Cell-Free Supernatant from Lactobacillus and Streptococcus Strains Modulate Mucus Production via Nf-κB/CREB Pathway in Diesel Particle Matter-Stimulated NCI-H292 Airway Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. Inhibition of NO Production by CFS from Lactobacillus and Streptococcus Strains in LPS-induced RAW 264.7 Cells
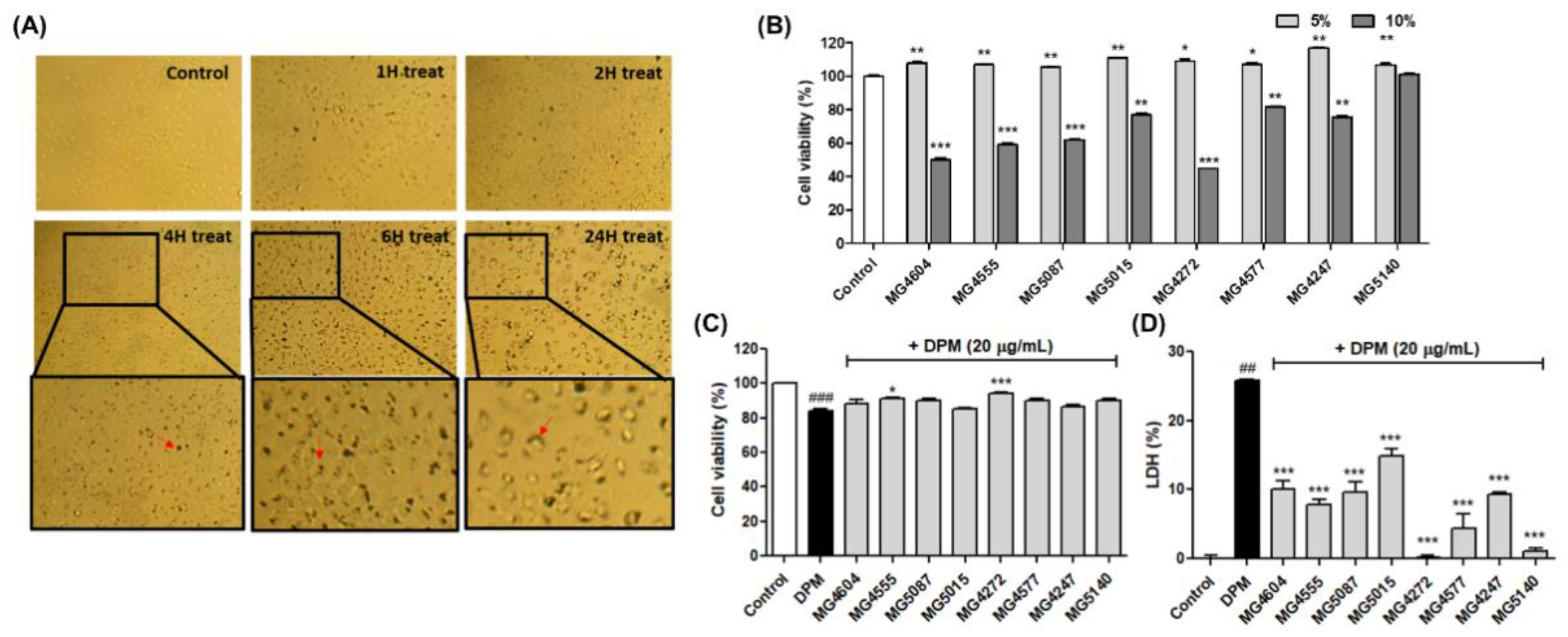
2.2. CFS from Lactobacillus and Streptococcus Strains Suppress Cytotoxicity in DPM-induced NCI-H292 Cells
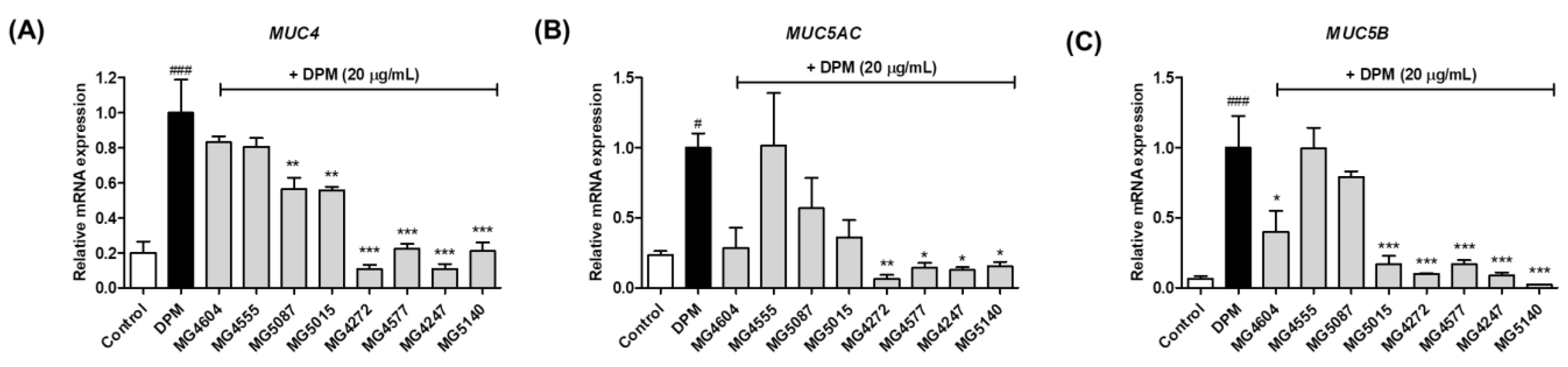
2.3. Inhibition of mRNA Expression Involved in Mucus Production by CFS from Lactobacillus and Streptococcus Strains in DPM-induced NCI-H292 Cells
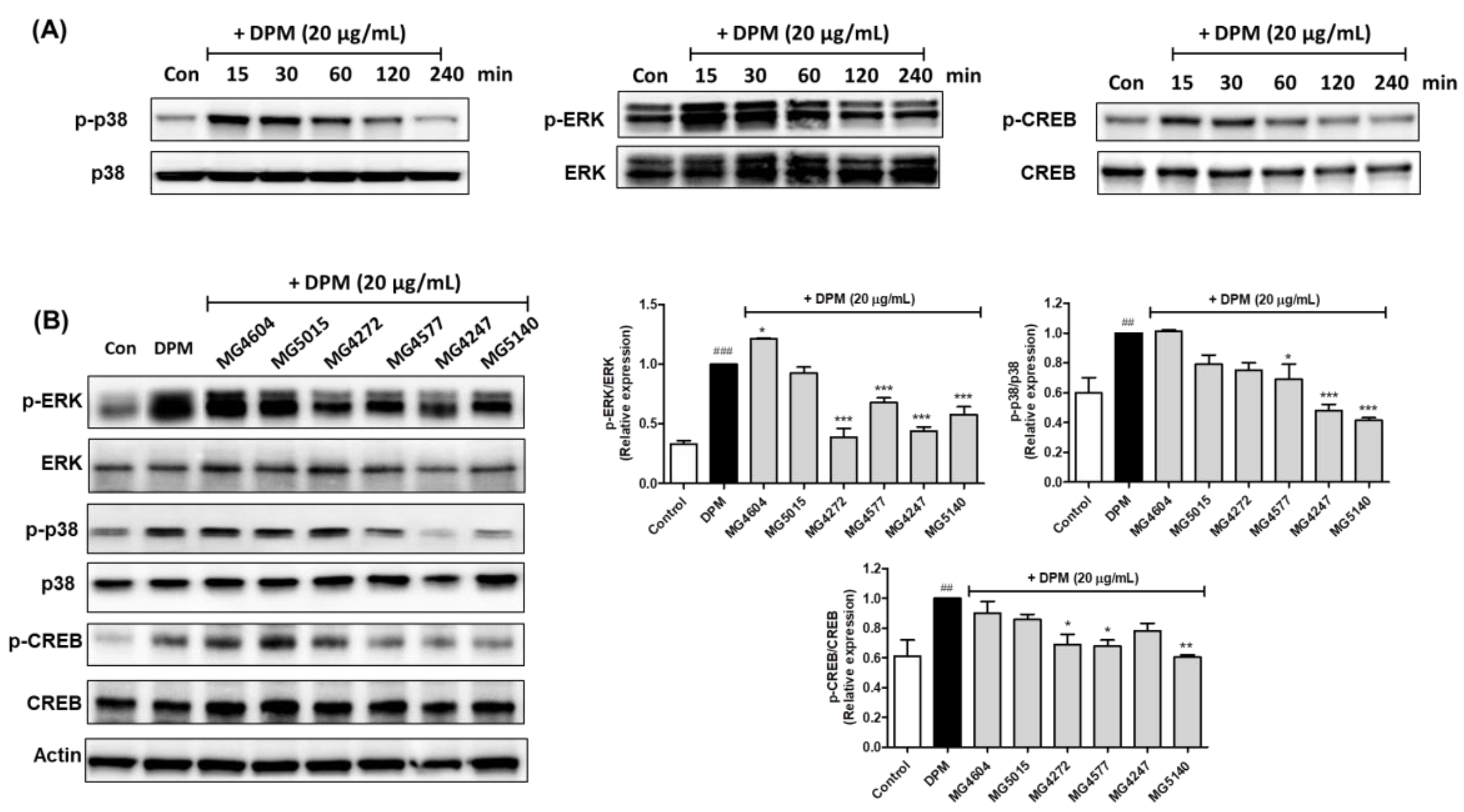
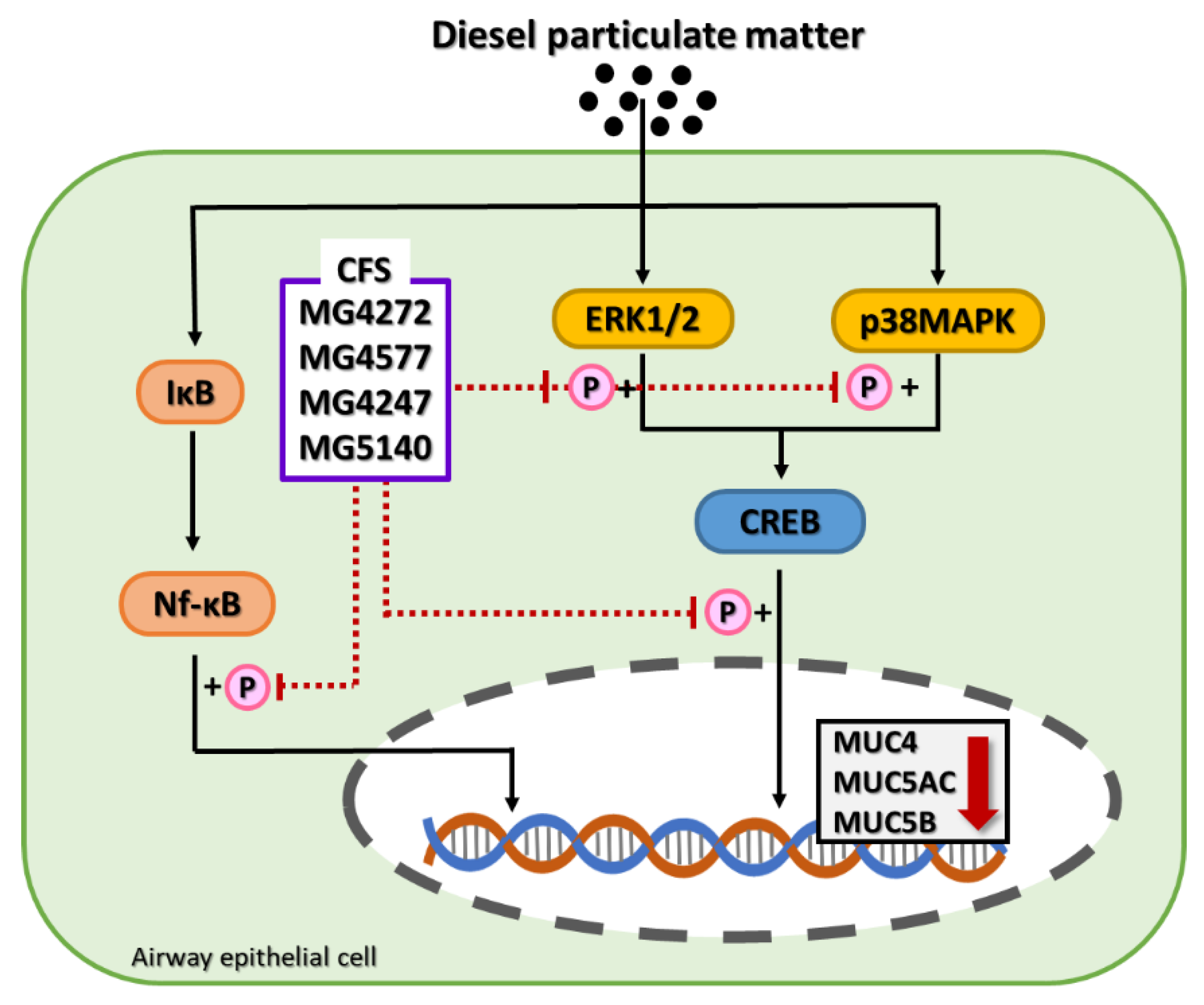
2.4. CFS Lactobacillus and Streptococcus Strains Modulate CREB Signaling Pathway in DPM-induced NCI-H292 Cells
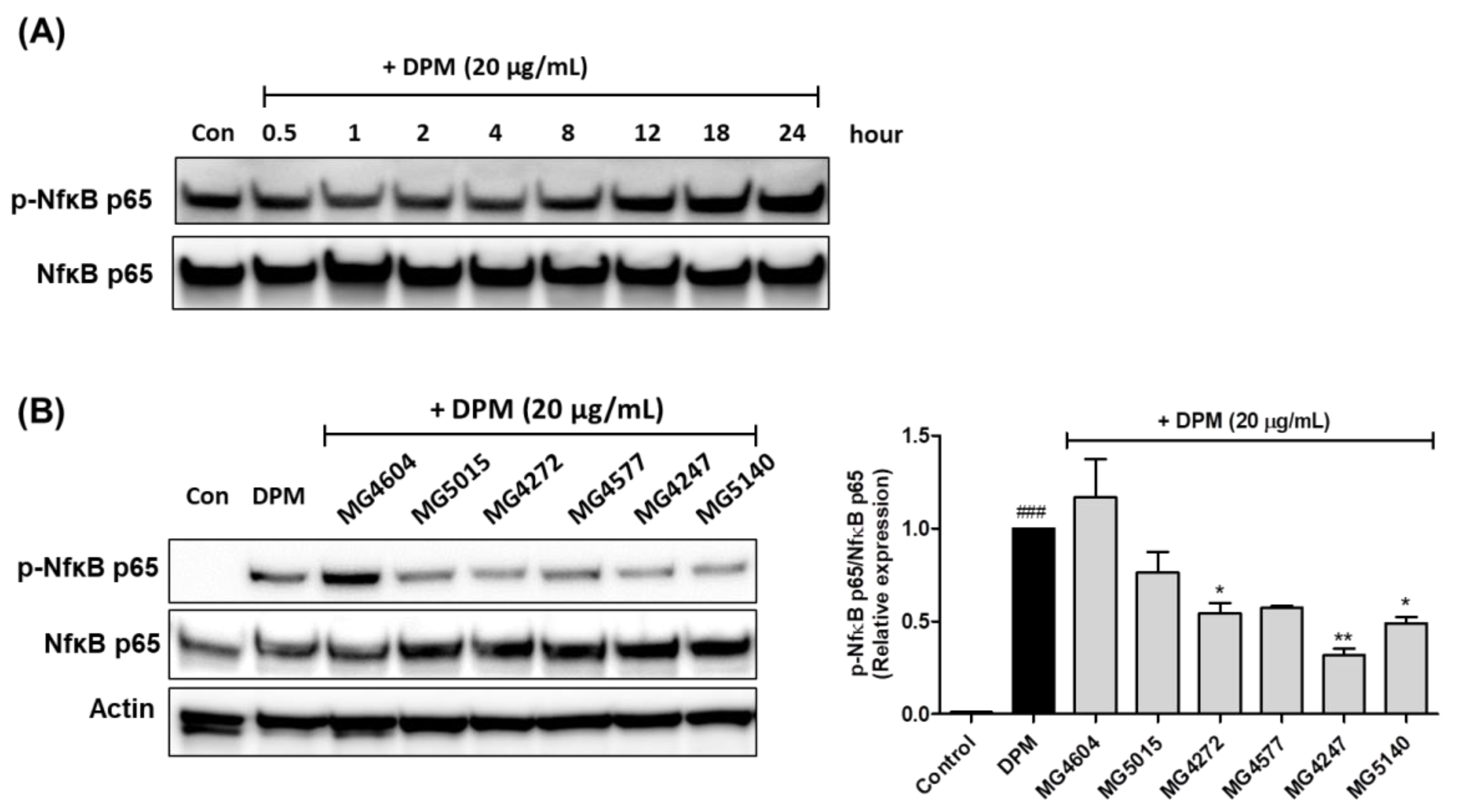
2.5. Effect on Phosphorylation of Nf-κB by CFS from Lactobacillus and Streptococcus Strains in DPM-induced NCI-H292 cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Cell-Free Supernatants (CFS) from Lactobacillus and Streptococcus Strains
4.3. Cell Culture
4.4. Cell Viability
4.5. Assessment of NO Production
4.6. Preparation of mRNA and Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Protein Extraction
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Agustí, A.; Edwards, L.D.; Rennard, S.I.; MacNee, W.; Tal-Singer, R.; Miller, B.E.; Vestbo, J.; Lomas, D.A.; Calverley, P.M.; Wouters, E. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: A novel phenotype. PLoS ONE 2012, 7, e37483. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.; Celli, B. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef] [PubMed]
