Computer-Aided Screening for Potential Coronavirus 3-Chymotrypsin-like Protease (3CLpro) Inhibitory Peptides from Putative Hemp Seed Trypsinized Peptidome
Abstract
1. Introduction
2. Results and Discussion
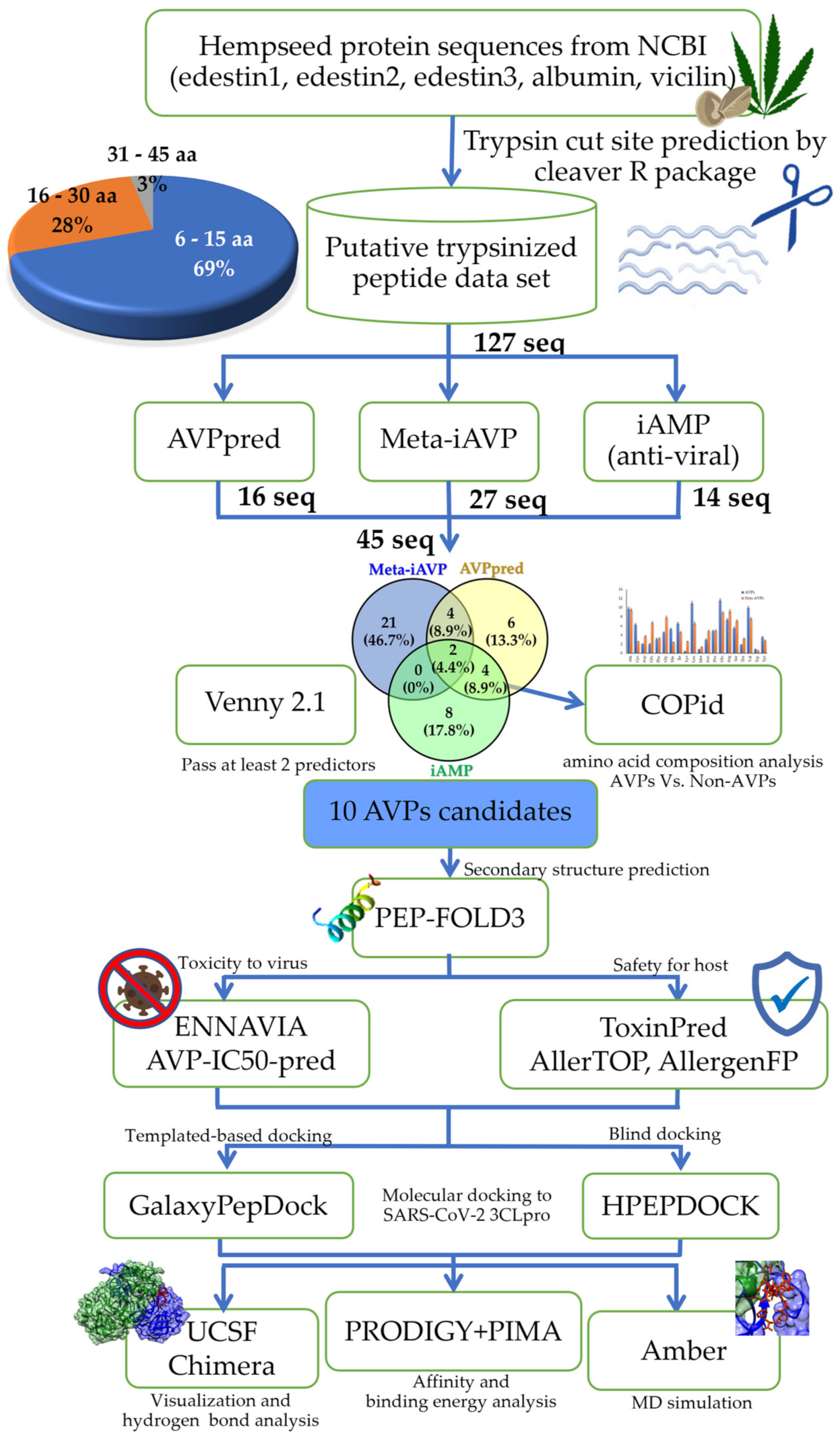
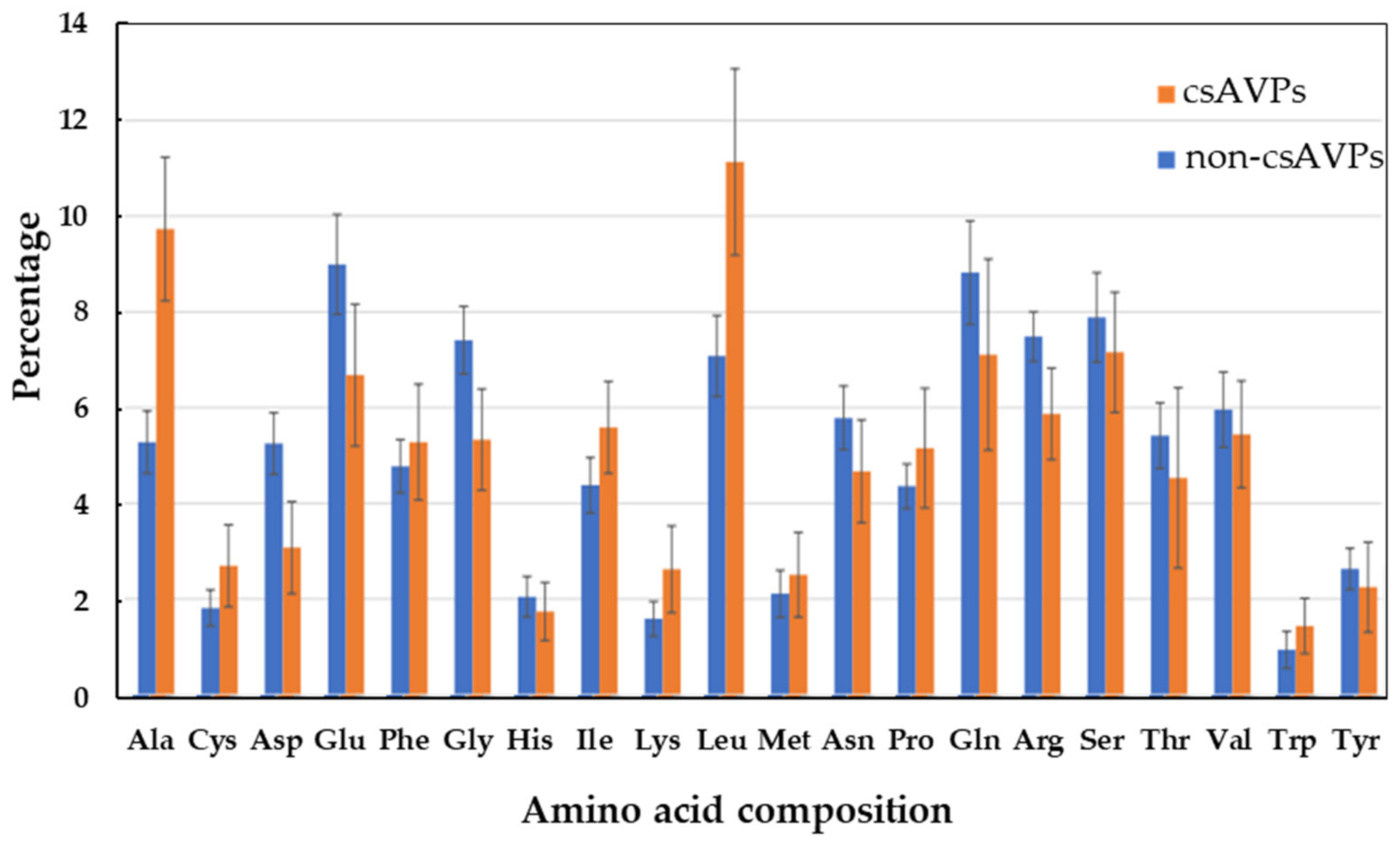
2.1. Hempseed Putative Antiviral Peptides Screening Using Computational Method
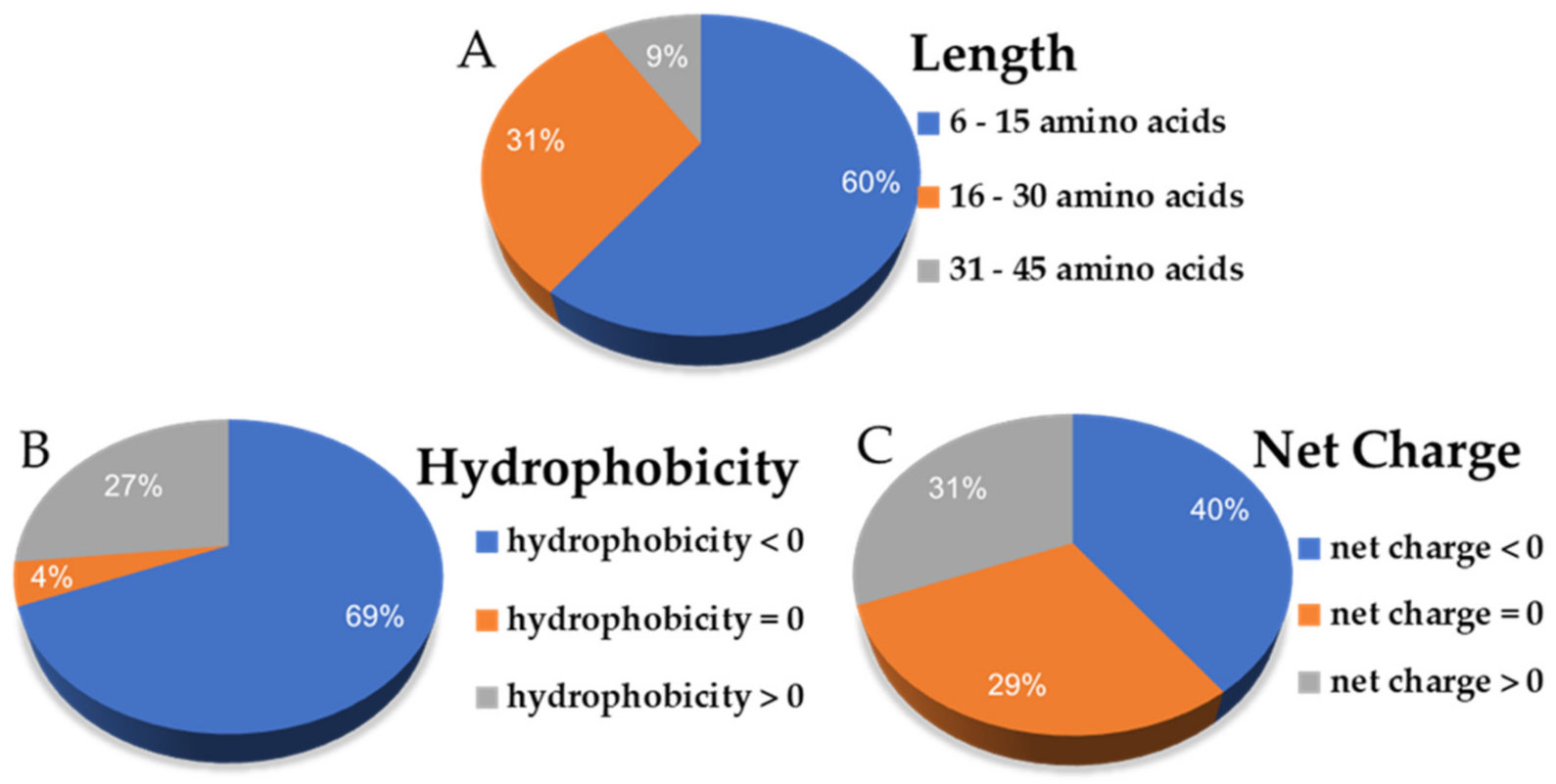
2.2. Predictive Antiviral Scores, IC50, and Physicochemical Properties of the Selected csAVPs
2.3. In silico Toxicity and Allergenicity Analysis of the Selected csAVPs
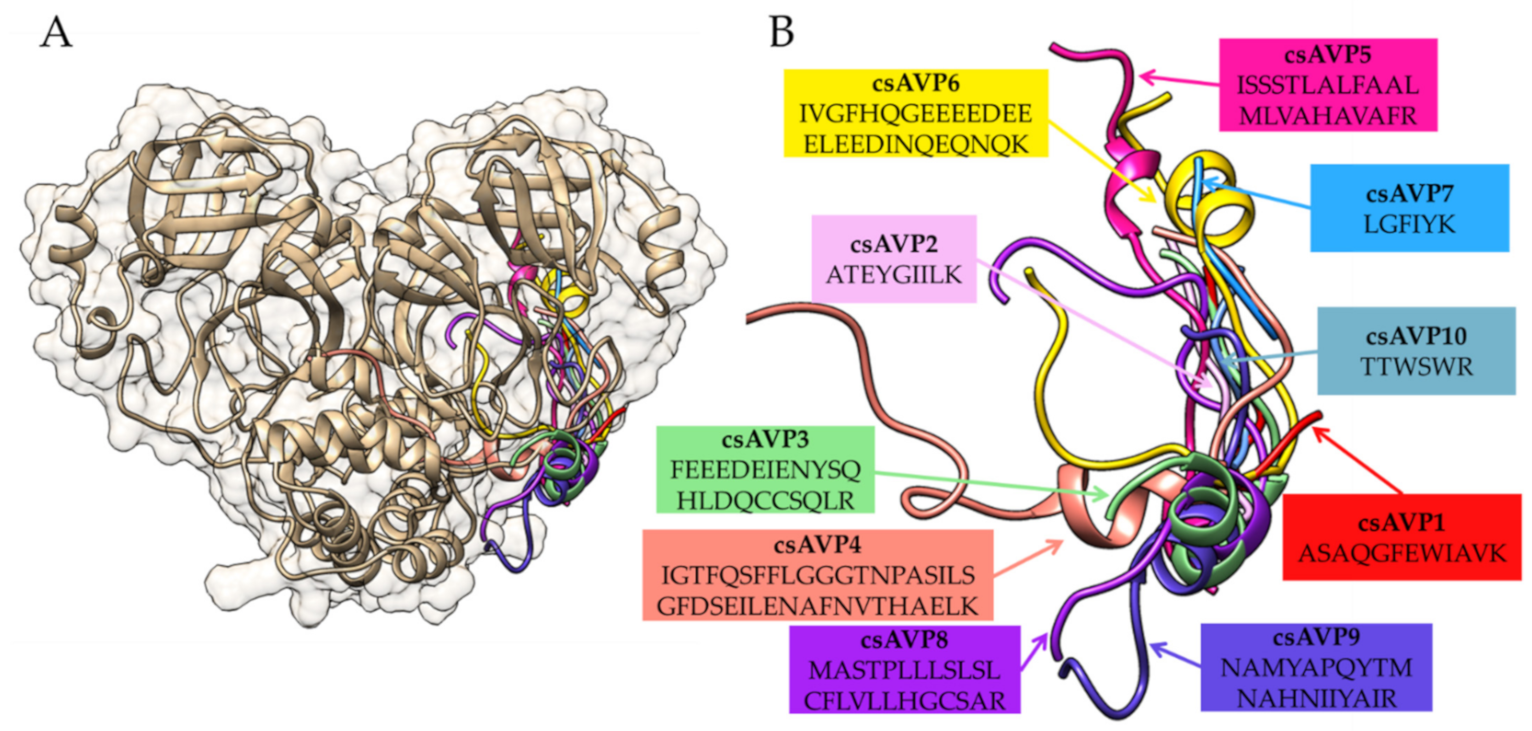
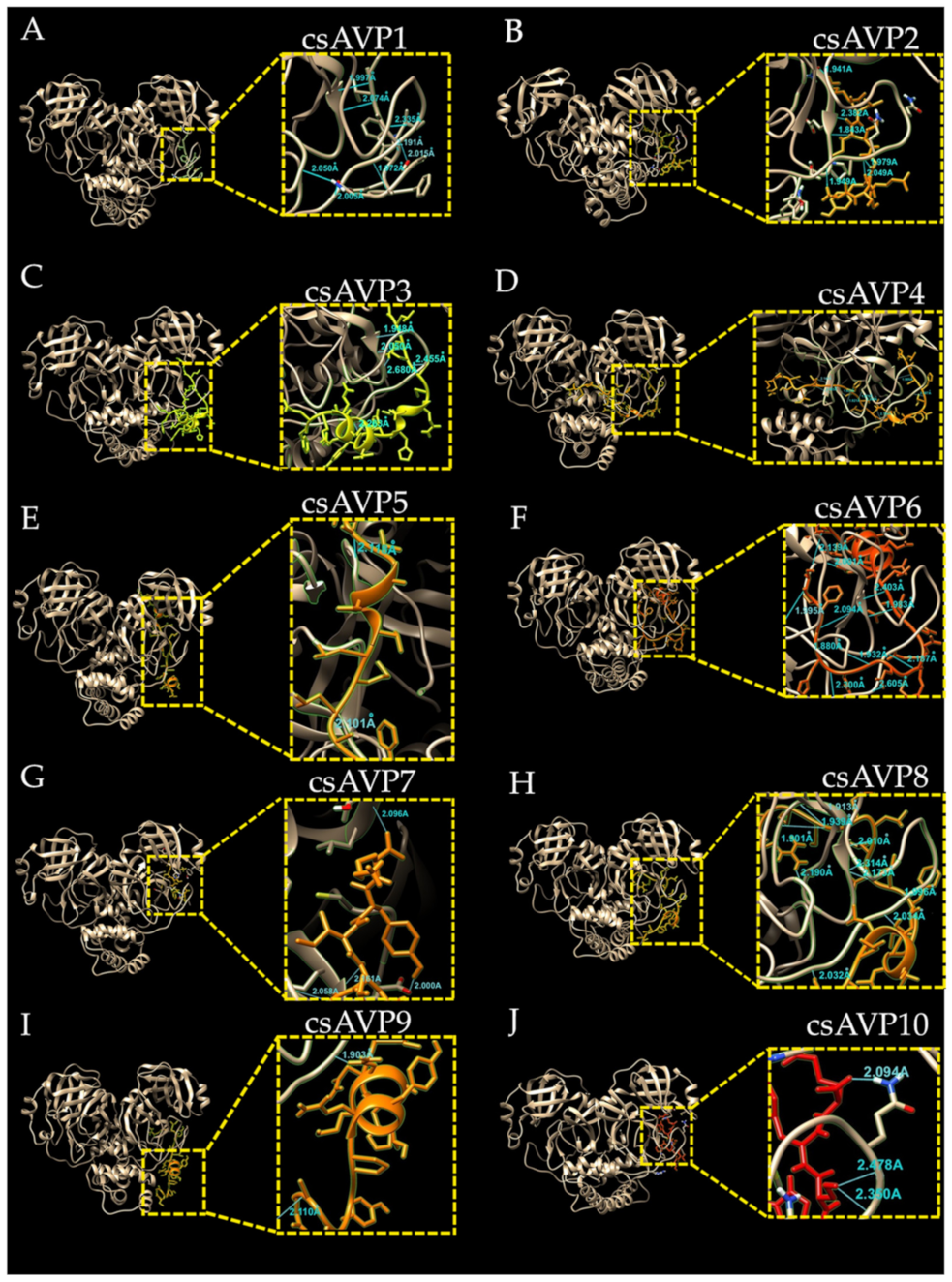
2.4. Molecular Docking of csAVPs with SARS-CoV-2 3CL Protease
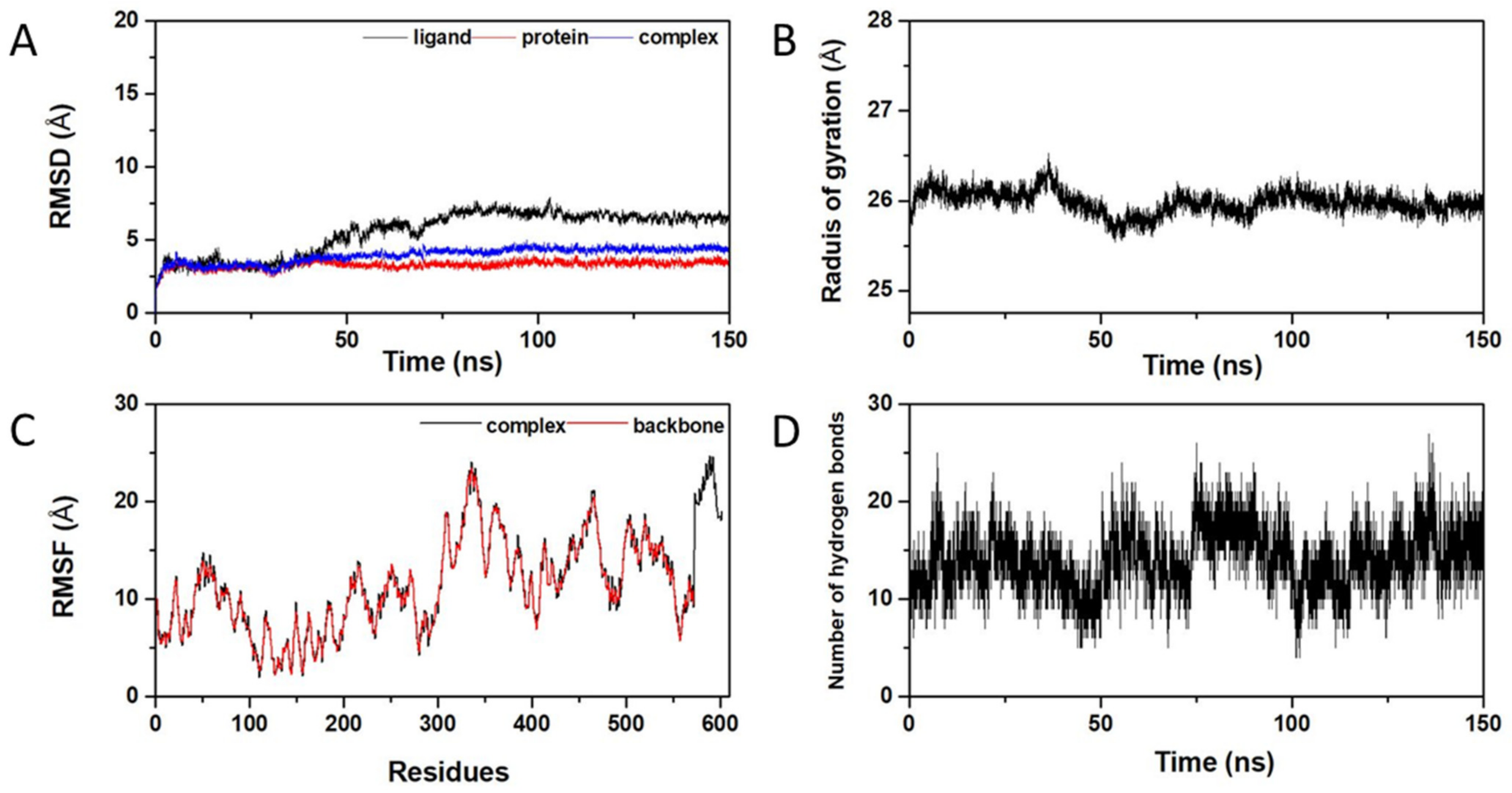
2.5. Molecular Dynamics Simulation of 3CLpro-csAVP4 Complex
2.6. Similarity of the csAVP4 against the Known Anti-Coronavirus Peptides Database
3. Materials and Methods
3.1. Preparation of the Hemp Seed Putative Hydrolyzed Peptidome
3.2. The Computer-Aided Prediction and Screening of Antiviral Peptides
3.3. Predictions of Allergenicity, Toxicity, and Physicochemical Properties
3.4. IC50 Prediction
3.5. Molecular Docking Simulation of Protein–Peptide
3.6. Molecular Dynamics Simulations Study
3.7. Similarity Searching of the csAVP4 against the Anti-Coronavirus Peptides Database
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, S.; Jiang, S.; Qi, X.; Bai, R.; Ye, X.Y.; Xie, T. Races of small molecule clinical trials for the treatment of COVID-19: An up-to-date comprehensive review. Drug Dev. Res. 2022, 83, 16–54. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.A.; Kim, H.; Xue, L. Why are vaccines against many human viral diseases still unavailable; an historic perspective? J. Med. Virol. 2020, 92, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Singh, M.; Ravichandiran, V.; Murty, U.; Srivastava, H.K. Peptide-like and small-molecule inhibitors against COVID-19. J. Biomol. Struct. Dyn. 2020, 39, 2904–2913. [Google Scholar] [CrossRef]
- Mousavi Maleki, M.S.; Rostamian, M.; Madanchi, H. Antimicrobial peptides and other peptide-like therapeutics as promising candidates to combat SARS-CoV-2. Expert Rev. Anti-Infect. Ther. 2021, 19, 1205–1217. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020, 6, 315–331. [Google Scholar] [CrossRef]
- Banerjee, R.; Perera, L.; Tillekeratne, L.V. Potential SARS-CoV-2 main protease inhibitors. Drug Discov. Today 2021, 26, 804–816. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Xi, K.; Grum-Tokars, V.; Xu, X.; Ratia, K.; Fu, W.; Houser, K.V.; Baker, S.C.; Johnson, M.E.; Mesecar, A.D. Structure-based design, synthesis, and biological evaluation of peptidomimetic SARS-CoV 3CLpro inhibitors. Bioorganic Med. Chem. Lett. 2007, 17, 5876–5880. [Google Scholar] [CrossRef]
