Validation of HPLC Method for Analysis of Gamma-Aminobutyric and Glutamic Acids in Plant Foods and Medicinal Plants
Abstract
1. Introduction
2. Results and Discussion
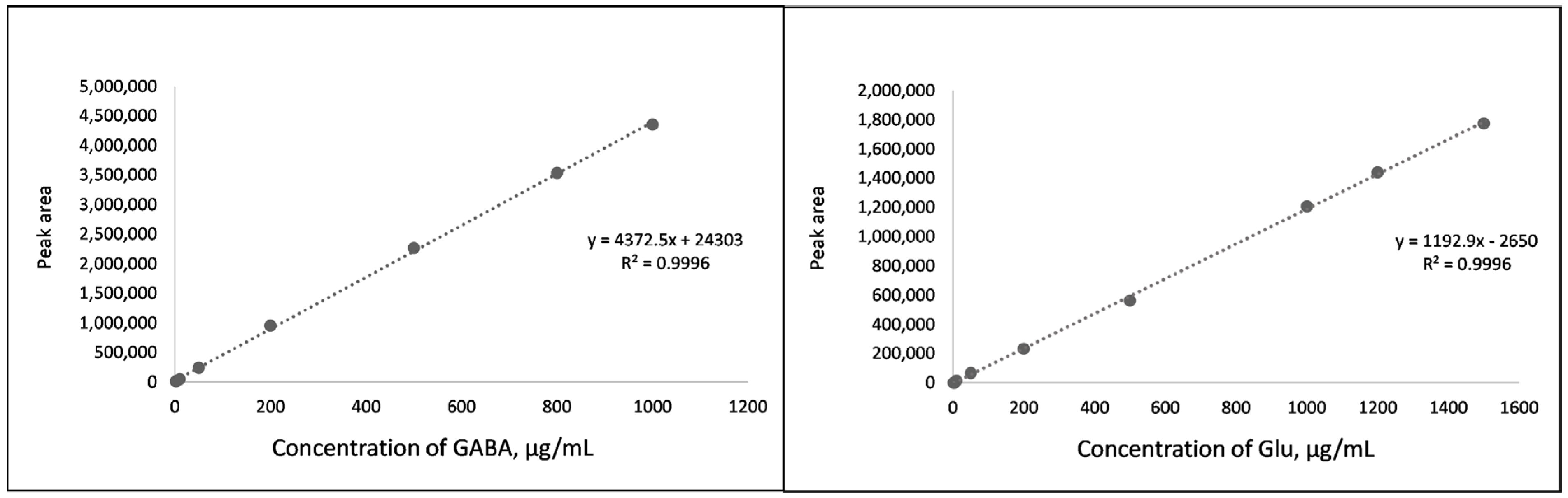
2.1. Method Validation
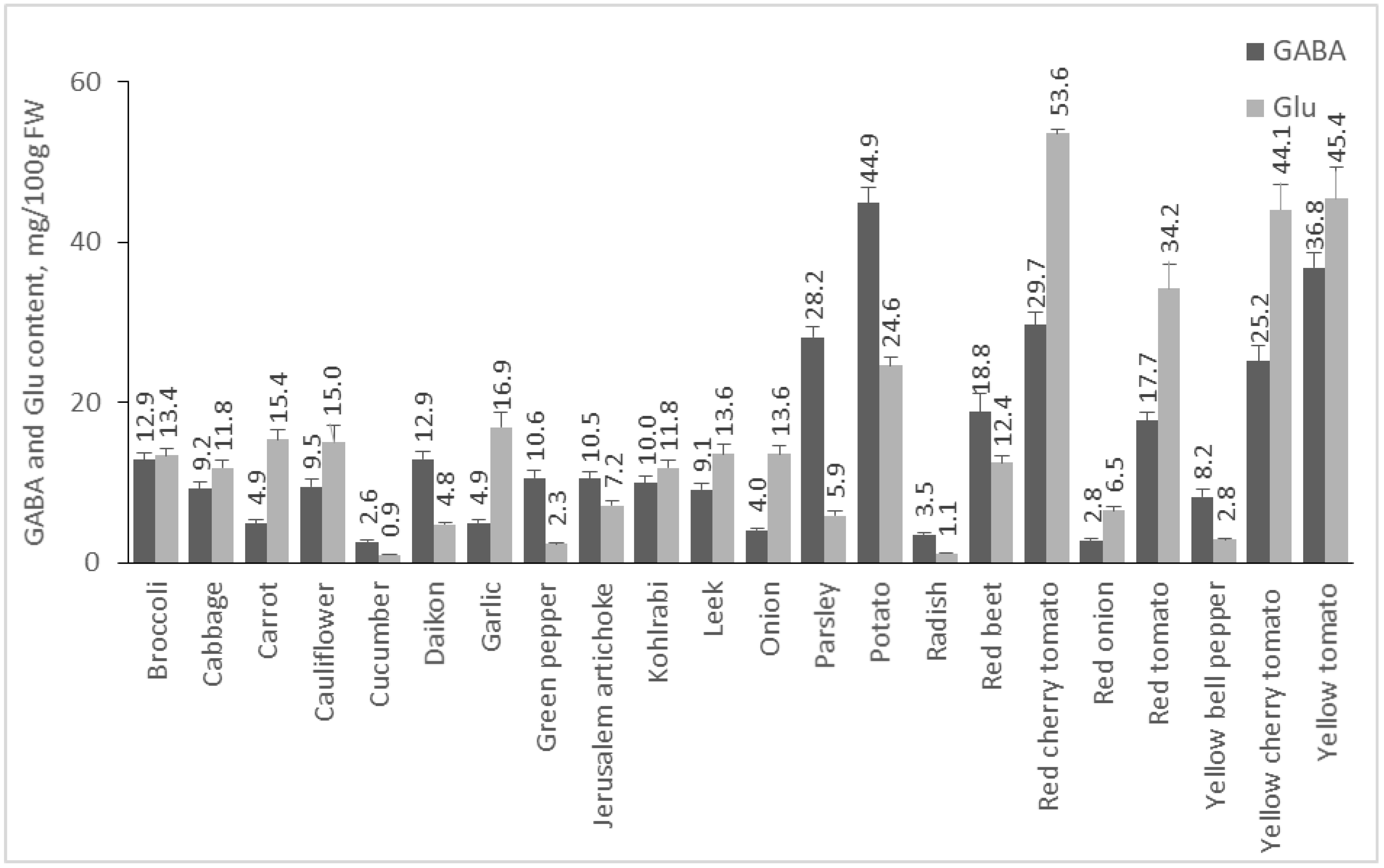
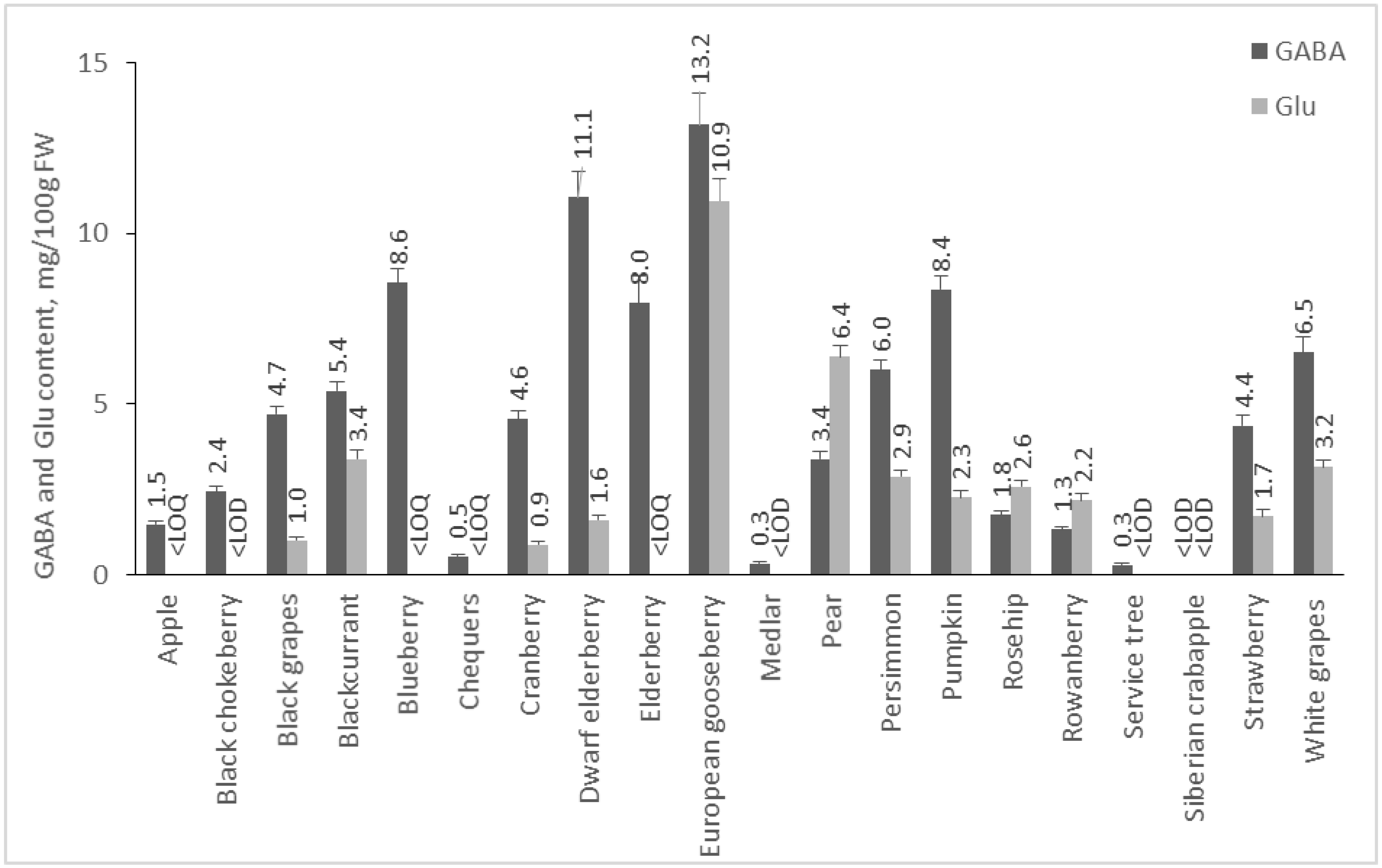
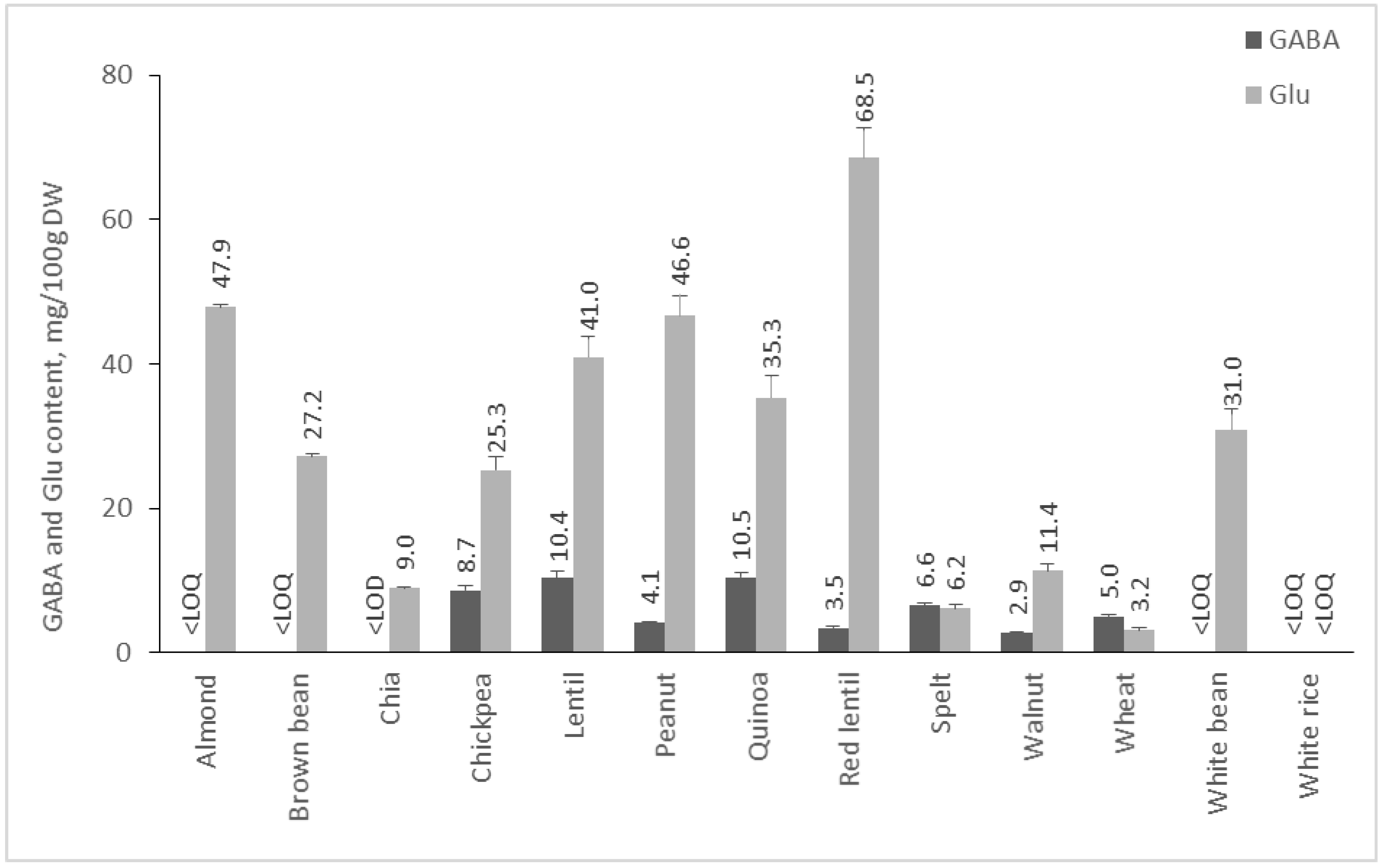
2.2. Content of GABA and Glu in Plant Foods
2.2.1. Content of GABA and Glu in Vegetables
2.2.2. Content of GABA and Glu in Fruits
2.2.3. Content of GABA and Glu in Cereals, Pseudocereals, Nuts, and Legumes
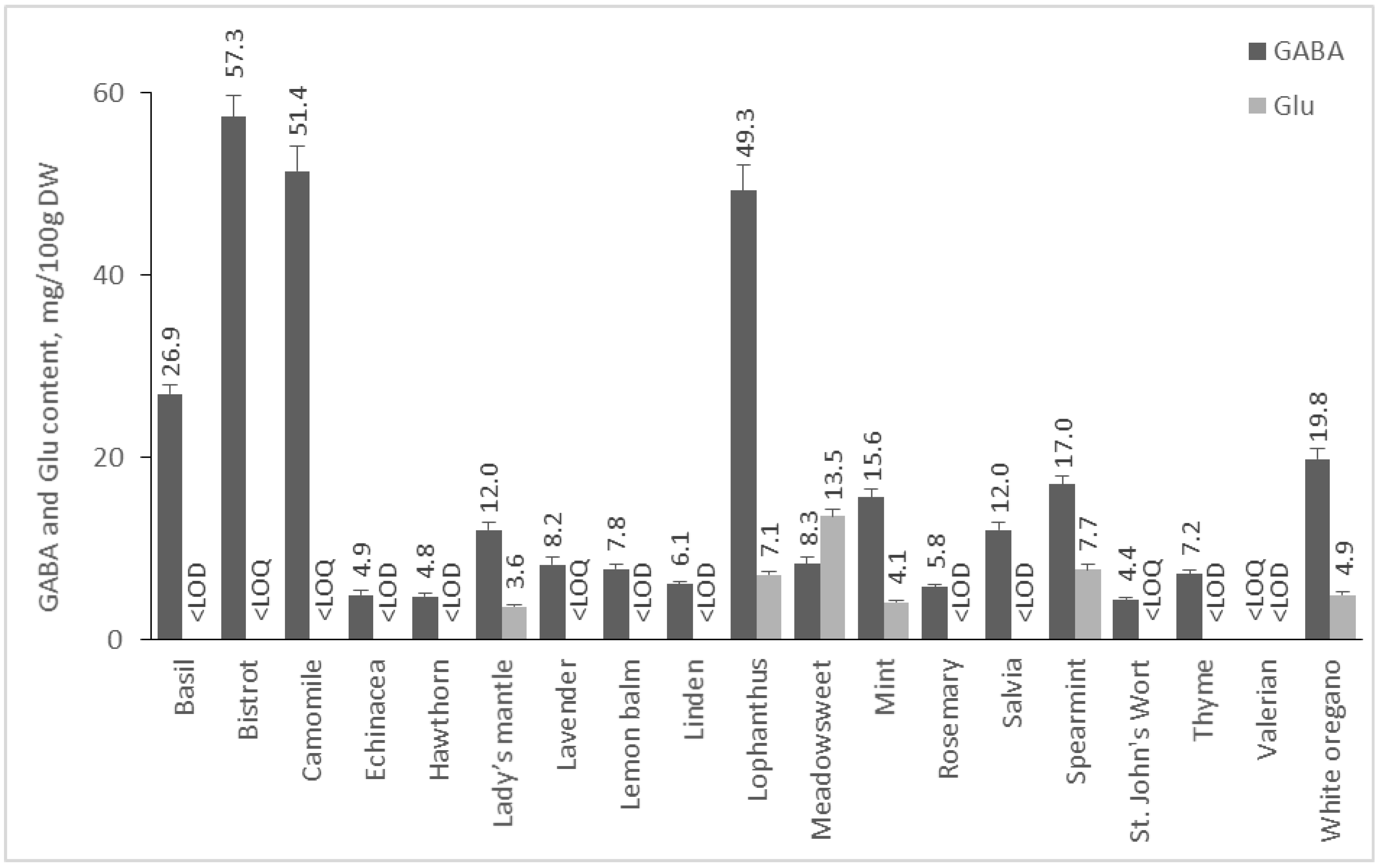
2.2.4. Content of GABA and Glu in Medicinal Plants
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Preparation of Extracts
3.4. Derivatization of GABA and Glu
3.5. HPLC Determination of GABA and Glu Derivatives
3.6. HPLC Validation
3.6.1. Linearity
3.6.2. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.6.3. Recovery
3.6.4. Precision
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wong, C.G.T.; Bottiglieri, T.; Snead, O.C. GABA, γ-hydroxybutyric acid, and neurological disease. Ann. Neurol. 2003, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, H.; Zhang, C.; Lu, Y.; Zhu, X.; Lu, Z. γ-Aminobutyric acid-rich yogurt fermented by Streptococcus salivarius subsp. thermophiles fmb5 apprars to have anti-diabetic effect on streptozotocin-induced diabetic mice. J. Funct. Foods 2016, 20, 267–275. [Google Scholar]
- Dhakal, R.; Bajpai, V.K.; Baek, K. Production of GABA (γ-aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef]