- Rovina, N.; Koutsoukou, A.; Koulouris, N.G. Inflammation and immune response in COPD: Where do we stand? Mediat. Inflamm. 2013, 2013, 413735. [Google Scholar] [CrossRef] [PubMed]
- Voynow, J.A.; Gendler, S.J.; Rose, M.C. Regulation of mucin genes in chronic inflammatory airway diseases. Am. J. Respir. Cell Mol. Biol. 2006, 34, 661–665. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, J. The function of mucins in the COPD airway. Curr. Respir. Care Rep. 2013, 2, 155–166. [Google Scholar] [CrossRef]
- Na, H.G.; Kim, Y.-D.; Choi, Y.S.; Bae, C.H.; Song, S.-Y. Diesel exhaust particles elevate MUC5AC and MUC5B expression via the TLR4-mediated activation of ERK1/2, p38 MAPK, and NF-κB signaling pathways in human airway epithelial cells. Biochem. Biophys. Res. Commun. 2019, 512, 53–59. [Google Scholar] [CrossRef]
- Milara, J.; Morell, A.; Ballester, B.; Armengot, M.; Morcillo, E.; Cortijo, J. MUC4 impairs the anti-inflammatory effects of corticosteroids in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2017, 139, 855–862.e13. [Google Scholar] [CrossRef]
- Koga, Y.; Tsurumaki, H.; Aoki-Saito, H.; Sato, M.; Yatomi, M.; Takehara, K.; Hisada, T. Roles of cyclic AMP response element binding activation in the ERK1/2 and p38 MAPK signalling pathway in central nervous system, cardiovascular system, osteoclast differentiation and mucin and cytokine production. Int. J. Mol. Sci. 2019, 20, 1346. [Google Scholar] [CrossRef]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I.; NIHR RESPIRE Global Respiratory Health Unit. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.-C. Recent insights into particulate matter (PM2. 5)-mediated toxicity in humans: An overview. Int. J. Environ. Health Res. 2022, 19, 7511. [Google Scholar] [CrossRef]