- Chou, K.-C.; Wei, D.-Q.; Zhong, W.-Z. Binding mechanism of coronavirus main proteinase with ligands and its implication to drug design against SARS. Biochem. Biophys. Res. Commun. 2003, 308, 148–151. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Xi, K.; Johnson, M.E.; Baker, S.C.; Mesecar, A.D. Progress in anti-SARS coronavirus chemistry, biology and chemotherapy. Annu. Rep. Med. Chem. 2006, 41, 183–196. [Google Scholar]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Citarella, A.; Scala, A.; Piperno, A.; Micale, N. SARS-CoV-2 Mpro: A potential target for peptidomimetics and small-molecule inhibitors. Biomolecules 2021, 11, 607. [Google Scholar] [CrossRef]
- Macip, G.; Garcia-Segura, P.; Mestres-Truyol, J.; Saldivar-Espinoza, B.; Pujadas, G.; Garcia-Vallvé, S. A review of the current landscape of SARS-CoV-2 main protease inhibitors: Have we hit the bullseye yet? Int. J. Mol. Sci. 2021, 23, 259. [Google Scholar] [CrossRef]
- Yang, H.; Xie, W.; Xue, X.; Yang, K.; Ma, J.; Liang, W.; Zhao, Q.; Zhou, Z.; Pei, D.; Ziebuhr, J. Design of wide-spectrum inhibitors targeting coronavirus main proteases. PLoS Biol. 2005, 3, e324. [Google Scholar]
- Sun, X.; Sun, Y.; Li, Y.; Wu, Q.; Wang, L. Identification and Characterization of the Seed Storage Proteins and Related Genes of Cannabis sativa L. Front. Nutr. 2021, 8, 678421. [Google Scholar] [CrossRef]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility-corrected amino acid score method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef]
- Santos-Sánchez, G.; Álvarez-López, A.I.; Ponce-España, E.; Carrillo-Vico, A.; Bollati, C.; Bartolomei, M.; Lammi, C.; Cruz-Chamorro, I. Hempseed (Cannabis sativa) protein hydrolysates: A valuable source of bioactive peptides with pleiotropic health-promoting effects. Trends Food Sci. Technol. 2022, 127, 303–318. [Google Scholar] [CrossRef]
- Li, J.; Bollati, C.; Bartolomei, M.; Mazzolari, A.; Arnoldi, A.; Vistoli, G.; Lammi, C. Hempseed (Cannabis sativa) Peptide H3 (IGFLIIWV) Exerts Cholesterol-Lowering Effects in Human Hepatic Cell Line. Nutrients 2022, 14, 1804. [Google Scholar] [CrossRef]
- Girgih, A.T.; Udenigwe, C.C.; Aluko, R.E. Reverse-phase HPLC separation of hemp seed (Cannabis sativa L.) protein hydrolysate produced peptide fractions with enhanced antioxidant capacity. Plant Foods Hum. Nutr. 2013, 68, 39–46. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Malomo, S.; Offengenden, M.; Wu, J.; Aluko, R.E. Structural and functional characterization of hemp seed (Cannabis sativa L.) protein-derived antioxidant and antihypertensive peptides. J. Funct. Foods 2014, 6, 384–394. [Google Scholar] [CrossRef]
- Lu, R.-R.; Qian, P.; Sun, Z.; Zhou, X.-H.; Chen, T.-P.; He, J.-F.; Zhang, H.; Wu, J. Hempseed protein derived antioxidative peptides: Purification, identification and protection from hydrogen peroxide-induced apoptosis in PC12 cells. Food Chem. 2010, 123, 1210–1218. [Google Scholar] [CrossRef]