- Al-Wadei, H.A.N.; Ullah, M.F.; Al-Wadei, M. GABA (γ-aminobutyric acid), a non-protein amino acid counters the β-adrenergic cascadeactivated oncogenic signaling in pancreatic cancer: A review of experimental evidence. Mol. Nutr. Food 2011, 55, 1745–1758. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.H.; Oh, S.H. Effects of germinated brown rice extracts with enhanced levels of GABA on cancer cell proliferation and apoptosis. J. Med. Food 2004, 7, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Shirai, T.; Ochiai, H.; Kasao, M.; Hayakawa, K.; Kimura, M.; Sansawa, H. Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 2003, 57, 490–495. [Google Scholar] [CrossRef]
- Kuriyama, K.; Sze, P.Y. Blood-brain barrier to H3-γ-aminobutyric acid in normal and amino oxyacetic acid-treated animals. Neuropharmacology 1971, 10, 103–108. [Google Scholar] [CrossRef]
- Boonstra, E.; Kleijn, R.; Colzato, L.S.; Alkemade, A.; Forstmann, B.U.; Nieuwenhuis, S. Neurotransmitters as food supplements: The effects of GABA on brain and behavior. Front. Psychol. 2015, 6, 1520. [Google Scholar] [CrossRef]
- Oh, S.H.; Moon, Y.J.; Oh, C.H. γ-Aminobutyric acid (GABA) content of selected uncooked foods. Prev. Nurt. Food Sci. 2003, 1, 75–78. [Google Scholar] [CrossRef]
- Ramos-Ruiz, R.; Poirot, E.; Flores-Mosquera, M. GABA, a non-protein amino acid ubiquitous in food matrices. Cogent Food Agric. 2018, 4, 1–89. [Google Scholar] [CrossRef]
- Briguglio, M.; Dell’Osso, B.; Panzica, G.; Malgaroli, A.; Banfi, G.; Zanaboni Dina, C.; Galentino, R.; Porta, M. Dietary Neuro-transmitters: A narrative review on current knowledge. Nutrients 2018, 10, 591. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.Y.; Tan, J.S.; Cheng, L.H. Gamma aminobutyric acid (GABA) enrichment in plant-based food–A mini review. Food Rev. Int. 2022, in press. [Google Scholar] [CrossRef]
