Z-Scheme CuOx/Ag/TiO2 Heterojunction as Promising Photoinduced Anticorrosion and Antifouling Integrated Coating in Seawater
Abstract
1. Introduction
2. Results and Discussion
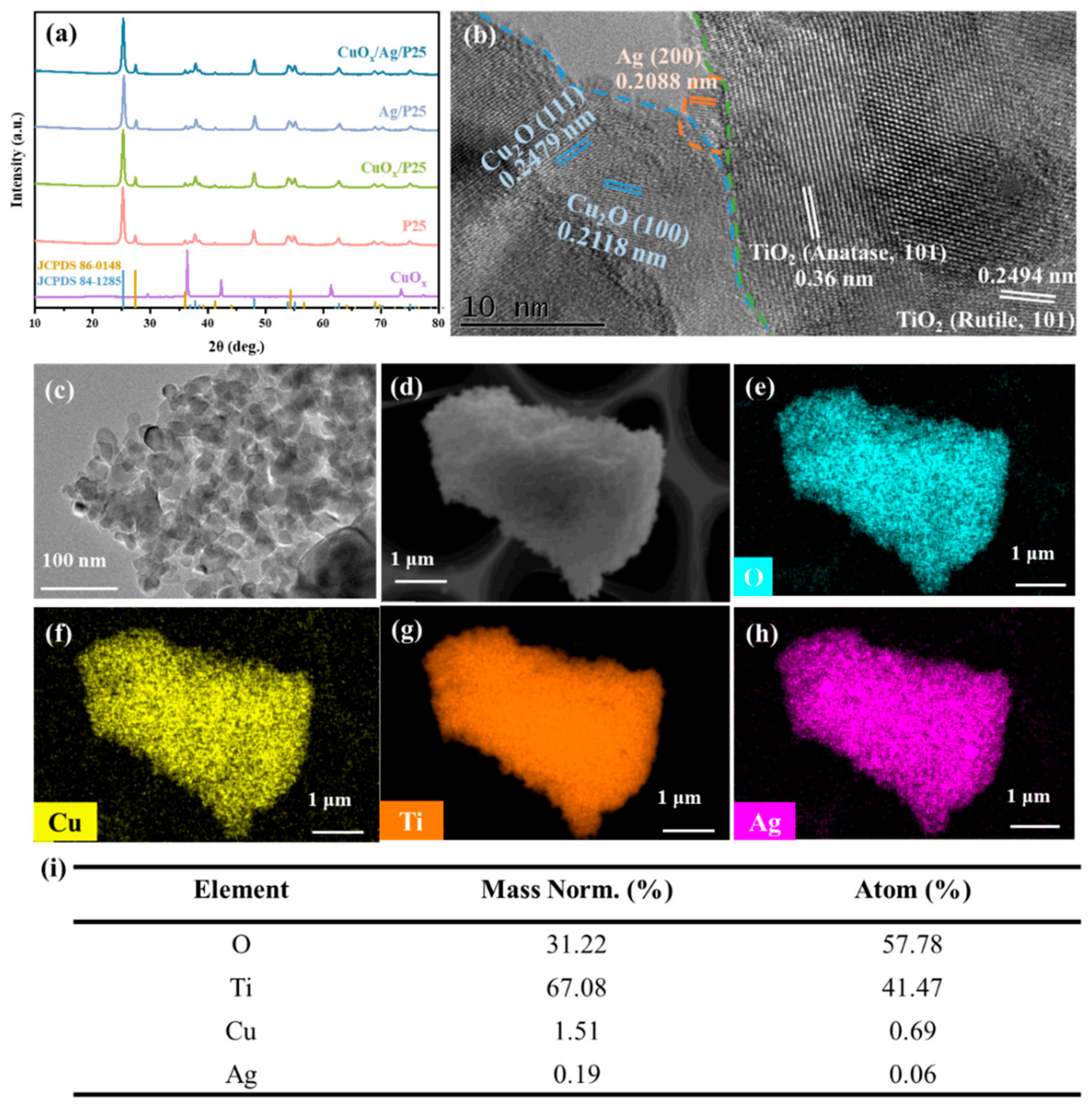
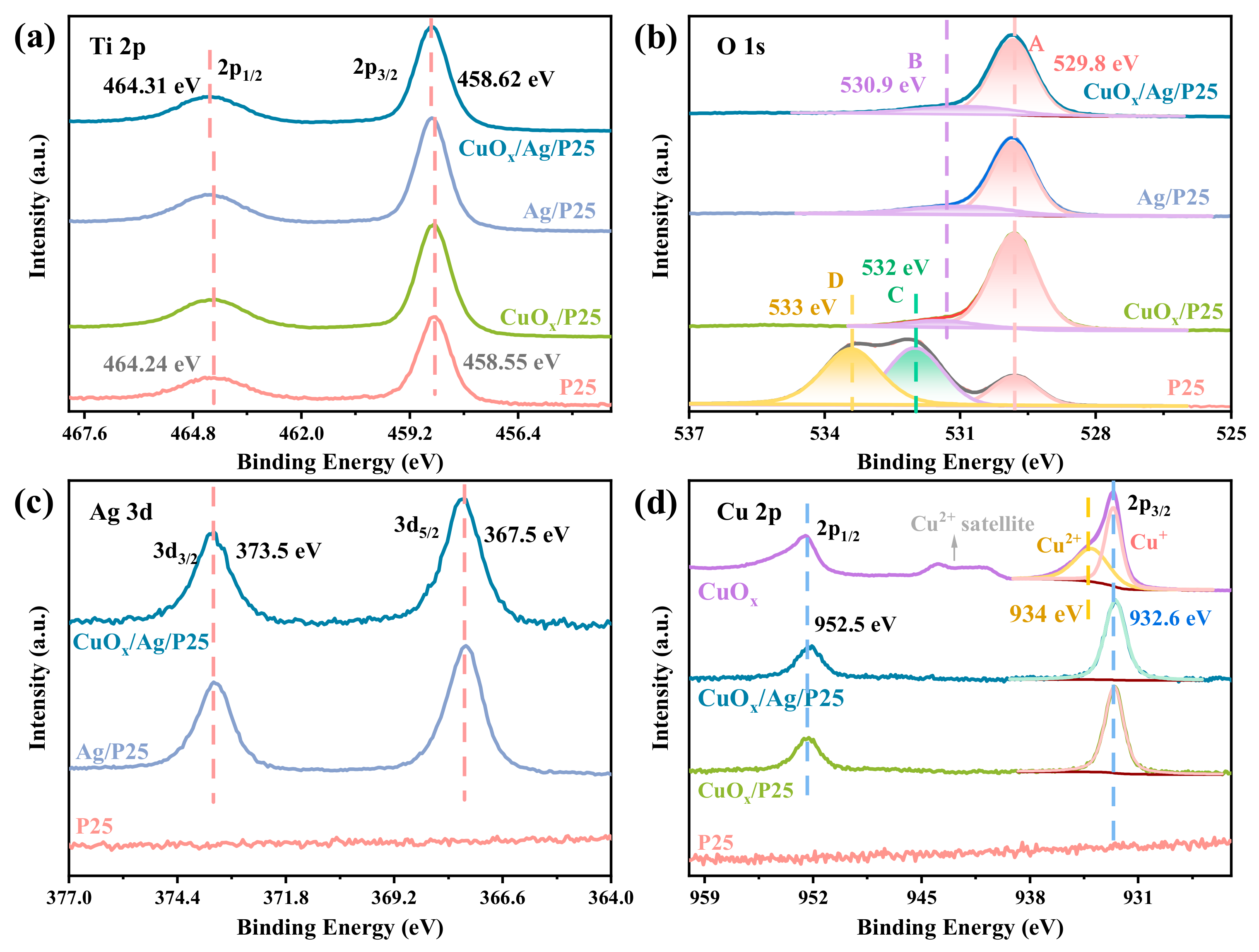
2.1. Structures, Compositions, and Morphologies
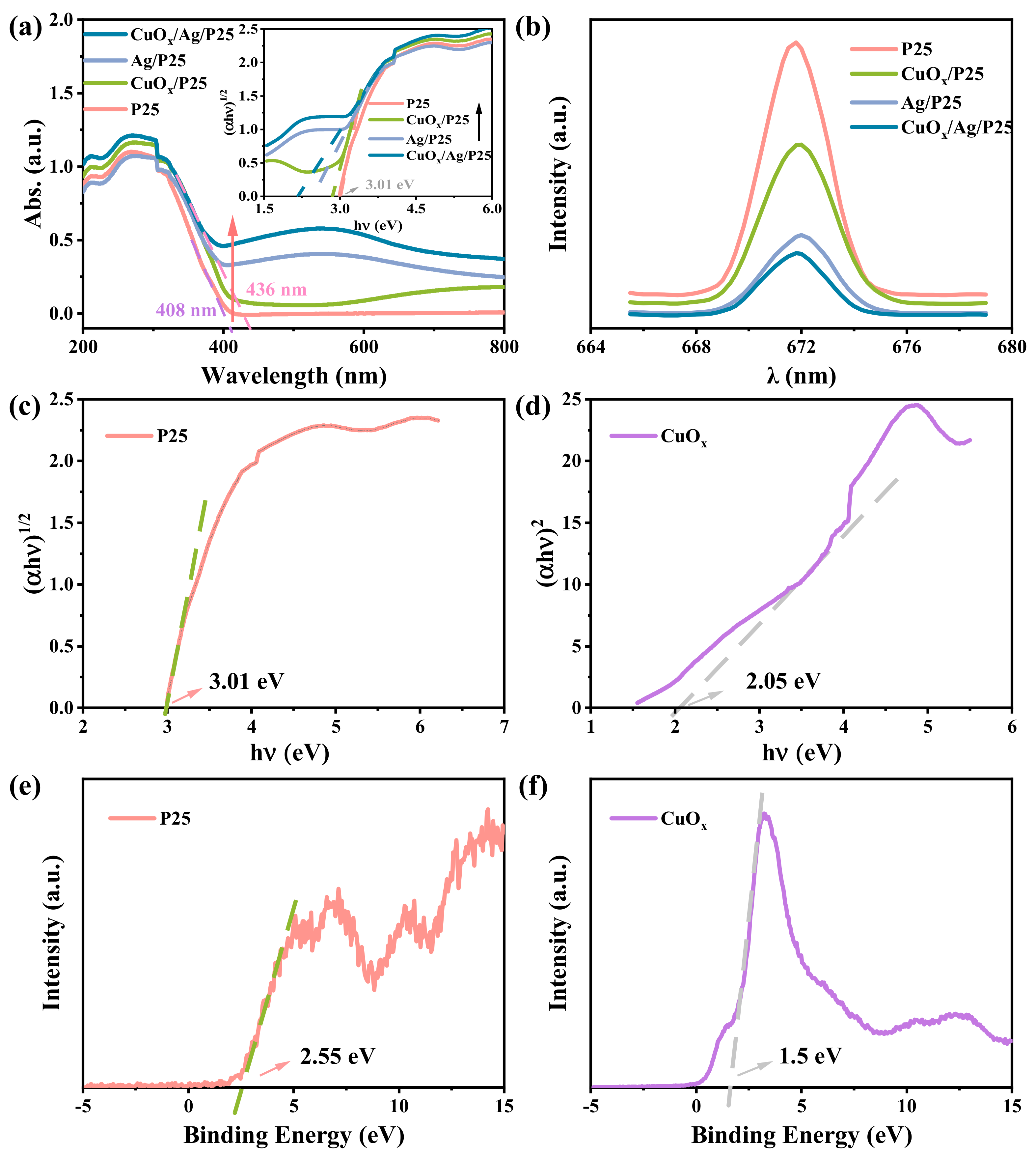
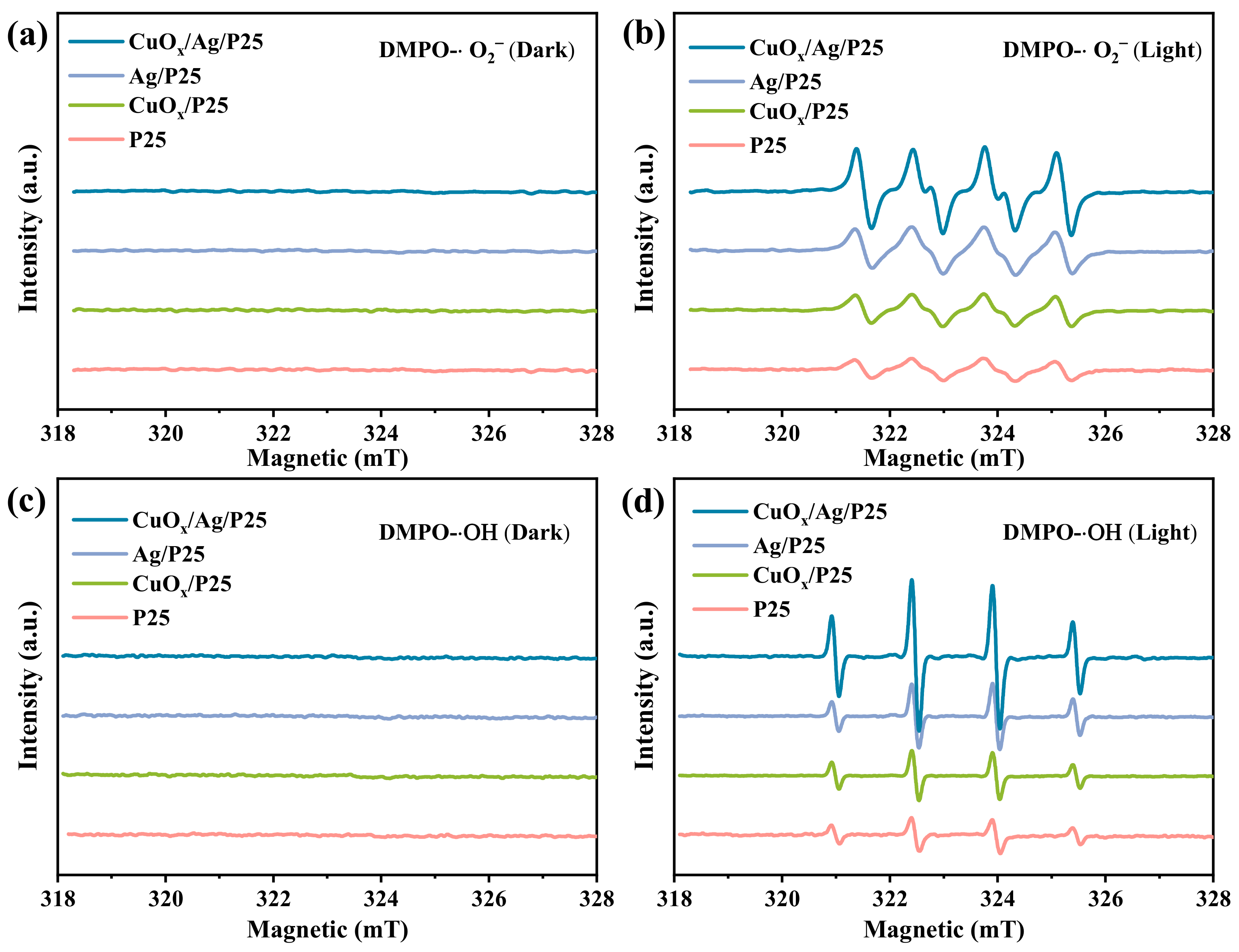
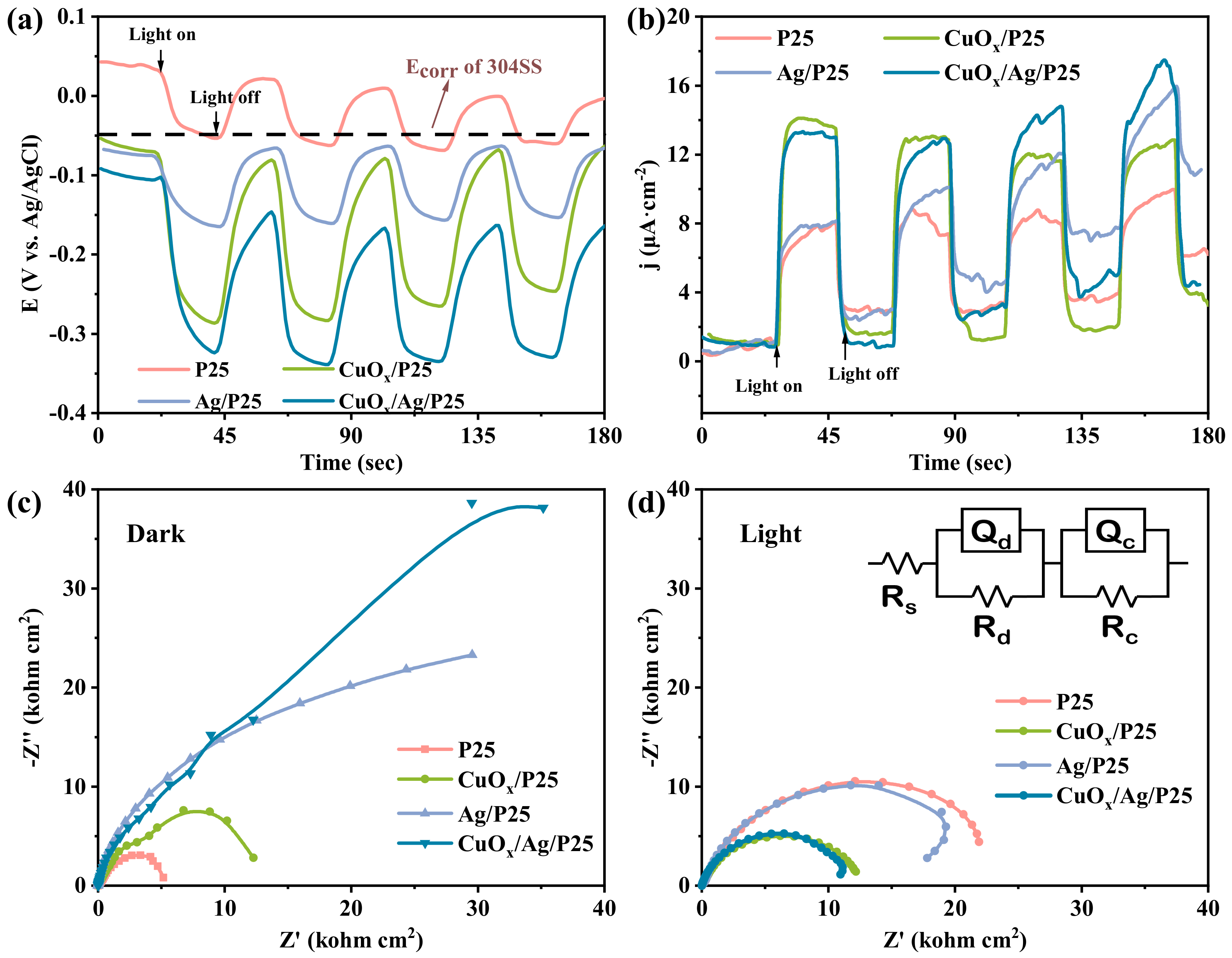
2.2. Photoelectric Characterization
2.3. Photoelectrochemical Cathodic Protection Performance Evaluation
2.4. Antibacterial Performance Evaluation
3. Discussion
4. Experimental Section
4.1. Synthesis of CuOx/Ag/TiO2
4.2. Preparation of Coatings
4.3. Characterizations
4.4. Photoelectrochemical Measurements
4.5. Antibacterial Performance Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, X.T.; Xu, H.; Nan, Y.B.; Sun, X.; Duan, J.Z.; Huang, Y.L.; Hou, B.R. Research progress of TiO2 photocathodic protection to metals in marine environment. J. Oceanol. Limnol. 2020, 38, 1018–1044. [Google Scholar] [CrossRef]
