Expanded Ligands Based upon Iron(II) Coordination Compounds of Asymmetrical Bis(terpyridine) Domains
Abstract
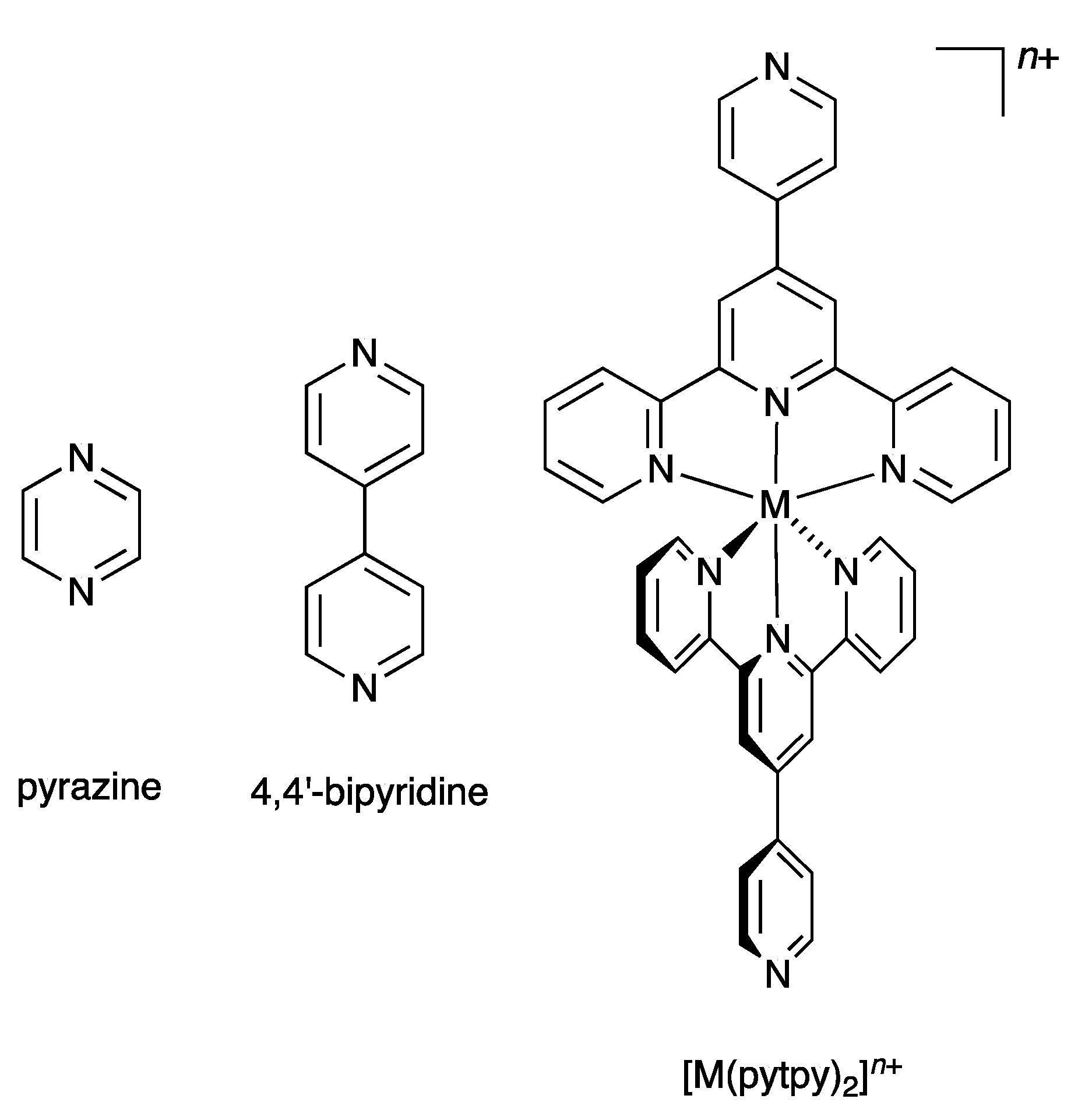
1. Introduction
2. Results and Discussion
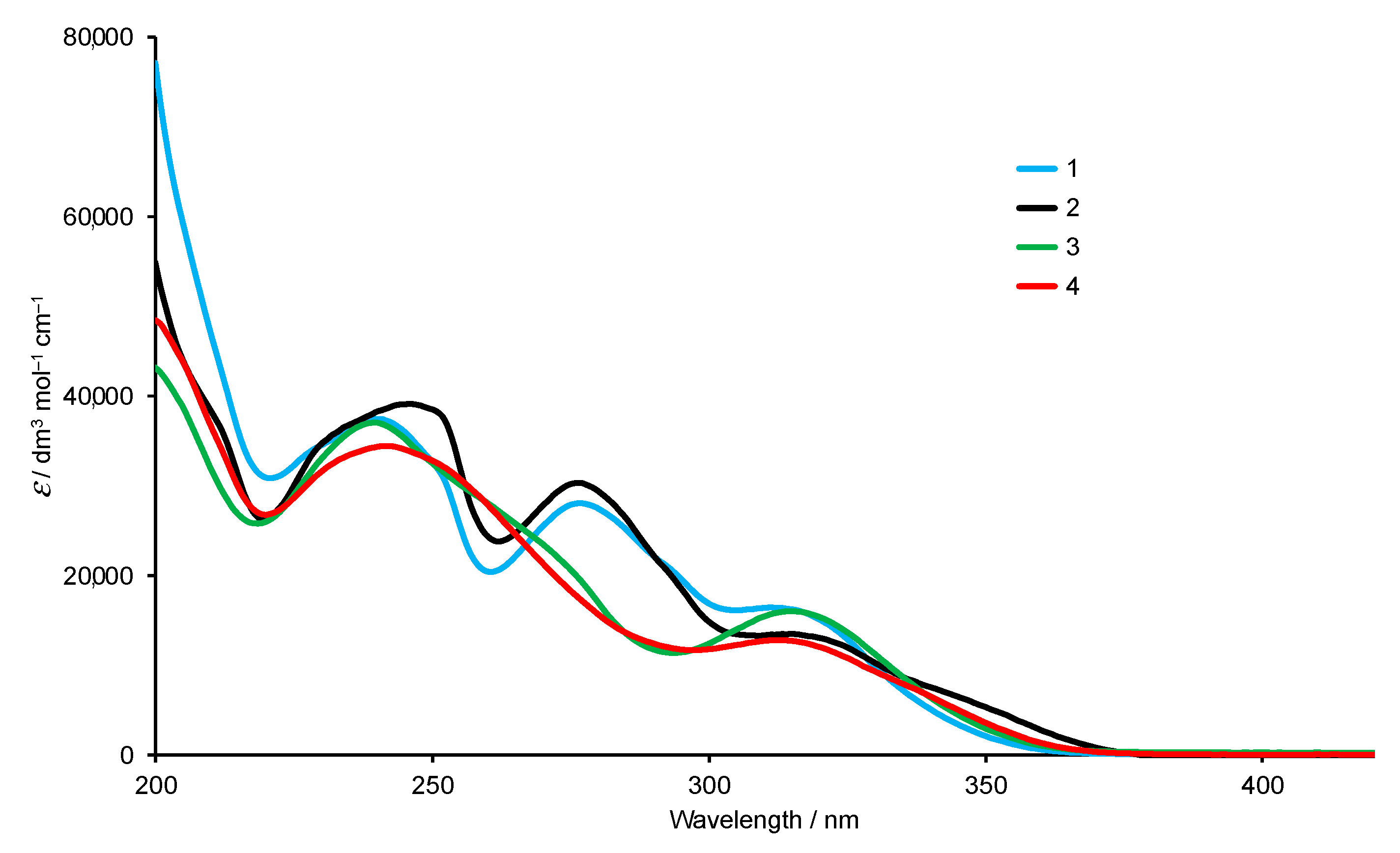
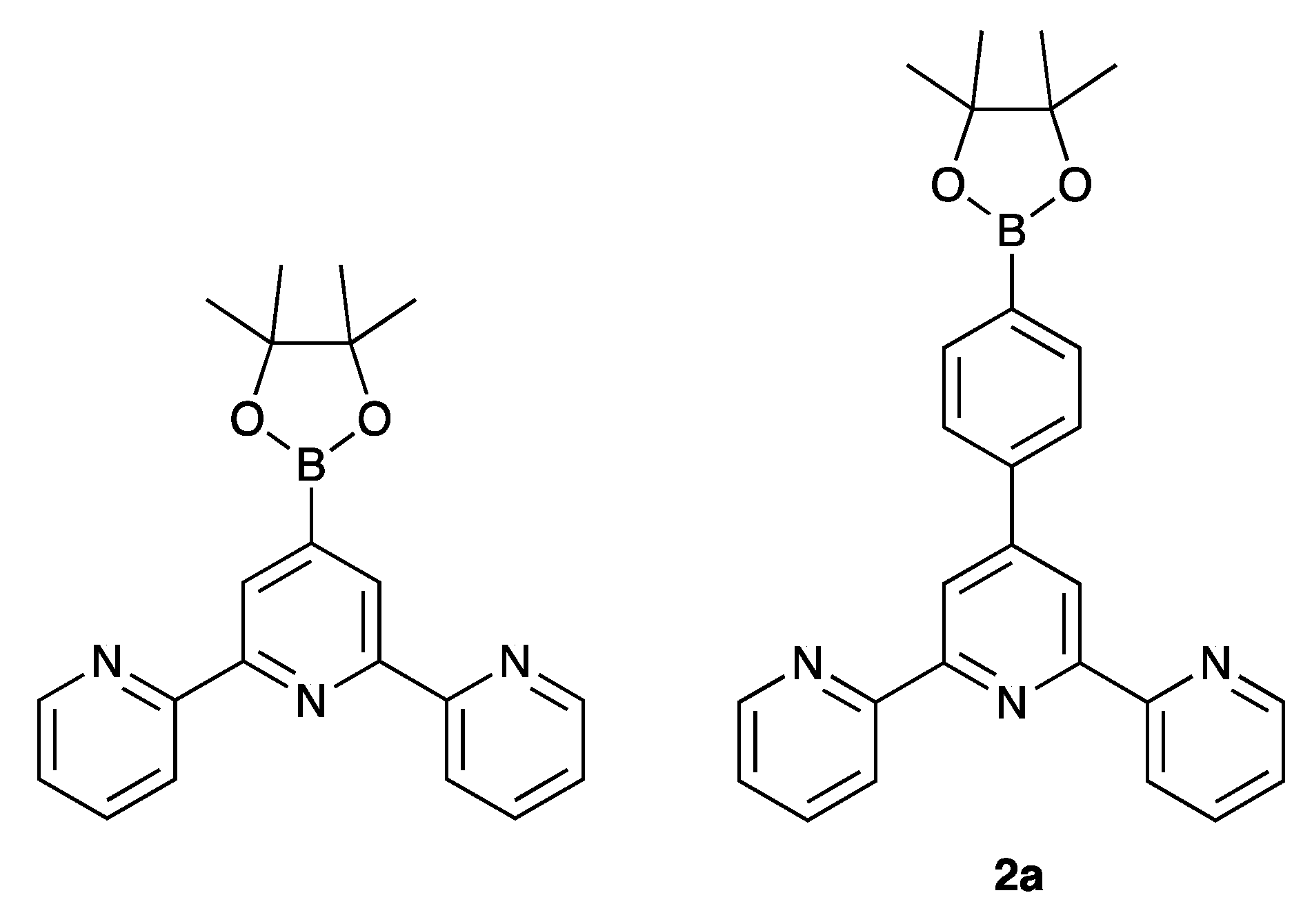
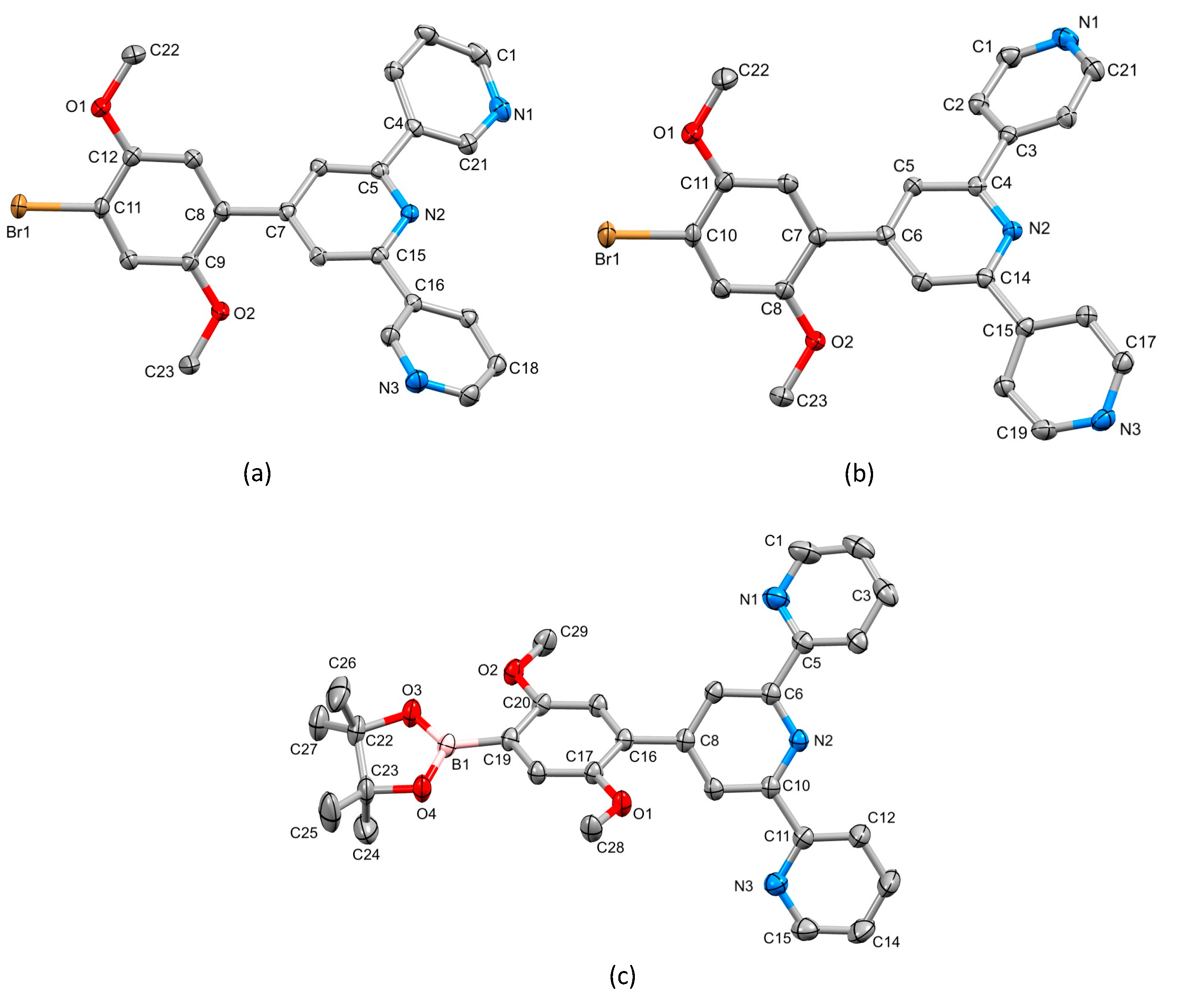
2.1. Synthesis and Characterization of Terpyridine Precursors 1–4
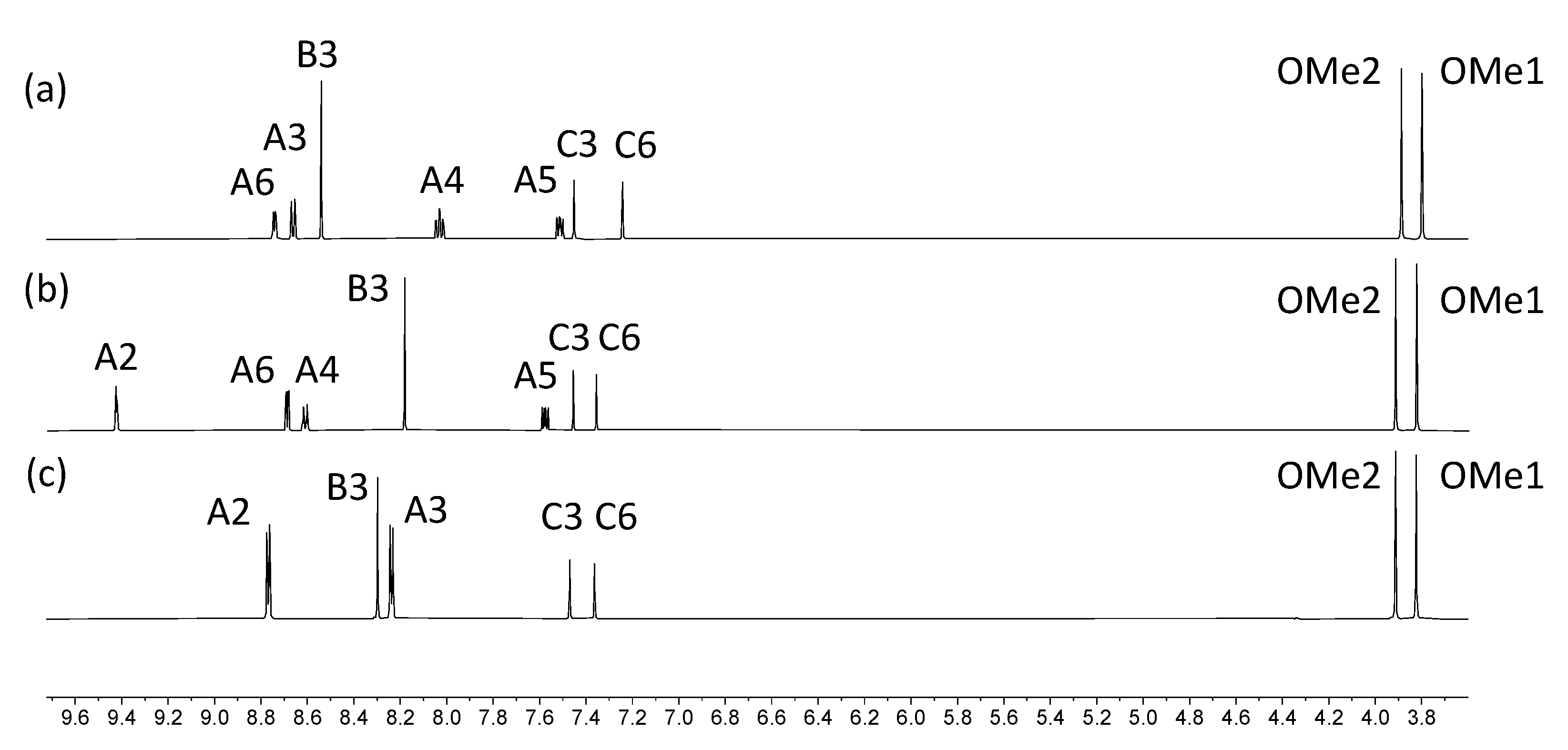
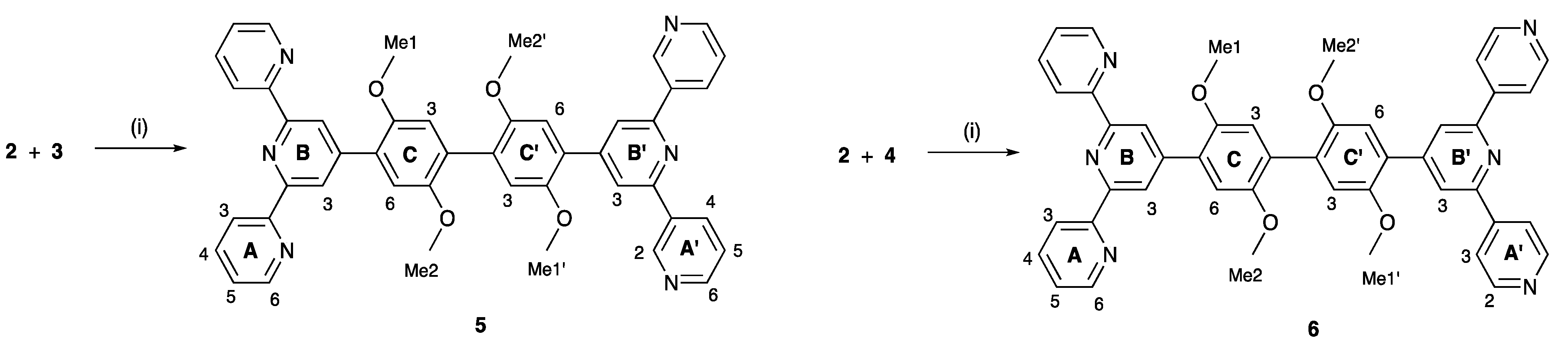
2.2. Synthesis and Characterization of the Asymmetrical Bis(Terpyridine) Ligands 5 and 6
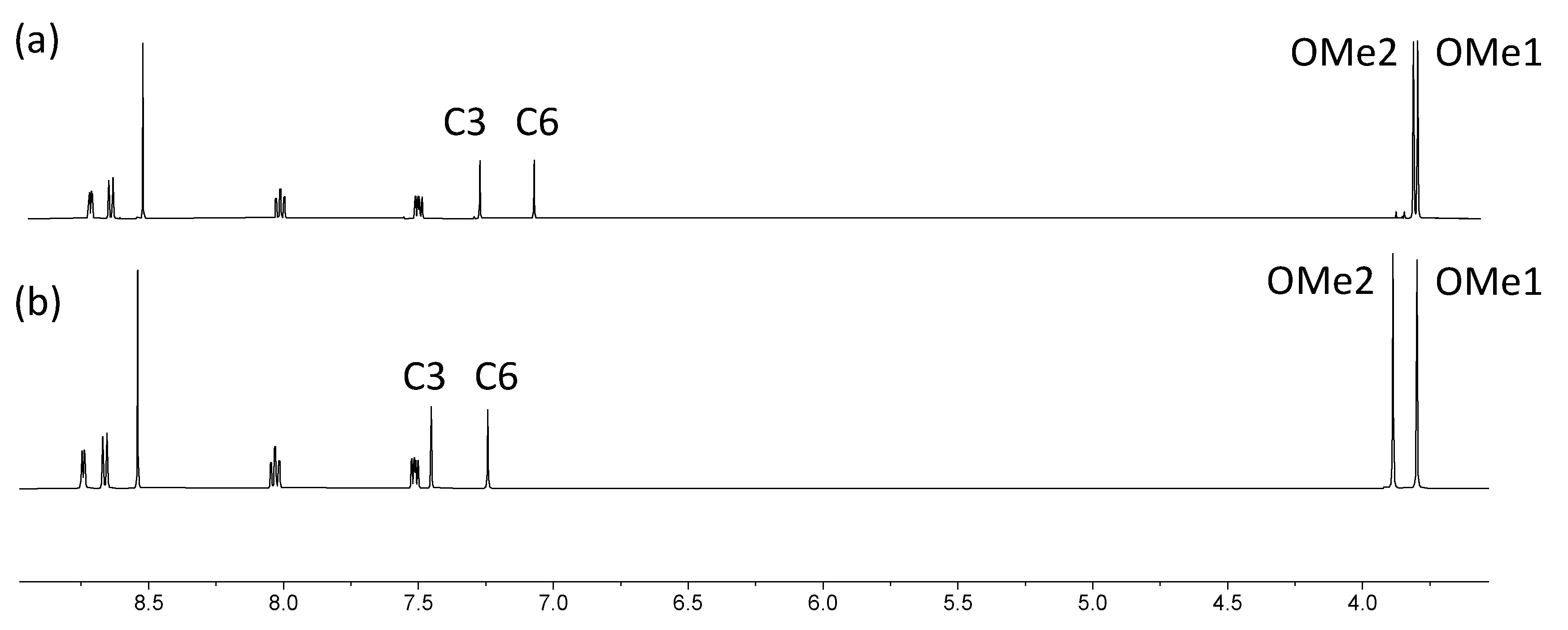
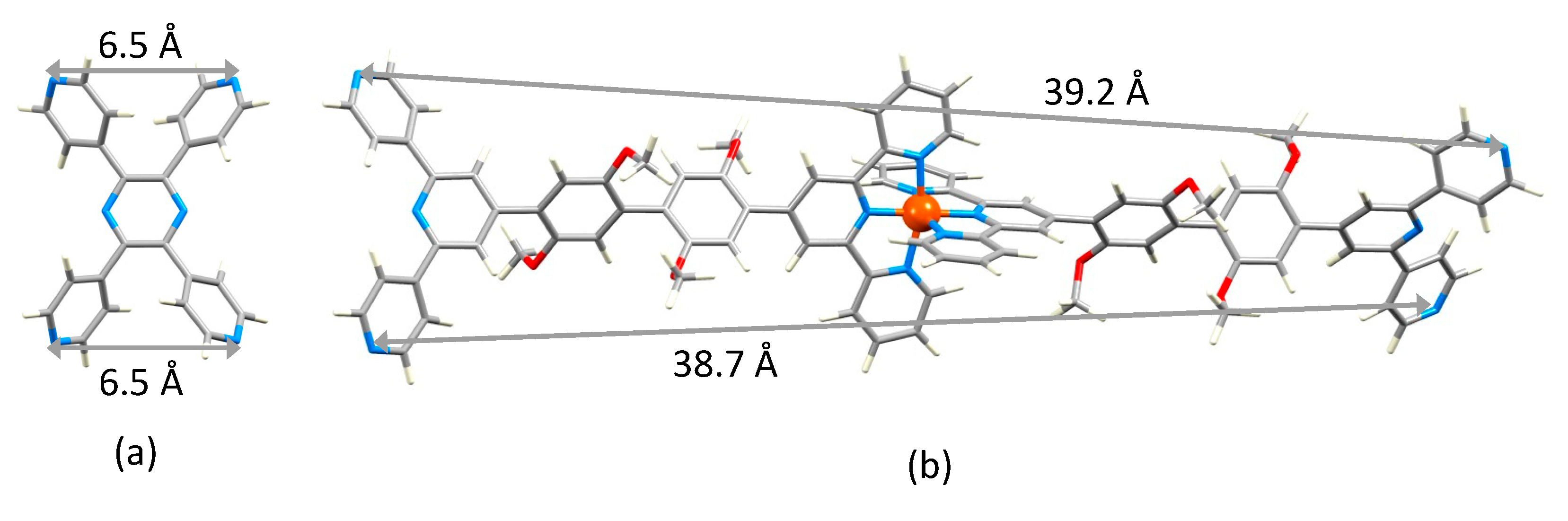
2.3. Expanded Ligands [Fe(5)2]2+ and [Fe(6)2]2+
3. Materials and Methods
3.1. General
3.2. Compound 1
3.3. Compound 2
3.4. Compound 3
3.5. Compound 4
3.6. Compound 5
3.7. Compound 6
3.8. [Fe(5)2][NO3]2
3.9. [Fe(6)2][BF4]2
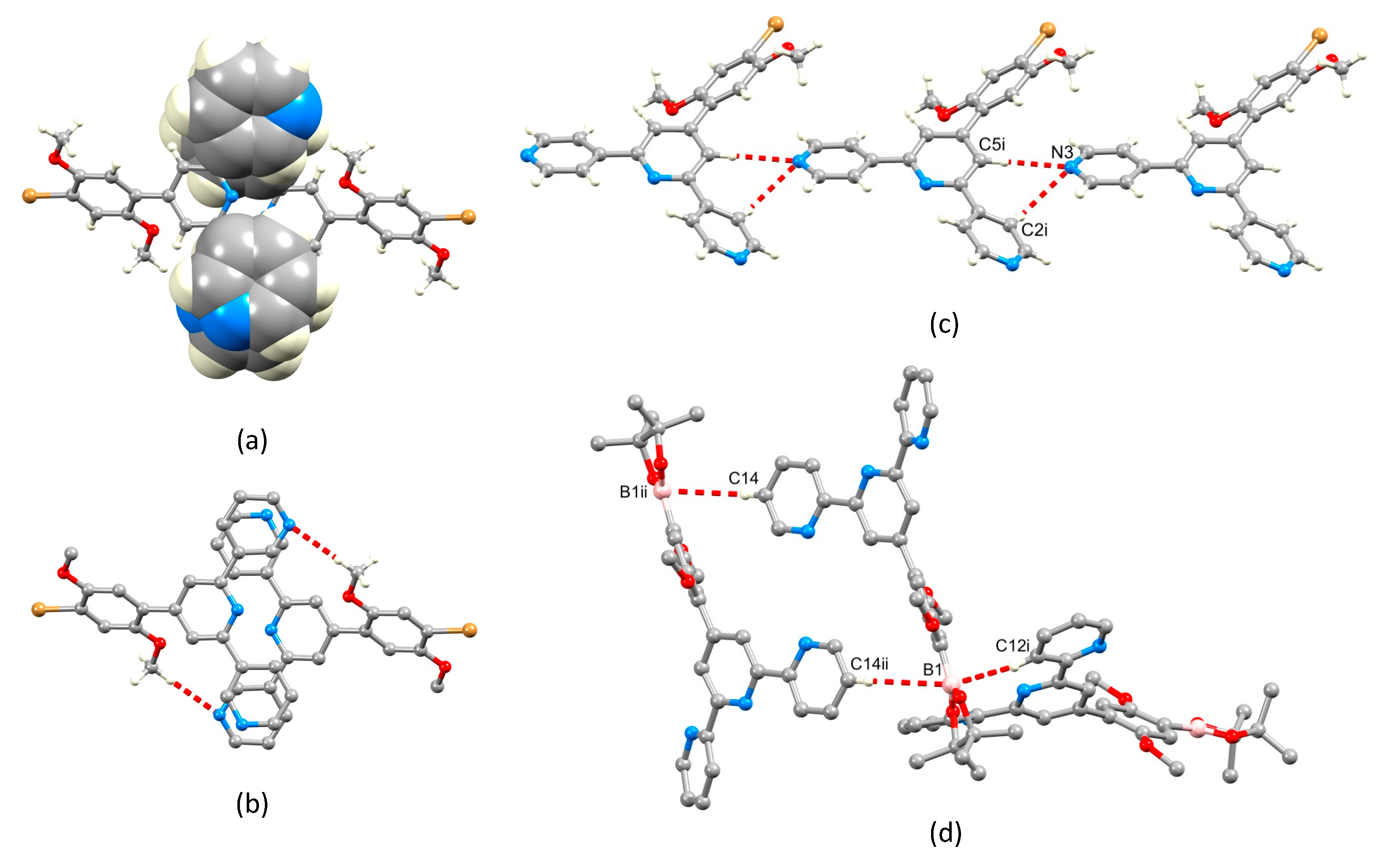
3.10. Crystallography
3.11. Compound 2
3.12. Compound 3