- Oketch-Rabah, H.A.; Madden, E.F.; Roe, A.L.; Betz, J.M. United States pharmacopeia (USP) safety review of gamma-aminobutyric acid (GABA). Nutrients 2021, 13, 2742. [Google Scholar] [CrossRef] [PubMed]
- Ubuka, T. Glutamic acid. In Handbook of Hormones: Comparative Endocrinology for Basic and Clinical Research, 2nd ed.; Ando, H., Ukena, K., Nagata, S., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 1063–1065. [Google Scholar]
- Zhang, H.; Wang, Z.Y.; Yang, X.; Zhao, H.T.; Zhang, Y.C.; Dong, A.J.; Jing, J.; Wang, J. Determination of free amino acids and 18 elements in freeze-dried strawberry and blueberry fruit using an amino acid analyzer and ICP-MS with microwave digestion. Food. Chem. 2014, 147, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Eulenburg, V.; Kreis, W.; Villmann, C.; Pischetsrieder, M. Three-step test system for the identification of novel GABA receptor modulating food plants. Plant. Foods. Hum. Nutr. 2016, 71, 355–360. [Google Scholar] [CrossRef]
- Zhang, S.; Takeda, Y.; Hagioka, S.; Takata, K.; Aoe, H.; Nakatsuka, H.; Yokoyama, M.; Morita, K. Measurement of GABA and glutamate in vivo levels with high sensitivity and frequency. Brain. Res. Protoc. 2005, 14, 61–66. [Google Scholar] [CrossRef]
- Hyun, T.K.; Seung, H.E.; Yong, C.J.; Sang, H.H.; Kim, J.S. Identification of glutamate decarboxylases as a γ-aminobutyric acid (GABA) biosynthetic enzyme in soybean. Ind. Crops Prod. 2013, 49, 864–870. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, H.; Song, Y.; Gu, Z. Effects of soaking and aeration treatment on γ-aminobutyric acid accumulation in germinated soybean (Glycine max L.). Eur. Food Res. Technol. 2011, 232, 787–795. [Google Scholar] [CrossRef]