- Zhang, X.F.; Li, M.Y.; Kong, L.F.; Wang, M.; Yan, L.; Xiao, F.J. Research progress in metal photoelectrochemical cathodic protection materials and its anticorrosion function realization. Surf. Technol. 2021, 50, 128–140. [Google Scholar] [CrossRef]
- Tian, J.; Chen, Z.Y.; Jing, J.P.; Feng, C.; Sun, M.M.; Li, W.B. Photoelectrochemical cathodic protection of Cu2O/TiO2 p-n heterojunction under visible light. J. Oceanol. Limnol. 2020, 38, 1517–1531. [Google Scholar] [CrossRef]
- Vedaprakash, L.; Dineshram, R.; Ratnam, K.; Lakshmi, K.; Jayaraj, K.; Babu, S.M.; Venkatesan, R.; Shanmugam, A. Experimental studies on the effect of different metallic substrates on marine biofouling. Colloids Surf. B 2013, 106, 1–10. [Google Scholar] [CrossRef]
- Lee, J.S.; Little, B.J. A mechanistic approach to understanding microbiologically influenced corrosion by metal-depositing bacteria. Corrosion 2019, 75, 6–11. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Raja, P.B.; Ismail, M.; Ghoreishiamiri, S.; Mirza, J.; Ismail, M.C.; Kakooei, S.; Rahim, A.A. Reviews on corrosion inhibitors: A short view. Chem. Eng. Commun. 2016, 203, 1145–1156. [Google Scholar] [CrossRef]