- Safiri, S.; Carson-Chahhoud, K.; Noori, M.; Nejadghaderi, S.A.; Sullman, M.J.; Heris, J.A.; Ansarin, K.; Mansournia, M.A.; Collins, G.S.; Kolahi, A.-A. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ 2022, 378, e069679. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.; Merenstein, D.; Merrifield, C.; Hutkins, R. Probiotics for human use. Nutr. Bull. 2018, 43, 212–225. [Google Scholar] [CrossRef]
- Mortaz, E.; Adcock, I.M.; Folkerts, G.; Barnes, P.J.; Paul Vos, A.; Garssen, J. Probiotics in the management of lung diseases. Mediat. Inflamm. 2013, 2013, 751068. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-K.; Paik, H.-D. Prophylactic effects of probiotics on respiratory viruses including COVID-19: A review. Food Sci. Biotechnol. 2021, 30, 773–781. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Gonzalez, E.G.; Lamas, A.; Mondragon, A.d.C.; Regal, P.; Miranda, J.M. Probiotics as a possible strategy for the prevention and treatment of allergies: A narrative review. Foods 2021, 10, 701. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Sheikh, M.; Eskandarzadeh, S.; Tarlak, F.; Tripathi, A.D.; Khosravi-Darani, K.; Sadanov, A. The Effect of probiotics on various diseases and their therapeutic role: An update review. J. Pure Appl. Microbiol. 2021, 15, 1042–1059. [Google Scholar] [CrossRef]
- Secher, T.; Maillet, I.; Mackowiak, C.; Le Bérichel, J.; Philippeau, A.; Panek, C.; Boury, M.; Oswald, E.; Saoudi, A.; Erard, F. The probiotic strain Escherichia coli Nissle 1917 prevents papain-induced respiratory barrier injury and severe allergic inflammation in mice. Sci. Rep. 2018, 8, 11245. [Google Scholar] [CrossRef]