- Kook, M.C.; Seo, M.J.; Cheigh, C.I.; Pyun, Y.R.; Cho, S.C.; Park, H. Enhanced production of γ-aminobutyric acid using rice bran extracts by Lactobacillus sakei B2-16. J. Microbiol. Biotchnol. 2010, 20, 763–766. [Google Scholar]
- Bai, Q.Y.; Chai, M.Q.; Gu, Z.X.; Cao, X.H.; Li, Y.; Liu, K.L. Effects of components in culture medium on glutamate decarboxylase activity and gamma-aminobutyric acid accumulation in foxtail millet (Setaria italica L.) during germination. Food Chem. 2009, 116, 152–157. [Google Scholar] [CrossRef]
- Ham, T.H.; Chu, S.H.; Han, S.J.; Ryu, S.N. γ-Aminobutyric acid metabolism in plant under environment stresses. Korean J. Crop Sci. 2012, 57, 144–150. [Google Scholar] [CrossRef]
- Kinnersley, A.M.; Turano, F.J. Gamma-aminobutyric acid (GABA) and plant responses to stress. Crit. Rev. Plant Sci. 2000, 19, 479–509. [Google Scholar] [CrossRef]
- Steward, F.C.; Thompson, J.F.; Dent, C.E. Gamma-aminobutyric acid. A constituent of the potato tuber? Science 1949, 110, 439–440. [Google Scholar]
- Nakamura, K.; Nara, K.; Noguchi, T.; Ohshiro, T.; Koga, H. Contents of gamma-aminobutyric acid (GABA) in potatoes and processed potato products. J. Jpn. Soc. Food Sci. Technol. 2006, 53, 514–517. [Google Scholar] [CrossRef][Green Version]
- Saito, T.; Matsukura, C.; Sugiyama, M.; Watahiki, A.; Ohshima, I.; Iijima, Y.; Konishi, C.; Fujii, T.; Inai, S.; Fukuda, N.; et al. Screening for gamma-aminobutyric acid (GABA)-rich tomato varieties. Engei Gakkai Zasshi 2008, 77, 242–250. [Google Scholar] [CrossRef]