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra, C.S. Research progress in organic zinc rich primer coatings for cathodic protection of metals-A comprehensive review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- Bu, Y.Y.; Chen, Z.Y.; Ao, J.P.; Hou, J.; Sun, M.X. Study of the photoelectrochemical cathodic protection mechanism for steel based on the SrTiO3-TiO2 composite. J. Alloys Compd. 2018, 731, 1214–1224. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Lyu, Y.H.; Wang, R.L.; Xie, C.; Zhou, H.J.; Jiang, S.P.; Wang, S.Y. Crystalline TiO2 protective layer with graded oxygen defects for efficient and stable silicon-based photocathode. Nat. Commun. 2018, 9, 3572. [Google Scholar] [CrossRef]
- Lu, X.Y.; Liu, L.; Ge, J.W.; Cui, Y.; Wang, F.H. Morphology controlled synthesis of Co(OH)2/TiO2 p-n heterojunction photoelectrodes for efficient photocathodic protection of 304 stainless steel. Appl. Surf. Sci. 2021, 537, 148002. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, Y.F. One-step facile preparation of ZnO nanorods as high-performance photoanodes for photoelectrochemical cathodic protection. Electrochim. Acta 2018, 276, 311–318. [Google Scholar] [CrossRef]
- Jing, J.P.; Chen, Z.Y.; Bu, Y.Y.; Sun, M.M.; Zheng, W.Q.; Li, W.B. Significantly enhanced photoelectrochemical cathodic protection performance of hydrogen treated Cr-doped SrTiO3 by Cr6+ reduction and oxygen vacancy modification. Electrochim. Acta 2019, 304, 386–395. [Google Scholar] [CrossRef]
- Ge, M.Z.; Li, Q.S.; Cao, C.Y.; Huang, J.Y.; Li, S.H.; Zhang, S.N.; Chen, Z.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y.K. One-dimensional TiO2 nanotube photocatalysts for solar water splitting. Adv. Sci. 2017, 4, 1600152. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- An, X.Q.; Liu, H.J.; Qu, J.H.; Moniz, S.J.; Tang, J.W. Photocatalytic mineralisation of herbicide 2,4,5-trichlorophenoxyacetic acid: Enhanced performance by triple junction Cu-TiO2-Cu2O and the underlying reaction mechanism. New J. Chem. 2015, 39, 314–320. [Google Scholar] [CrossRef]
- Wang, M.Y.; Sun, L.; Lin, Z.Q.; Cai, J.H.; Xie, K.P.; Lin, C.J. p-n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211–1220. [Google Scholar] [CrossRef]