- DE SÁ Fialho, A.K.C.; Miranda, M.T.F.; Carvalho, J.L.C.; Brito, A.A.; Albertini, R.; Aimbire, F. Role of probiotics Bifidobacterium breve and Lactobacillus rhamnosus on inflammation lung in an experimental model of chronic obstructive pulmonary disease. FASEB J. 2019, 33, 516.4. [Google Scholar] [CrossRef]
- Adamson, J.; Haswell, L.E.; Phillips, G.; Gaça, M.D. In vitro models of chronic obstructive pulmonary disease (COPD). In Bronchitis; Martin-Loeches, I., Ed.; InTech: Vienna, Austria, 2011; pp. 41–66. [Google Scholar]
- Newland, N.; Richter, A. Agents associated with lung inflammation induce similar responses in NCI-H292 lung epithelial cells. Toxicol. In Vitro 2008, 22, 1782–1788. [Google Scholar] [CrossRef]
- Jo, S.; Na, H.G.; Choi, Y.S.; Bae, C.H.; Song, S.-Y.; Kim, Y.-D. Saponin attenuates diesel exhaust particle (DEP)-induced MUC5AC expression and pro-inflammatory cytokine upregulation via TLR4/TRIF/NF-κB signaling pathway in airway epithelium and ovalbumin (OVA)-sensitized mice. J. Ginseng Res. 2022, 46, 801–808. [Google Scholar] [CrossRef]
- Keulers, L.; Dehghani, A.; Knippels, L.; Garssen, J.; Papadopoulos, N.; Folkerts, G.; Braber, S.; van Bergenhenegouwen, J. Probiotics, prebiotics, and synbiotics to prevent or combat air pollution consequences: The gut-lung axis. Environ. Pollut. 2022, 302, 119066. [Google Scholar] [CrossRef] [PubMed]
