Wu, T.; Li, Y. Fabrication of Chitosan/Magnetite-Graphene Oxide Composites as a Novel Bioadsorbent for Adsorption and Detoxification of Cr (VI) from Aqueous Solution. Sci. Rep. 2018, 8, 15397. [Google Scholar] [CrossRef] [PubMed]
- El Rouby, W.M.A.; Farghali, A.A.; Sadek, M.A.; Khalil, W.F. Fast Removal of Sr(II) From Water by Graphene Oxide and Chitosan Modified Graphene Oxide. J. Inorg. Organomet. Polym. Mater. 2018, 28, 2336–2349. [Google Scholar] [CrossRef]
- Vo, T.S.; Vo, T.T.B.C.; Suk, J.W.; Kim, K. Recycling Performance of Graphene Oxide-Chitosan Hybrid Hydrogels for Removal of Cationic and Anionic Dyes. Nano Converg. 2020, 7, 4. [Google Scholar] [CrossRef]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Graphene Oxide-Chitosan Composite Material for Treatment of a Model Dye Effluent. ACS Omega 2018, 3, 13045–13054. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Huang, Z.W.; Li, Z.J.; Zheng, L.R.; Wu, W.S.; Chai, Z.F.; Shi, W.Q. Adsorption of Eu (III) and Th (IV) on Three-Dimensional Graphene-Based Macrostructure Studied by Spectroscopic Investigation. Environ. Pollut. 2019, 248, 82–89. [Google Scholar] [CrossRef]
- Panda, H.; Tiadi, N.; Mohanty, M.; Mohanty, C.R. Studies on Adsorption Behavior of an Industrial Waste for Removal of Chromium from Aqueous Solution. S. Afr. J. Chem. Eng. 2017, 23, 132–138. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Q.; Zhou, L.; Wen, T.; Wang, J. Mutual Effects of U (VI) and Eu (III) Immobilization on Interpenetrating 3-Dimensional MnO2/Graphene Oxide Composites. Sci. Total Environ. 2019, 695, 133696. [Google Scholar] [CrossRef]
- Yao, W.; Wu, Y.; Pang, H.; Wang, X.; Yu, S.; Wang, X. In-Situ Reduction Synthesis of Manganese Dioxide@polypyrrole Core/Shell Nanomaterial for Highly Efficient Enrichment of U (VI) and Eu (III). Sci. China Chem. 2018, 61, 812–823. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, X.; Duan, S.; Yu, X.; Huang, Y.; Hayat, T.; Li, J. The Adsorption of Eu (III) on Carbonaceous Nanofibers: Batch Experiments and Modeling Study. J. Mol. Liq. 2016, 222, 456–462. [Google Scholar] [CrossRef]
- Plancque, G.; Moulin, V.; Toulhoat, P.; Moulin, C. Europium speciation by time-resolved laser-induced fluorescence. Anal. Chim. Acta 2003, 478, 11–22. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Mengelizadeh, N.; Al Rawi, O.; Balarak, D. Capacity and Modeling of Acid Blue 113 Dye Adsorption onto Chitosan Magnetized by Fe2O3 Nanoparticles. J. Polym. Environ. 2021, 30, 344–359. [Google Scholar] [CrossRef]