- Westall, R.G. γ-aminobutyric acid and β-alanine in plant tissues: Isolation of γ-amino-butyric acid from beetroot (Beta vulgaris). Nature 1950, 165, 717–718. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Lee, M.K.; Chun, J.H.; Seo, J.M.; Lee, S.W.; Al-Dhabi, N.A.; Kim, S.J. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.). Food Chem. 2014, 145, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.A.; Lopez-Ayerra, B.; Martinez-Tome, M.; Garcia-Carmona, F. Effect of industrial processing on amino acid content of broccoli. J. Sci. Food Agric. 2001, 81, 1299–1305. [Google Scholar] [CrossRef]
- Kato, R.; Hayashi, S.; Kobayashi, T.; Takahashi, H.; Kimura, N.; Takahashi, A.; Kumakura, K.; Matsuoka, H. Behavior analysis of gamma-aminobutyrate and glutamate decarboxylase activity in salted radish roots (Takuanzuke). J. Jpn. Soc. Food Sci. J. 2015, 62, 492–500. [Google Scholar] [CrossRef][Green Version]
- Marcy, J.E.; Carroll, D.E.; Young, C.T. Changes in free amino-acid and total nitrogen concentrations during maturation of muscadine grapes (V-Rotundifolia). J. Food Sci. 1981, 46, 543. [Google Scholar] [CrossRef]
- Murch, S.J.; Hall, B.A.; Le, C.H.; Saxena, P.K. Changes in the levels of indoleamine phytochemicals during veraison and ripening of wine grapes. J. Pineal. Res. 2010, 49, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Deewatthanawong, R.; Nock, J.F.; Watkins, C.B. Gamma-aminobutyric acid (GABA) accumulation in four strawberry cultivars in response to elevated CO2 storage. Postharvest Biol. Technol. 2010, 57, 92–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Pengmin, L.