- Gautier, T.; Gall, D.-L.; Sweidan, A.; Tamanai-Shacoori, Z.; Jolivet-Gougeon, A.; Loréal, O.; Bousarghin, L. Next-generation probiotics and their metabolites in COVID-19. Microorganisms 2021, 9, 941. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.L.; Sa, A.K.; Britto, A.; Ferreira, M.; Anatriello, E.; Keller, A.; Aimbire, F. Bifidobacterium breve significantly reduces cigarette smoke-induced COPD in C57Bl/6 mice. Eur. Respir. J. 2018, 52, 4244. [Google Scholar] [CrossRef]
- Nam, W.; Kim, H.; Bae, C.; Kim, J.; Nam, B.; Lee, Y.; Kim, J.; Park, S.; Lee, J.; Sim, J. Lactobacillus HY2782 and Bifidobacterium HY8002 decrease airway hyperresponsiveness induced by chronic PM2. 5 inhalation in mice. J. Med. Food 2020, 23, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, T.; Si, B.; Du, H.; Liu, Y.; Waqas, A.; Huang, S.; Zhao, G.; Chen, S.; Xu, A. Intratracheally instillated diesel PM2. 5 significantly altered the structure and composition of indigenous murine gut microbiota. Ecotoxicol. Environ. Saf. 2021, 210, 111903. [Google Scholar] [CrossRef]
- Eze, F.I.; Uzor, P.F.; Ikechukwu, P.; Obi, B.C.; Osadebe, P.O. In vitro and in vivo models for anti-inflammation: An evaluative review. INNOSC Theranostics Pharmacol. Sci. (ITPS) 2019, 2, 3–15. [Google Scholar] [CrossRef]
- Korhonen, R.; Korpela, R.; Saxelin, M.; Mäki, M.; Kankaanranta, H.; Moilanen, E. Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation 2001, 25, 223–232. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kang, J.-H.; Jung, Y.-R.; Kang, C.-H. Lactobacillus gasseri MG4247 and Lacticaseibacillus paracasei MG4272 and MG4577 modulate allergic inflammatory response in RAW 264.7 and RBL-2H3 cells. Probiotics Antimicrob. Proteins, 2011; ahead of print. [Google Scholar] [CrossRef]
- Shekhawat, J.; Gauba, K.; Gupta, S.; Purohit, P.; Mitra, P.; Garg, M.; Misra, S.; Sharma, P.; Banerjee, M. Interleukin-6 perpetrator of the COVID-19 cytokine storm. Indian J. Clin. Biochem. 2021, 36, 440–450. [Google Scholar] [CrossRef]