- Gao, J.; Li, T.; Chen, D.; Gu, H.; Mao, X. Identification and molecular docking of antioxidant peptides from hemp seed protein hydrolysates. LWT 2021, 147, 111453. [Google Scholar] [CrossRef]
- Bollati, C.; Cruz-Chamorro, I.; Aiello, G.; Li, J.; Bartolomei, M.; Santos-Sánchez, G.; Ranaldi, G.; Ferruzza, S.; Sambuy, Y.; Arnoldi, A. Investigation of the intestinal trans-epithelial transport and antioxidant activity of two hempseed peptides WVSPLAGRT (H2) and IGFLIIWV (H3). Food Res. Int. 2022, 152, 110720. [Google Scholar] [CrossRef] [PubMed]
- Orio, L.P.; Boschin, G.; Recca, T.; Morelli, C.F.; Ragona, L.; Francescato, P.; Arnoldi, A.; Speranza, G. New ACE-inhibitory peptides from hemp seed (Cannabis sativa L.) proteins. J. Agric. Food Chem. 2017, 65, 10482–10488. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Santos-Sánchez, G.; Bollati, C.; Bartolomei, M.; Li, J.; Arnoldi, A.; Lammi, C. Hempseed (Cannabis sativa) Peptides WVSPLAGRT and IGFLIIWV Exert Anti-inflammatory Activity in the LPS-Stimulated Human Hepatic Cell Line. J. Agric. Food Chem. 2022, 70, 577–583. [Google Scholar] [CrossRef]
- Ren, Y.; Liang, K.; Jin, Y.; Zhang, M.; Chen, Y.; Wu, H.; Lai, F. Identification and characterization of two novel α-glucosidase inhibitory oligopeptides from hemp (Cannabis sativa L.) seed protein. J. Funct. Foods 2016, 26, 439–450. [Google Scholar] [CrossRef]
- Nayak, A.P.; Green, B.J.; Sussman, G.; Berlin, N.; Lata, H.; Chandra, S.; ElSohly, M.A.; Hettick, J.M.; Beezhold, D.H. Characterization of Cannabis sativa allergens. Ann. Allergy Asthma Immunol. 2013, 111, 32–37.e34. [Google Scholar] [CrossRef]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional quality and potential functionality for human health and nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Tallei, T.E.; Adam, A.A.; Elseehy, M.M.; El-Shehawi, A.M.; Mahmoud, E.A.; Tania, A.D.; Niode, N.J.; Kusumawaty, D.; Rahimah, S.; Effendi, Y. Fruit bromelain-derived peptide potentially restrains the attachment of SARS-CoV-2 variants to hACE2: A pharmacoinformatics approach. Molecules 2022, 27, 260. [Google Scholar] [CrossRef]
- Harnkit, N.; Khongsonthi, T.; Masuwan, N.; Prasartkul, P.; Noikaew, T.; Chumnanpuen, P. Virtual Screening for SARS-CoV-2 Main Protease Inhibitory Peptides from the Putative Hydrolyzed Peptidome of Rice Bran. Antibiotics 2022, 11, 1318. [Google Scholar] [CrossRef]
- Mahmud, S.; Paul, G.K.; Biswas, S.; Afrose, S.; Mita, M.A.; Hasan, M.R.; Shimu, M.S.S.; Hossain, A.; Promi, M.M.; Ema, F.K. Prospective role of peptide-based antiviral therapy against the main protease of SARS-CoV-2. Front. Mol. Biosci. 2021, 8, 628585. [Google Scholar] [CrossRef]