- Hamza, M.F.; Roux, J.C.; Guibal, E. Uranium and Europium Sorption on Amidoxime-Functionalized Magnetic Chitosan Micro-Particles. Chem. Eng. J. 2018, 344, 124–137. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Lujanienė, G.; Šemčuk, S.; Lečinskytė, A.; Kulakauskaitė, I.; Mažeika, K.; Valiulis, D.; Pakštas, V.; Skapas, M.; Tumėnas, S. Magnetic Graphene Oxide Based Nano-Composites for Removal of Radionuclides and Metals from Contaminated Solutions. J. Environ. Radioact. 2017, 166, 166–174. [Google Scholar] [CrossRef]
- Lujanienė, G.; Šemčuk, S.; Kulakauskaitė, I.; Mažeika, K.; Valiulis, D.; Juškėnas, R.; Tautkus, S. Sorption of Radionuclides and Metals to Graphene Oxide and Magnetic Graphene Oxide. J. Radioanal. Nucl. Chem. 2015, 307, 2267–2275. [Google Scholar] [CrossRef]
- Lujanienė, G.; Beneš, P.; Štamberg, K.; Vopalka, D.; Radžiūtė, E. Study of Pu (IV) and Am (III) Sorption to Clay Minerals: Laboratory Experiments and Modeling. Proc. Radiochem. 2011, 1, 237–244. [Google Scholar] [CrossRef]
- Lujanienė, G.; Šapolaitė, J.; Radžiūtė, E.; Aninkevičius, V. Plutonium Oxidation State Distribution in Natural Clay and Goethite. J. Radioanal. Nucl. Chem. 2009, 282, 793–797. [Google Scholar] [CrossRef]
- Lujanienė, G.; Beneš, P.; Štamberg, K.; Jokšas, K.; Kulakauskaitė, I. Pu and Am Sorption to the Baltic Sea Bottom Sediments. J. Radioanal. Nucl. Chem. 2013, 295, 1957–1967. [Google Scholar] [CrossRef]
- Lujanienė, G.; Štamberg, K.; Pakštas, V.; Juškėnas, R.; Kulakauskaitė, I.; Šemčuk, S.; Mažeika, K.; Vopálka, D. Study of Pu Sorption Behavior in Natural Clay. J. Radioanal. Nucl. Chem. 2015, 304, 53–59. [Google Scholar] [CrossRef]




| Sample | T, K | M, % | Doublet | Hyperfine Field Distribution/Sextets | |||||
|---|---|---|---|---|---|---|---|---|---|
| Id, % | δ, mm/s | Δ, mm/s | IB, % | δ, mm/s | Δ, mm/s | B, T | |||
| MGH | 296 | 100 | 81 | 0.36 ± 0.01 | 0.66 ± 0.01 | 19 | 0.36 * | 0 * | 25.4 ** |
| 80 | 13 | 0.48 ± 0.01 | 0.51 ± 0.04 | 87 | 0.46 ± 0.01 | –0.14 ± 0.02 | 37.2 ** | ||
| 11 | 0 | - | - | 61 | 0.49 ± 0.01 | –0.07 ± 0.02 | 51.0 ± 0.2 | ||
| 39 | 0.45 ± 0.01 | –0.06 ± 0.03 | 46.8 ± 0.4 | ||||||
| GO-MGH | 296 | 32 | 81 | 0.36 ± 0.01 | 0.72 ± 0.01 | 19 | 0.36 * | 0 * | 34.6 ** |
| GO-MGH-CS I | 296 | 15 | 80 | 0.36 ± 0.01 | 0.68 ± 0.01 | 20 | 0.30 ± 0.06 | 0 * | 29.9 ** |
| GO-MGH-CS II | 296 | 17 | 74 | 0.37 ± 0.01 | 0.73 ± 0.01 | 26 | 0.37 * | 0 * | 35.2 ** |
| GO-MGH-CS III | 296 | 0.6 | 100 | 0.37 ± 0.02 | 0.68 ± 0.03 | - | - | - | - |
| Models | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| Parameters | R2 | qm, mg/g | KL | RL | R2 | KF | 1/n |
| GO-MGH | 0.998 | 80 | 0.013 | 0.278 | 0.659 | 2.097 | 0.642 |
| GO-CS | 0.995 | 77 | 0.016 | 0.238 | 0.540 | 2.681 | 0.611 |
| GO-MGH-CS I | 0.982 | 32 | 0.017 | 0.227 | 0.871 | 1.000 | 0.649 |
| GO-MGH-CS II | 0.999 | 147 | 0.024 | 0.172 | 0.967 | 4.794 | 0.695 |