- Hacievliyagil, S.; Mutlu, L.C.; Temel, I. Airway inflammatory markers in chronic obstructive pulmonary disease patients and healthy smokers. Niger. J. Clin. Pract. 2013, 16, 76–81. [Google Scholar] [CrossRef]
- Machado, A.; Marques, A.; Burtin, C. Extra-pulmonary manifestations of COPD and the role of pulmonary rehabilitation: A symptom-centered approach. Expert Rev. Respir. Med. 2021, 15, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Thai, P.; Loukoianov, A.; Wachi, S.; Wu, R. Regulation of airway mucin gene expression. Annu. Rev. Physiol. 2008, 70, 405. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-H.; Kang, J.-H.; Kim, J.A.; Shin, J.-M.; Lee, H.-M. Diesel exhaust particles enhance MUC4 expression in NCI-H292 cells and nasal epithelial cells via the p38/CREB pathway. Int. Arch. Allergy Immunol. 2016, 171, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Schuliga, M. NF-kappaB signaling in chronic inflammatory airway disease. Biomolecules 2015, 5, 1266–1283. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, L.; Donato, M.T.; Gómez-Lechón, M.J. General cytotoxicity assessment by means of the MTT assay. In Protocols in In Vitro Hepatocyte Research; Springer: Berlin/Heidelberg, Germany, 2015; pp. 333–348. [Google Scholar]
- Yucel, A.A.; Gulen, S.; Dincer, S.; Yucel, A.E.; Yetkin, G.I. Comparison of two different applications of the Griess method for nitric oxide measurement. J. Exp. Integr. Med. 2012, 2, 167–171. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71. [Google Scholar]