- Mujwar, S.; Sun, L.; Fidan, O. In silico evaluation of food-derived carotenoids against SARS-CoV-2 drug targets: Crocin is a promising dietary supplement candidate for COVID-19. J. Food Biochem. 2022, 46, e14219. [Google Scholar] [CrossRef]
- Shinu, P.; Sharma, M.; Gupta, G.L.; Mujwar, S.; Kandeel, M.; Kumar, M.; Nair, A.B.; Goyal, M.; Singh, P.; Attimarad, M. Computational Design, Synthesis, and Pharmacological Evaluation of Naproxen-Guaiacol Chimera for Gastro-Sparing Anti-Inflammatory Response by Selective COX2 Inhibition. Molecules 2022, 27, 6905. [Google Scholar] [CrossRef]
- Kciuk, M.; Mujwar, S.; Szymanowska, A.; Marciniak, B.; Bukowski, K.; Mojzych, M.; Kontek, R. Preparation of Novel Pyrazolo [4, 3-e] tetrazolo [1, 5-b][1, 2, 4] triazine Sulfonamides and Their Experimental and Computational Biological Studies. Int. J. Mol. Sci. 2022, 23, 5892. [Google Scholar] [CrossRef]
- Chang, K.Y.; Yang, J.-R. Analysis and prediction of highly effective antiviral peptides based on random forests. PLoS ONE 2013, 8, e70166. [Google Scholar] [CrossRef]
- Skalickova, S.; Heger, Z.; Krejcova, L.; Pekarik, V.; Bastl, K.; Janda, J.; Kostolansky, F.; Vareckova, E.; Zitka, O.; Adam, V. Perspective of use of antiviral peptides against influenza virus. Viruses 2015, 7, 5428–5442. [Google Scholar] [CrossRef]
- Ahmed, A.; Siman-Tov, G.; Hall, G.; Bhalla, N.; Narayanan, A. Human antimicrobial peptides as therapeutics for viral infections. Viruses 2019, 11, 704. [Google Scholar] [CrossRef]
- Nyanguile, O. Peptide antiviral strategies as an alternative to treat lower respiratory viral infections. Front. Immunol. 2019, 10, 1366. [Google Scholar] [CrossRef]
- Sala, A.; Ardizzoni, A.; Ciociola, T.; Magliani, W.; Conti, S.; Blasi, E.; Cermelli, C. Antiviral activity of synthetic peptides derived from physiological proteins. Intervirology 2018, 61, 166–173. [Google Scholar] [CrossRef]
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019, 76, 3525–3542. [Google Scholar] [CrossRef] [PubMed]
- Shoombuatong, W.; Schaduangrat, N.; Nantasenamat, C. Unraveling the bioactivity of anticancer peptides as deduced from machine learning. EXCLI J. 2018, 17, 734. [Google Scholar] [PubMed]
- Chen, W.; Ding, H.; Feng, P.; Lin, H.; Chou, K.-C. iACP: A sequence-based tool for identifying anticancer peptides. Oncotarget 2016, 7, 16895. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, R.; Birkedal, B.; Owens, R.; Anantharamaiah, G.; Segrest, J.; Compans, R. Antiviral effects of apolipoprotein AI and its synthetic amphipathic peptide analogs. Virology 1990, 176, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, B.; Hasanshahi, Z.; Hashempour, T. HIV capsid and protease, new targets of melittin. Int. J. Pept. Res. Ther. 2020, 26, 2057–2065. [Google Scholar] [CrossRef]