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, X.; Liu, Z.; Wu, X.; Bao, C.; Sun, Y.; Su, N.; Cui, J. Transcriptome analysis reveals the molecular mechanism of GABA accumulation during quinoa (Chenopodium quinoa Willd.) germination. J. Agric. Food Chem. 2021, 69, 12171–12186. [Google Scholar] [CrossRef] [PubMed]
- Rozan, P.; Kuo, Y.H.; Lambein, F. Amino acids in seeds and seedlings of the genus Lens. Phytochemistry 2001, 58, 281–289. [Google Scholar] [CrossRef]
- Hermanussen, M.; Gonder, U.; Jakobs, C.; Stegemann, D.; Hoffmann, G. Patterns of free amino acids in german convenience food products: Marked mismatch between label information and composition. Eur. J. Clin. Nutr. 2010, 64, 88–98. [Google Scholar] [CrossRef]
- Rozan, P.; Kuo, Y.H.; Lambein, F. Free amino acids present in commercially available seedlings sold for human consumption. A potential hazard for consumers. J. Agric. Food Chem. 2000, 48, 716–723. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef]
- Khalil, A.W.; Zeb, A.; Mahmood, F.; Tariq, S.; Khattak, A.B.; Shah, H. Comparison of sprout quality characteristics of desi and kabuli type chickpea cultivars (Cicer arietinum L.). Swiss Soc. Food Sci. Technol. 2007, 40, 937–945. [Google Scholar] [CrossRef]
- Ohm, J.B.; Lee, C.W.; Cho, K. Germinated wheat: Phytochemical composition and mixing characteristics. Cereal Chem. 2016, 93, 612–617. [Google Scholar] [CrossRef]
- Ferreira, C.D.; Bubolz, V.K.; Silva, J.; Dittgen, C.L.; Ziegler, V.; Raphaelli, C.O.; Oliveira, M. Changes in the chemical composition and bioactive compounds of chickpea (Cicer arietinum L.) fortified by germination. LWT 2019, 111, 363–369. [Google Scholar] [CrossRef]