- Sun, W.X.; Wei, N.; Cui, H.Z.; Lin, Y.; Wang, X.Z.; Tian, J.; Li, J.; Wen, J. 3D ZnIn2S4 nanosheet/TiO2 nanowire arrays and their efficient photocathodic protection for 304 stainless steel. Appl. Surf. Sci. 2018, 434, 1030–1039. [Google Scholar] [CrossRef]
- Liu, D.; Yang, C.T.; Zhou, E.Z.; Yang, H.Y.; Li, Z.; Xu, D.K.; Wang, F.H. Progress in microbiologically influenced corrosion of metallic materials in marine environment. Surf. Technol. 2019, 48, 166–174. [Google Scholar] [CrossRef]
- Mehtab, A.; Banerjee, S.; Mao, Y.; Ahmad, T. Type-II CuFe2O4/graphitic carbon nitride heterojunctions for high-efficiency photocatalytic and electrocatalytic hydrogen generation. ACS Appl. Mater. Inter. 2022, 14, 44317–44329. [Google Scholar] [CrossRef]
- Jasrotia, R.; Verma, A.; Verma, R.; Ahmed, J.; Godara, S.K.; Kumar, G.; Mehtab, A.; Ahmad, T.; Kalia, S. Photocatalytic dye degradation efficiency and reusability of Cu-substituted Zn-Mg spinel nanoferrites for wastewater remediation. J. Water Process. Eng. 2022, 48, 102865. [Google Scholar] [CrossRef]