3.13. Compound 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Damhus, T.; Hartshorn, R.M.; Hutton, A.T. Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005, IUPAC Red Book; Connelly, N.G., Damhus, T., Hartshorn, R.M., Hutton, A.T., Eds.; RSC Publishing: Cambridge, UK, 2005. [Google Scholar]
- Constable, E.C. A Journey from Solution Self-Assembly to Designed Interfacial Assembly. Adv. Inorg. Chem. 2018, 71, 79–134. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. More hydra than Janus—Non-classical coordination modes in complexes of oligopyridine ligands. Coord. Chem. Rev. 2017, 350, 84–104. [Google Scholar] [CrossRef]
- Gelmini, L.; Stephan, D.W. The facile preparation of early transition metal/late transition metal heterobimetallic complexes; (η5-C5H5)2Zr(PPh2)2 as a ‘metalloligand’ for Ni, Pd and Pt. Inorg. Chim. Acta 1986, 111, L17–L18. [Google Scholar] [CrossRef]
- Kumar, G.; Kumar, G.; Gupta, R. Effect of pyridyl donors from organic ligands versus metalloligands on material design. Inorg. Chem. Front. 2021, 8, 1334–1373. [Google Scholar] [CrossRef]
- Li, F.; Lindoy, L.F. Metalloligand Strategies for Assembling Heteronuclear Nanocages—Recent Developments. Aust. J. Chem. 2019, 72, 731–741. [Google Scholar] [CrossRef]
- Dzhardimalieva, G.I.; Uflyand, I.E. Design and synthesis of coordination polymers with chelated units and their application in nanomaterials science. RSC Adv. 2017, 7, 42242–42288. [Google Scholar] [CrossRef]
- Kumar, G.; Gupta, R. Molecularly designed architectures—The metalloligand way. Chem. Soc. Rev. 2013, 42, 9403–9453. [Google Scholar] [CrossRef]
- Chen, B.; Xiang, S.; Qian, G. Metal—Organic Frameworks with Functional Pores for Recognition of Small Molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef]
- Constable, E.C. Expanded ligands—An assembly principle for supramolecular chemistry. Coord. Chem. Rev. 2008, 252, 842–855. [Google Scholar] [CrossRef]
- Constable, E.C.; Schofield, E. Metal-directed assembly of a box-like structure. Chem. Commun. 1998, 403–404. [Google Scholar] [CrossRef]