- Mao, J.J.; Li, Q.S.; Soeller, I.; Rockwell, K.; Xie, S.X.; Amsterdam, J.D. Long-term chamomile therapy of generalized anxiety disor-der: A study protocol for a randomized, double-blind, placebo-controlled trial. J. Clin. Trials. 2014, 4, 188. [Google Scholar] [PubMed]
- Mechan, A.O.; Fowler, A.; Seifert, N.; Rieger, H.; Wöhrle, T.; Etheve, S.; Wyss, A.; Schüler, G.; Colletto, B.; Kilpert, C.; et al. Monoamine reuptake inhibition and mood-enhancing potential of a specified oregano extract. Br. J. Nutr. 2011, 105, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, M.; Sajjadi, S.E.; Vaezi, A. Evaluation of anxiolytic and sedative effect of essential oil and hydroalcoholic extract of Ocimum basilicum L. and chemical composition of its essential oil. Res. Pharm. Sci. 2015, 10, 535–543. [Google Scholar] [PubMed]
- Ríos, J.L.; Schinella, G.R.; Moragrega, I. Phenolics as GABAA receptor ligands: An updated review. Molecules 2022, 27, 1770. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, Y.; Wei, Z.Z.; Yuan, W.X.; Li, Y.L.; Zhang, C.H.; Xue, X.T.; Zhou, H.J. Determination and comparison of γ-aminobutyric acid (GABA) content in pu-erh and other types of Chinese tea. J Agric. Food Chem. 2011, 59, 3641–3648. [Google Scholar] [CrossRef]
- Gong, J.; Huang, J.; Xiao, G.; You, Y.; Yuan, H.; Chen, F.; Liu, S.; Mao, J.; Li, B. Determination of γ-aminobutyric acid in Chinese rice wines and its evolution during fermentation. J. Inst. Brew. 2017, 123, 417–422. [Google Scholar] [CrossRef]
- Validation of analytical procedures: Text and methodology Q2(R1). In ICH Harmonized Tripartite Guideline; U.S. Food & Drug Administration: Silver Spring, MD, USA, 2005.