- Jasrotia, R.; Verma, A.; Verma, R.; Godara, S.K.; Ahmed, J.; Mehtab, A.; Ahmad, T.; Puri, P.; Kalia, S. Photocatalytic degradation of malachite green pollutant using novel dysprosium modified Zn-Mg photocatalysts for wastewater remediation. Ceram. Int. 2022, 48, 29111–29120. [Google Scholar] [CrossRef]
- Bhat, S.A.; Hassan, T.; Majid, S. Heavy metal toxicity and their harmful effects on living organisms-a review. Int. J. Med. Sci. Diagn. Res. 2019, 3, 106–122. [Google Scholar] [CrossRef]
- Wang, R.; Liu, R.X.; Luo, S.J.; Wu, J.X.; Zhang, D.H.; Yue, T.L.; Sun, J.; Zhang, C.; Zhu, L.Y.; Wang, J.L. Band structure engineering enables to UV-Visible-NIR photocatalytic disinfection: Mechanism, pathways and DFT calculation. Chem. Eng. J. 2021, 421, 129596. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Ma, C.C.; Wang, W.; Zhang, M.T.; Hao, X.P.; Chen, S.G. Fabrication of Cu2O-Ag nanocomposites with enhanced durability and bactericidal activity. J. Colloid Interf. Sci. 2019, 557, 156–167. [Google Scholar] [CrossRef]
- You, J.H.; Guo, Y.Z.; Guo, R.; Liu, X.W. A review of visible light-active photocatalysts for water disinfection: Features and prospects. Chem. Eng. J. 2019, 373, 624–641. [Google Scholar] [CrossRef]