- Kreutzer, A.G.; Krumberger, M.; Diessner, E.M.; Parrocha, C.M.T.; Morris, M.A.; Guaglianone, G.; Butts, C.T.; Nowick, J.S. A cyclic peptide inhibitor of the SARS-CoV-2 main protease. Eur. J. Med. Chem. 2021, 221, 113530. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Srinivasa, M.G.; Siddappa BC, R.; Mandal, S.P.; Dixit, S.R.; Pujar, G.; Bangera Sheshappa, M. Isolation and characterization of ACE-I inhibitory peptides from ribbonfish for a potential inhibitor of the main protease of SARS-CoV-2: An in silico analysis. Proteins Struct. Funct. Bioinform. 2022, 90, 982–992. [Google Scholar] [CrossRef]
- Schaduangrat, N.; Nantasenamat, C.; Prachayasittikul, V.; Shoombuatong, W. Meta-iAVP: A sequence-based meta-predictor for improving the prediction of antiviral peptides using effective feature representation. Int. J. Mol. Sci. 2019, 20, 5743. [Google Scholar] [CrossRef]
- Bulet, P.; Stöcklin, R.; Menin, L. Anti-microbial peptides: From invertebrates to vertebrates. Immunol. Rev. 2004, 198, 169–184. [Google Scholar] [CrossRef]
- Oren, Z.; Shai, Y. Mode of action of linear amphipathic α-helical antimicrobial peptides. Pept. Sci. 1998, 47, 451–463. [Google Scholar] [CrossRef]
- Li, S.; Guo, S.; Li, F.; Xiang, J. Functional diversity of anti-lipopolysaccharide factor isoforms in shrimp and their characters related to antiviral activity. Mar. Drugs 2015, 13, 2602–2616. [Google Scholar] [CrossRef]
- Scott, M.G.; Hancock, R.E. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit. Rev. Immunol. 2000, 20, 24. [Google Scholar] [CrossRef]
- Wang, C.-K.; Shih, L.-Y.; Chang, K.Y. Large-scale analysis of antimicrobial activities in relation to amphipathicity and charge reveals novel characterization of antimicrobial peptides. Molecules 2017, 22, 2037. [Google Scholar] [CrossRef]
- Chowdhury, A.S.; Reehl, S.M.; Kehn-Hall, K.; Bishop, B.; Webb-Robertson, B.-J.M. Better understanding and prediction of antiviral peptides through primary and secondary structure feature importance. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Timmons, P.B.; Hewage, C.M. ENNAVIA is a novel method which employs neural networks for antiviral and anti-coronavirus activity prediction for therapeutic peptides. Brief. Bioinform. 2021, 22, bbab258. [Google Scholar] [CrossRef]
- Qureshi, A.; Tandon, H.; Kumar, M. AVP-IC50Pred: Multiple machine learning techniques-based prediction of peptide antiviral activity in terms of half maximal inhibitory concentration (IC50). Pept. Sci. 2015, 104, 753–763. [Google Scholar] [CrossRef]
- Robles-Loaiza, A.A.; Pinos-Tamayo, E.A.; Mendes, B.; Ortega-Pila, J.A.; Proaño-Bolaños, C.; Plisson, F.; Teixeira, C.; Gomes, P.; Almeida, J.R. Traditional and Computational Screening of Non-Toxic Peptides and Approaches to Improving Selectivity. Pharmaceuticals 2022, 15, 323. [Google Scholar] [CrossRef]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v. 2—a server for in silico prediction of allergens. J. Mol. Modeling 2014, 20, 1–6. [Google Scholar] [CrossRef]