- Meeploy, M.; Deewatthanawong, R. Determination of γ-aminobutyric acid (GABA) in rambutan fruit cv. Rongrian by HPLC-ELSD and separation of GABA from rambutan fruit using Dowex 50W-X8 column. J. Chromatogr. Sci. 2016, 54, 445–452. [Google Scholar] [CrossRef]
- Tong, T.; Liu, Y.J.; Kang, J.; Zhang, C.M.; Kang, S.G. Antioxidant activity and main chemical components of a novel fermented tea. Molecules 2019, 24, 2917. [Google Scholar] [CrossRef]
| Added Glu, (mg) | Recovered Glu, (mg) | Recovery, (%) | Mean, (%) | RSD, (%) |
|---|---|---|---|---|
| 1.1 | 1.07 | 97.2 | 97.8 | 0.68 |
| 1.1 | 1.08 | 97.8 | ||
| 1.1 | 1.08 | 98.0 | ||
| 1.1 | 1.09 | 98.8 | ||
| 1.1 | 1.07 | 97.1 | ||
| 0.5 | 0.52 | 103.3 | 103.2 | 3.29 |
| 0.5 | 0.50 | 99.4 | ||
| 0.5 | 0.54 | 108.6 | ||
| 0.5 | 0.51 | 101.7 | ||
| 0.5 | 0.51 | 102.7 |
| Added GABA, (mg) | Recovered GABA, (mg) | Recovery, (%) | Mean, (%) | RSD, (%) |
|---|---|---|---|---|
| 1.8 | 1.91 | 106.2 | 102.6 | 3.72 |
| 1.8 | 1.88 | 104.3 | ||
| 1.8 | 1.73 | 96.4 | ||
| 1.8 | 1.88 | 104.4 | ||
| 1.8 | 1.83 | 101.8 | ||
| 0.9 | 0.95 | 105.6 | 104.9 | 0.73 |
| 0.9 | 0.95 | 105.2 | ||
| 0.9 | 0.95 | 105.3 | ||
| 0.9 | 0.93 | 103.7 | ||
| 0.9 | 0.94 | 104.8 |
| Sample № | Sample Mass, (g) | Extracted GABA, (mg/g) | Extracted Glu, (mg/g) |
|---|---|---|---|
| 1 | 1.003 | 1.76 | 1.09 |
| 2 | 1.002 | 1.77 | 1.09 |
| 3 | 1.008 | 1.74 | 1.09 |
| 4 | 1.009 | 1.74 | 1.10 |
| 5 | 1.027 | 1.81 | 1.08 |
| 6 | 1.017 | 1.83 | 1.09 |
| 7 | 1.030 | 1.85 | 1.09 |
| 8 | 1.021 | 1.85 | 1.08 |
| 9 | 1.032 | 1.87 | 1.10 |
| 10 | 1.031 | 1.86 | 1.10 |
| MEAN | - | 1.81 | 1.09 |
| SD | - | 0.049 | 0.006 |
| RSD | - | 2.73 | 0.55 |
| Day | Added GABA, (mg) | Recovered GABA, (mean ± SD), (mg) | Precision, (RSD) | Accuracy, (% recovery) |
|---|---|---|---|---|
| 1 | 1.8 | 1.85 ± 0.07 | 3.72 | 102.6 |
| 0.9 | 0.94 ± 0.01 | 0.73 | 104.9 | |
| 2 | 1.8 | 1.83 ± 0.05 | 2.55 | 101.7 |
| 0.9 | 0.91 ± 0.04 | 4.64 | 101.4 | |
| Day | Added Glu, (mg) | Recovered Glu, (mean ± SD) (mg) | Precision, (RSD) | Accuracy, (% recovery) |
| 1 | 1.1 | 1.08 ± 0.01 | 0.68 | 97.8 |
| 0.5 | 0.52 ± 0.02 | 3.29 | 103.2 | |
| 2 | 1.1 | 1.05 ± 0.02 | 2.24 | 95.8 |
| 0.5 | 0.51 ± 0.02 | 4.83 | 101.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pencheva, D.; Teneva, D.; Denev, P. Validation of HPLC Method for Analysis of Gamma-Aminobutyric and Glutamic Acids in Plant Foods and Medicinal Plants. Molecules 2023, 28, 84. https://doi.org/10.3390/molecules28010084
Pencheva D, Teneva D, Denev P. Validation of HPLC Method for Analysis of Gamma-Aminobutyric and Glutamic Acids in Plant Foods and Medicinal Plants. Molecules. 2023; 28(1):84. https://doi.org/10.3390/molecules28010084
Chicago/Turabian StylePencheva, Daniela, Desislava Teneva, and Petko Denev. 2023. "Validation of HPLC Method for Analysis of Gamma-Aminobutyric and Glutamic Acids in Plant Foods and Medicinal Plants" Molecules 28, no. 1: 84. https://doi.org/10.3390/molecules28010084
APA StylePencheva, D., Teneva, D., & Denev, P. (2023). Validation of HPLC Method for Analysis of Gamma-Aminobutyric and Glutamic Acids in Plant Foods and Medicinal Plants. Molecules, 28(1), 84. https://doi.org/10.3390/molecules28010084