| GO-MGH-CS III | 0.998 | 160 | 0.018 | 0.217 | 0.920 | 5.100 | 0.658 |
| Adsorbents | Conditions | qm (mg/g) | Refs. |
|---|---|---|---|
| Chitosan nanoparticles | pH = 3; T = 25 °C. | 115 | [42] |
| Maghemite | pH = 6; T = 25 °C. | 34 | [41] |
| Magnetite nanoparticles | pH = 5; T = 25 °C. | 0.24 | [39] |
| GO | pH = 5; T = 25 °C. | 68 | [71] |
| MnO2/graphene oxide | 84 | ||
| Magnetic amidoxime-functionalised MCM-41 | pH = 6; T = 25 °C. | 27 | [2] |
| Manganese dioxide@polypyrrole core/shell nanomaterial | pH = 6.5; T = 25 °C. | 55 | [72] |
| Carbonaceous nanofibers | pH = 4.5; T = 25 °C. | 63 | [73] |
| GO-MGH | pH = 5; T = 25 °C. | 80 | This work |
| GO-CS | 77 | ||
| GO-MGH-CS I | 32 | ||
| GO-MGH-CS II | 147 | ||
| GO-MGH-CS III | 160 |
| Models | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| Parameters | R2 | K1 (min−1) | qe Theo (mg/g) | qe Exp (mg/g) | R2 | K2 [g/(mg min−1)] | qe (mg/g) |
| GO-MGH | 0.964 | 0.035 | 2.964 | 3.091 | 0.914 | 0.014 | 3.254 |
| GO-CS | 0.873 | 0.061 | 3.404 | 3.606 | 0.976 | 0.026 | 3.644 |
| GO-MGH-CS I | 0.904 | 0.050 | 2.689 | 2.763 | 0.931 | 0.027 | 2.872 |
| GO-MGH-CS II | 0.768 | 0.048 | 4.266 | 4.306 | 0.824 | 0.018 | 4.521 |
| GO-MGH-CS III | 0.851 | 0.048 | 3.995 | 4.249 | 0.914 | 0.016 | 4.320 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lujanienė, G.; Novikau, R.; Joel, E.F.; Karalevičiūtė, K.; Šemčuk, S.; Mažeika, K.; Talaikis, M.; Pakštas, V.; Tumėnas, S.; Mažeika, J.; et al. Preparation of Graphene Oxide-Maghemite-Chitosan Composites for the Adsorption of Europium Ions from Aqueous Solutions. Molecules 2022, 27, 8035. https://doi.org/10.3390/molecules27228035
Lujanienė G, Novikau R, Joel EF, Karalevičiūtė K, Šemčuk S, Mažeika K, Talaikis M, Pakštas V, Tumėnas S, Mažeika J, et al. Preparation of Graphene Oxide-Maghemite-Chitosan Composites for the Adsorption of Europium Ions from Aqueous Solutions. Molecules. 2022; 27(22):8035. https://doi.org/10.3390/molecules27228035
Chicago/Turabian StyleLujanienė, Galina, Raman Novikau, Edith Flora Joel, Karolina Karalevičiūtė, Sergej Šemčuk, Kęstutis Mažeika, Martynas Talaikis, Vidas Pakštas, Saulius Tumėnas, Jonas Mažeika, and et al. 2022. "Preparation of Graphene Oxide-Maghemite-Chitosan Composites for the Adsorption of Europium Ions from Aqueous Solutions" Molecules 27, no. 22: 8035. https://doi.org/10.3390/molecules27228035
APA StyleLujanienė, G., Novikau, R., Joel, E. F., Karalevičiūtė, K., Šemčuk, S., Mažeika, K., Talaikis, M., Pakštas, V., Tumėnas, S., Mažeika, J., & Jokšas, K. (2022). Preparation of Graphene Oxide-Maghemite-Chitosan Composites for the Adsorption of Europium Ions from Aqueous Solutions. Molecules, 27(22), 8035. https://doi.org/10.3390/molecules27228035