- Dimitrov, I.; Naneva, L.; Doytchinova, I.; Bangov, I. AllergenFP: Allergenicity prediction by descriptor fingerprints. Bioinformatics 2014, 30, 846–851. [Google Scholar] [CrossRef]
- Weng, G.; Gao, J.; Wang, Z.; Wang, E.; Hu, X.; Yao, X.; Cao, D.; Hou, T. Comprehensive evaluation of fourteen docking programs on protein–peptide complexes. J. Chem. Theory Comput. 2020, 16, 3959–3969. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Al-Karmalawy, A.A.; Abdel-Aziz, T.M.; Elhady, S.S.; Darwish, K.M.; Hassan, A.H. Investigating the structure–activity relationship of marine natural polyketides as promising SARS-CoV-2 main protease inhibitors. RSC Adv. 2021, 11, 31339–31363. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Sood, D.; van der Spek, P.J.; Sharma, H.S.; Chandra, R. Molecular binding mechanism and pharmacology comparative analysis of noscapine for repurposing against SARS-CoV-2 protease. J. Proteome Res. 2020, 19, 4678–4689. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, G.A.; Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: New York, NY, USA, 1997; Volume 12. [Google Scholar]
- Sterri, S.H.; Fonnum, F. Role of Carboxylesterases in Therapeutic Intervention of Nerve Gas Poisoning. In Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2009; Chapter 68; pp. 1033–1040. [Google Scholar]
- Fakih, T.M. Dermaseptin-based antiviral peptides to prevent COVID-19 through in silico molecular docking studies against SARS-CoV-2 spike protein. Pharm. Sci. Res. 2020, 7, 8. [Google Scholar] [CrossRef]
- Lee, H.; Heo, L.; Lee, M.S.; Seok, C. GalaxyPepDock: A protein–peptide docking tool based on interaction similarity and energy optimization. Nucleic Acids Res. 2015, 43, W431–W435. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Jin, B.; Li, H.; Huang, S.-Y. HPEPDOCK: A web server for blind peptide–protein docking based on a hierarchical algorithm. Nucleic Acids Res. 2018, 46, W443–W450. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.; Dror, R.; Shaw, D. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Vishvakarma, V.K.; Singh, M.B.; Jain, P.; Kumari, K.; Singh, P. Hunting the main protease of SARS-CoV-2 by plitidepsin: Molecular docking and temperature-dependent molecular dynamics simulations. Amino Acids 2022, 54, 205–213. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Li, B.; Lu, C.; Yang, S.; Long, J.; Chen, H.; Huang, J.; He, B. A database of anti-coronavirus peptides. Sci. Data 2022, 9, 294. [Google Scholar] [CrossRef]
- Gibb, S. Cleaver: Cleavage of Polypeptide Sequences, R package version 1.34.1; 2022. Available online: https://github.com/sgibb/cleaver/ (accessed on 1 February 2022).