- Hnasko, T.S.; Hnasko, R.M. The western blot. In ELISA; Springer: Berlin/Heidelberg, Germany, 2015; pp. 87–96. [Google Scholar]

| Gene † | Sequence (5′-3′) | Product (bp) | |
|---|---|---|---|
| MUC4 | Forward | GACTTGGAGCTCTTTGAGAATGG | 139 |
| Reverse | TGCAATGGCAGACCACAGTCC | ||
| MUC5AC | Forward | CTCCTACCAATGCTCTGTA | 131 |
| Reverse | GTTGCAGAAGCAGGTTTG | ||
| MUC5B | Forward | GACAGAGACGACAATGAG | 154 |
| Reverse | CCTGATGTTTTCAAAAGTTTC | ||
| GADPH | Forward | ACCCACTCCTCCACCTTTG | 178 |
| Reverse | CTCTTGTGCTCTTGCTGGG | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Kang, C.-H. Cell-Free Supernatant from Lactobacillus and Streptococcus Strains Modulate Mucus Production via Nf-κB/CREB Pathway in Diesel Particle Matter-Stimulated NCI-H292 Airway Epithelial Cells. Molecules 2023, 28, 61. https://doi.org/10.3390/molecules28010061
Lee JY, Kang C-H. Cell-Free Supernatant from Lactobacillus and Streptococcus Strains Modulate Mucus Production via Nf-κB/CREB Pathway in Diesel Particle Matter-Stimulated NCI-H292 Airway Epithelial Cells. Molecules. 2023; 28(1):61. https://doi.org/10.3390/molecules28010061
Chicago/Turabian StyleLee, Ji Yeon, and Chang-Ho Kang. 2023. "Cell-Free Supernatant from Lactobacillus and Streptococcus Strains Modulate Mucus Production via Nf-κB/CREB Pathway in Diesel Particle Matter-Stimulated NCI-H292 Airway Epithelial Cells" Molecules 28, no. 1: 61. https://doi.org/10.3390/molecules28010061
APA StyleLee, J. Y., & Kang, C.-H. (2023). Cell-Free Supernatant from Lactobacillus and Streptococcus Strains Modulate Mucus Production via Nf-κB/CREB Pathway in Diesel Particle Matter-Stimulated NCI-H292 Airway Epithelial Cells. Molecules, 28(1), 61. https://doi.org/10.3390/molecules28010061