- Constable, E.C.; Dunphy, E.L.; Housecroft, C.E.; Kylberg, W.; Neuburger, M.; Schaffner, S.; Schofield, E.R.; Smith, C.B. Structural development of free or coordinated 4′-(4-pyridyl)-2,2′:6′,2″-terpyridine ligands through N-alkylation: New strategies for metallomacrocycle formation. Chem. Eur. J. 2006, 12, 4600–4610. [Google Scholar] [CrossRef] [PubMed]
- Beves, J.E.; Constable, E.C.; Housecroft, C.E.; Kepert, C.J.; Price, D.J. The first example of a coordination polymer from the expanded 4,4′-bipyridine ligand [Ru(pytpy)2]2+ (pytpy = 4′-(4-pyridyl)-2,2′:6′,2″-terpyridine). CrystEngCommun 2007, 9, 456–459. [Google Scholar] [CrossRef]
- Beves, J.E.; Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Schaffner, S. A palladium (II) complex of 4′-(4-pyridyl)-2,2′:6′,2″-terpyridine: Lattice control through an interplay of stacking and hydrogen bonding effects. Inorg. Chem. Commun. 2007, 10, 1185–1188. [Google Scholar] [CrossRef]
- Beves, J.E.; Constable, E.C.; Housecroft, C.E.; Kepert, C.J.; Price, D.J.; Schaffner, S. The conjugate acid of bis{4′-(4-pyridyl)-2,2′:6′,2″-terpyridine}iron(II) as a self-complementary hydrogen-bonded building block. CrystEngCommun 2007, 9, 1073–1077. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Schaffner, S.; Schaper, F. Preparation and structural characterisation of bis(4′-(3-pyridyl)-2,2′:6′,2″-terpyridine)ruthenium(II) hexafluorophosphate. Inorg. Chem. Commun. 2006, 9, 433–436. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Schaffner, S.; Schaper, F. The solid-state structure of bis(4′-(4-pyridyl)-2,2′:6′,2″-terpyridine)ruthenium hexafluorophosphate nitrate—an expanded 4,4′-bipyridine. Inorg. Chem. Commun. 2006, 9, 616–619. [Google Scholar] [CrossRef]
- Constable, E.C.; Dunphy, E.L.; Housecroft, C.E.; Neuburger, M.; Schaffner, S.; Schaper, F.; Batten, S.R. Expanded ligands: Bis(2,2′:6′,2″-terpyridine carboxylic acid)ruthenium(II) complexes as metallosupramolecular analogues of dicarboxylic acids. Dalton Trans. 2007, 4323–4332. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-hindered 4′-Aryl-2,2′:6′,2″-terpyridines. Synlett 2005, 1251–1254. [Google Scholar] [CrossRef]