- Kumar, M.; Thakur, V.; Raghava, G.P. COPid: Composition based protein identification. Silico Biol. 2008, 8, 121–128. [Google Scholar]
- Beaufays, J.; Lins, L.; Thomas, A.; Brasseur, R. In silico predictions of 3D structures of linear and cyclic peptides with natural and non-proteinogenic residues. J. Pept. Sci. 2012, 18, 17–24. [Google Scholar] [CrossRef]
- Zhou, X.; Zhong, F.; Lin, C.; Hu, X.; Zhang, Y.; Xiong, B.; Yin, X.; Fu, J.; He, W.; Duan, J.; et al. Structure of SARS-CoV-2 main protease in the apo state. Sci. China Life Sci. 2021, 64, 656–659. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Mathew, O.K.; Sowdhamini, R. PIMA: Protein-Protein interactions in Macromolecular Assembly-a web server for its Analysis and Visualization. Bioinformation 2016, 12, 9. [Google Scholar] [CrossRef]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A web server for predicting the binding affinity of protein–protein complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Uberuaga, B.P.; Anghel, M.; Voter, A.F. Synchronization of trajectories in canonical molecular-dynamics simulations: Observation, explanation, and exploitation. J. Chem. Phys. 2004, 120, 6363–6374. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
| Peptide ID | Secondary Structure | Sequences (from Protein) | Length | AVPpred | Meta-iAVP | iAMP pred | ENNAVIA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M3 * | M4 * | A * | B * | C * | D * | ||||||
| csAVP1 |  | ASAQGFEWIAVK (edestin2) | 12 | 53.13 | 26.77 | 0.946 | 0.527 | 0.000 | 0.000 | 0.000 | 0.000 |
| csAVP2 |  | ATEYGIILK (vicilin) | 9 | 43.36 | 70.76 | 0.038 | 0.718 | 0.001 | 0.333 | 0.000 | 0.325 |
| csAVP3 |  | FEEEDEIENYSQHLDQCCSQLR (albumin) | 22 | 53.34 | 45.49 | 0.362 | 0.558 | 0.128 | 0.133 | 0.103 | 0.154 |
| csAVP4 |  | IGTFQSFFLGGGTNPASILSGFDSEILENAFNVTHAELK (vicilin) | 39 | 35.33 | 64.14 | 0.542 | 0.331 | 0.202 | 0.000 | 1.000 | 0.000 |
| csAVP5 |  | ISSSTLALFAALMLVAHAVAFR (albumin) | 22 | 53.46 | 70.85 | 1.000 | 0.211 | 0.884 | 0.000 | 0.768 | 0.100 |
| csAVP6 |  | IVGFHQGEEEEDEEELEEDINQEQNQK (vicilin) | 27 | 46.69 | 64.43 | 0.282 | 0.625 | 0.011 | 0.000 | 0.005 | 0.000 |
| csAVP7 |  | LGFIYK (vicilin) | 6 | 54.22 | 71.75 | 1.000 | 0.859 | 0.864 | 1.000 | 0.199 | 0.596 |
| csAVP8 |  | MASTPLLLSLSLCFLVLLHGCSAR (edestin3) | 24 | 49.95 | 64.74 | 0.996 | 0.029 | 0.888 | 0.000 | 0.999 | 0.000 |
| csAVP9 |  | NAMYAPQYTMNAHNIIYAIR (edestin3) | 20 | 37.99 | 49.6 | 0.696 | 0.461 | 0.422 | 0.369 | 0.908 | 0.502 |
| csAVP10 |  | TTWSWR (vicilin) | 6 | 42.03 | 49.7 | 0.008 | 0.655 | 0.316 | 1.000 | 0.000 | 0.995 |
| Peptide ID | Predicted IC50 (µM) | Average IC50 (µM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Virus Specific | Hybrid Model I * | Hybrid Model II * | |||||||||
| SVM | RF | SVM | RF | IBk | K Star | SVM | RF | IBk | K Star | ||
| csAVP1 | 46.19 | 0.01 | 104.96 | 29.05 | 42.80 | 0.22 | 104.76 | 29.56 | 0.22 | 0.01 | 35.78 |
| csAVP2 | 45.82 | 0.01 | 78.69 | 108.68 | 152.62 | 11.41 | 78.60 | 109.07 | 11.41 | 0.01 | 59.63 |
| csAVP3 | 45.90 | 0.01 | 32.67 | 38.96 | 57.24 | 17.01 | 32.76 | 39.57 | 17.01 | 0.01 | 28.11 |
| csAVP4 | 45.92 | 0.01 | 40.75 | 19.24 | 19.85 | 8.01 | 40.75 | 19.62 | 8.01 | 0.01 | 20.22 |
| csAVP5 | 48.16 | 0.01 | 49.29 | 17.59 | 31.74 | 11.01 | 49.21 | 17.82 | 11.01 | 0.01 | 23.59 |
| csAVP6 | 45.92 | 0.01 | 39.40 | 58.99 | 34.91 | 0.01 | 39.42 | 57.25 | 0.01 | 0.01 | 27.59 |
| csAVP7 | 45.63 | 0.01 | 51.16 | 48.57 | 19.85 | 313.01 | 51.15 | 48.32 | 70.01 | 0.01 | 64.77 |
| csAVP8 | 45.91 | 0.01 | 38.66 | 12.84 | 11.05 | 1.01 | 38.69 | 13.04 | 1.01 | 0.01 | 16.22 |
| csAVP9 | 45.74 | 0.01 | 46.33 | 48.26 | 34.78 | 14.01 | 46.25 | 48.17 | 14.01 | 0.01 | 29.76 |
| csAVP10 | 45.94 | 0.01 | 45.75 | 17.61 | 62.98 | 9.01 | 45.73 | 17.63 | 9.01 | 0.01 | 25.37 |
| Peptide ID | Hydrophobicity | Steric Hindrance | Sidebulk | Hydropathicity | Amphipathicity | Hydrophilicity | Net Hydrogen | Charge | pI | Mol wt |
|---|---|---|---|---|---|---|---|---|---|---|
| csAVP1 | 0.04 | 0.62 | 0.62 | 0.33 | 0.52 | −0.35 | 0.58 | 0.00 | 6.35 | 1306.65 |
| csAVP2 | 0.06 | 0.64 | 0.64 | 0.53 | 0.55 | −0.29 | 0.56 | 0.00 | 6.35 | 1007.33 |
| csAVP3 | −0.34 | 0.63 | 0.63 | −1.33 | 0.64 | 0.58 | 1.05 | −5.50 | 4.00 | 2716.18 |
| csAVP4 | 0.03 | 0.61 | 0.61 | 0.21 | 0.26 | −0.33 | 0.56 | −2.50 | 4.40 | 4131.18 |
| csAVP5 | 0.17 | 0.56 | 0.56 | 1.62 | 0.18 | −0.83 | 0.41 | 1.50 | 10.11 | 2290.08 |
| csAVP6 | −0.36 | 0.66 | 0.66 | −1.89 | 0.85 | 1.12 | 1.00 | −10.50 | 3.77 | 3245.71 |
| csAVP7 | 0.14 | 0.57 | 0.57 | 0.79 | 0.52 | −0.77 | 0.43 | 1.00 | 8.94 | 868.19 |
| csAVP8 | 0.12 | 0.54 | 0.54 | 1.43 | 0.16 | −0.81 | 0.42 | 1.50 | 8.40 | 2546.51 |
| csAVP9 | −0.06 | 0.62 | 0.62 | −0.17 | 0.26 | −0.70 | 0.85 | 1.50 | 8.84 | 2356.01 |
| csAVP10 | −0.27 | 0.55 | 0.55 | −1.42 | 0.41 | −0.72 | 1.50 | 1.00 | 10.11 | 835.99 |
| Peptide ID | Toxicity Predictions | Allergenicity Predictions | ||
|---|---|---|---|---|
| SVM Scores | Toxicity | AllerTOP | AllergenFP | |
| csAVP 1 | −0.75 | Non-toxin | Probable allergen | Probable non-allergen |
| csAVP 2 | −0.65 | Non-toxin | Probable allergen | Probable non-allergen |
| csAVP 3 | 0.24 | Toxin | Probable allergen | Probable non-allergen |
| csAVP 4 | −1.34 | Non-toxin | Probable non-allergen | Probable non-allergen |
| csAVP 5 | −1.18 | Non-toxin | Probable non-allergen | Probable non-allergen |
| csAVP 6 | −0.66 | Non-toxin | Probable non-allergen | Probable non-allergen |
| csAVP 7 | −1.11 | Non-toxin | Probable non-allergen | Probable allergen |
| csAVP 8 | −1.25 | Non-toxin | Probable non-allergen | Probable non-allergen |
| csAVP 9 | −0.68 | Non-toxin | Probable allergen | Probable non-allergen |
| csAVP 10 | −0.91 | Non-toxin | Probable non-allergen | Probable allergen |
| Peptide | Peptide Residues | 3CLpro Residue | Distance (Å) | Peptide | Peptide Residues | 3Clpro Residue | Distance (Å) |
|---|---|---|---|---|---|---|---|
| csAVP1 | VAL11 | GLU134 | 1.997 | csAVP6 | GLU15 | GLU134 | 2.403 |
| VAL11 | GLU134 | 2.074 | GLU6 | GLU134 | 1.983 | ||
| ALA10 | THR178 | 2.355 | PHE4 | HIS160 | 2.094 | ||
| TRP8 | GLN180 | 2.191 | GLU10 | ALA181 | 2.187 | ||
| GLY5 | ALA181 | 1.972 | PHE4 | ARG123 | 1.880 | ||
| GLN4 | ASN125 | 2.050 | HIS5 | THR186 | 2.300 | ||
| GLN4 | GLY183 | 2.005 | GLY7 | ASN125 | 1.932 | ||
| SER2 | ALA179 | 2.015 | GLN26 | GLY131 | 2.193 | ||
| csAVP2 | LYS9 | ASN111 | 1.941 | GLU17 | SER132 | 2.001 | |
| ILE7 | GLU134 | 2.382 | GLU8 | THR184 | 2.605 | ||
| ILE7 | GLU134 | 1.843 | csAVP7 | LYS6 | THR24 | 2.096 | |
| GLY5 | GLN180 | 1.979 | TYR5 | GLU134 | 2.000 | ||
| TYR4 | GLN180 | 2.049 | PHE3 | GLU134 | 2.161 | ||
| ALA1 | ALA182 | 1.949 | GLY2 | GLN177 | 2.058 | ||
| csAVP3 | GLN20 | GLU134 | 1.948 | csAVP8 | GLY20 | SER132 | 1.913 |
| GLN20 | GLU134 | 2.050 | GLY20 | GLY131 | 1.939 | ||
| SER19 | THR178 | 2.455 | LEU17 | GLU134 | 2.010 | ||
| TYR10 | THR184 | 2.263 | LEU17 | GLU134 | 2.314 | ||
| csAVP4 | GLU26 | GLU134 | 1.905 | VAL16 | GLU134 | 2.173 | |
| THR23 | GLN180 | 2.187 | PHE14 | GLN180 | 1.996 | ||
| SER13 | THR127 | 1.878 | ARG24 | GLY158 | 2.190 | ||
| PHE11 | ARG123 | 1.821 | ARG24 | GLY130 | 1.901 | ||
| LEU8 | GLN99 | 2.465 | SER11 | ALA181 | 2.034 | ||
| SER9 | THR103 | 2.145 | THR4 | THR184 | 2.032 | ||
| ASP12 | ALA121 | 1.935 | csAVP9 | ARG22 | ASN111 | 2.118 | |
| SER13 | THR127 | 2.205 | ALA11 | GLU134 | 2.101 | ||
| ASN18 | GLY183 | 2.724 | csAVP10 | SER4 | THR178 | 2.350 | |
| csAVP5 | ALA11 | GLU134 | 2.101 | SER4 | GLN177 | 2.478 | |
| ARG22 | ASN111 | 2.118 | ARG6 | GLN177 | 2.094 |
| Peptide ID | Binding Energy (kJ/mol) | Docking Scores (kJ/mol) | Binding Affinity and Dissociation Constant | ||||
|---|---|---|---|---|---|---|---|
| H-Bond. Ener. | Elec. Ener. | VDW. Ener. | GalaxyPepDock | HPEPDOCK | ΔG (kcal/mol) | Kd (M) at 25.0 °C | |
| csAVP1 | −31.64 | 4.46 | −140.78 | −167.96 | −202.55 | −11.0 | 9 × 10−9 |
| csAVP2 | −34.55 | 0.00 | −114.22 | −148.76 | −162.16 | −9.4 | 1.2 × 10−7 |
| csAVP3 | −24.70 | 8.04 | −183.80 | −200.46 | −182.57 | −13.9 | 6.10 × 10−11 |
| csAVP4 | −41.90 | 15.53 | −420.90 | −447.27 | −203.25 | −18.2 | 4.30 × 10−14 |
| csAVP5 | −12.54 | 0.00 | −215.81 | −228.36 | −193.07 | −13.4 | 1.60 × 10−10 |
| csAVP6 | −69.15 | −6.44 | −496.58 | −572.17 | −158.42 | −15.6 | 3.40 × 10−12 |
| csAVP7 | −28.83 | 0.46 | −94.16 | −122.52 | −167.37 | −9.4 | 1.20 × 10−7 |
| csAVP8 | −39.60 | −5.23 | −286.11 | −330.94 | −212.76 | −13.8 | 7.00 × 10−11 |
| csAVP9 | −12.16 | −24.15 | −180.11 | −216.42 | −229.77 | −11.4 | 4.70 × 10−9 |
| csAVP10 | −10.42 | 2.17 | −101.72 | −109.97 | −218.87 | −8.2 | 9.50 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasertsuk, K.; Prongfa, K.; Suttiwanich, P.; Harnkit, N.; Sangkhawasi, M.; Promta, P.; Chumnanpuen, P. Computer-Aided Screening for Potential Coronavirus 3-Chymotrypsin-like Protease (3CLpro) Inhibitory Peptides from Putative Hemp Seed Trypsinized Peptidome. Molecules 2023, 28, 50. https://doi.org/10.3390/molecules28010050
Prasertsuk K, Prongfa K, Suttiwanich P, Harnkit N, Sangkhawasi M, Promta P, Chumnanpuen P. Computer-Aided Screening for Potential Coronavirus 3-Chymotrypsin-like Protease (3CLpro) Inhibitory Peptides from Putative Hemp Seed Trypsinized Peptidome. Molecules. 2023; 28(1):50. https://doi.org/10.3390/molecules28010050
Chicago/Turabian StylePrasertsuk, Kansate, Kasidit Prongfa, Piyapach Suttiwanich, Nathaphat Harnkit, Mattanun Sangkhawasi, Pongsakorn Promta, and Pramote Chumnanpuen. 2023. "Computer-Aided Screening for Potential Coronavirus 3-Chymotrypsin-like Protease (3CLpro) Inhibitory Peptides from Putative Hemp Seed Trypsinized Peptidome" Molecules 28, no. 1: 50. https://doi.org/10.3390/molecules28010050
APA StylePrasertsuk, K., Prongfa, K., Suttiwanich, P., Harnkit, N., Sangkhawasi, M., Promta, P., & Chumnanpuen, P. (2023). Computer-Aided Screening for Potential Coronavirus 3-Chymotrypsin-like Protease (3CLpro) Inhibitory Peptides from Putative Hemp Seed Trypsinized Peptidome. Molecules, 28(1), 50. https://doi.org/10.3390/molecules28010050