- Sun, Q.; Tang, L.; Zhang, Z.; Zhang, K.; Xie, Z.; Chi, Z.; Zhang, H.; Yang, W. Bright NUV mechanofluorescence from a terpyridine-based pure organic crystal. Chem. Commun. 2018, 54, 94–97. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 5th ed.; Pearson: Harlow, UK, 2018; p. 413. ISBN 978-1-292-13414-7. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Schwalbe, M.; Metzinger, R.; Teets, T.S.; Nocera, D.G. Terpyridine–Porphyrin Hetero-Pacman Compounds. Chem. Eur. J. 2012, 18, 15449–15458. [Google Scholar] [CrossRef] [PubMed]
- Janiak, C. A critical account on π–π stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualising crystal structures. Acta Cryst. 2002, B58, 389–397. [Google Scholar] [CrossRef]
- Spartan; Version 18; Wavefunction Inc.: Irvine, CA, USA, 2020.
- Chen, Y.; Yang, T.; Huang, J.; Yong, H.-Y. Two Zn(II)-based coordination polymers: Treatment effect on the cardiac arrest induced by anesthesia by regulating Sirt1 expression. Inorg. Nano-Metal Chem. 2020, 51, 1471–1476. [Google Scholar] [CrossRef]
- Software for the Integration of CCD Detector System Bruker Analytical X-ray Systems; Bruker axs: Madison, WI, USA, 2013.
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with ShelXL. Acta Cryst. 2015, C27, 3–8. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Yang, J.; Clegg, J.K.; Jiang, Q.; Lui, X.; Yan, H.; Zhong, W.; Beves, J.E. Multi-pyridine decorated Fe(ii) and Ru(ii) complexes by Pd(0)-catalysed cross couplings: New building blocks for metallosupramolecular assemblies. Dalton Trans. 2013, 42, 15625–15636. [Google Scholar] [CrossRef][Green Version]
- Liu, S.-L.; Chen, Q.-W.; Zhang, Z.-W.; Chen, Q.; Wei, L.-Q.; Lin, N. Efficient heterogeneous catalyst of Fe(II)-based coordination complexes for Friedel-Crafts alkylation reaction. J. Solid State Chem. 2022, 310, 123045. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, D.; Prescimone, A.; Housecroft, C.E.; Constable, E.C. Expanded Ligands Based upon Iron(II) Coordination Compounds of Asymmetrical Bis(terpyridine) Domains. Molecules 2023, 28, 82. https://doi.org/10.3390/molecules28010082
Rocco D, Prescimone A, Housecroft CE, Constable EC. Expanded Ligands Based upon Iron(II) Coordination Compounds of Asymmetrical Bis(terpyridine) Domains. Molecules. 2023; 28(1):82. https://doi.org/10.3390/molecules28010082
Chicago/Turabian StyleRocco, Dalila, Alessandro Prescimone, Catherine E. Housecroft, and Edwin C. Constable. 2023. "Expanded Ligands Based upon Iron(II) Coordination Compounds of Asymmetrical Bis(terpyridine) Domains" Molecules 28, no. 1: 82. https://doi.org/10.3390/molecules28010082
APA StyleRocco, D., Prescimone, A., Housecroft, C. E., & Constable, E. C. (2023). Expanded Ligands Based upon Iron(II) Coordination Compounds of Asymmetrical Bis(terpyridine) Domains. Molecules, 28(1), 82. https://doi.org/10.3390/molecules28010082