- Wen, B.; Waterhouse, G.I.; Jia, M.Y.; Jiang, X.H.; Zhang, Z.M.; Yu, L.M. The feasibility of polyaniline-TiO2 coatings for photocathodic antifouling: Antibacterial effect. Synth. Met. 2019, 257, 116175. [Google Scholar] [CrossRef]
- Kong, J.J.; Rui, Z.B.; Liu, S.H.; Liu, H.W.; Ji, H.B. Homeostasis in CuxO/SrTiO3 hybrid allows highly active and stable visible light photocatalytic performance. Chem. Commun. 2017, 53, 12329–12332. [Google Scholar] [CrossRef]
- Yin, Z.; Wang, Y.; Song, C.Q.; Zheng, L.H.; Ma, N.; Liu, X.; Li, S.W.; Lin, L.L.; Li, M.Z.; Xu, Y.; et al. Hybrid Au-Ag nanostructures for enhanced plasmon-driven catalytic selective hydrogenation through visible light irradiation and surface-enhanced raman scattering. J. Am. Chem. Soc. 2018, 140, 864–867. [Google Scholar] [CrossRef]
- Ji, W.K.; Rui, Z.B.; Ji, H.B. Z-scheme Ag3PO4/Ag/SrTiO3 heterojunction for visible-light induced photothermal synergistic VOCs degradation with enhanced performance. Ind. Eng. Chem. Res. 2019, 58, 13950–13959. [Google Scholar] [CrossRef]
- Ghasemi, N.; Jamali-Sheini, F.; Zekavati, R. CuO and Ag/CuO nanoparticles: Biosynthesis and antibacterial properties. Mater. Lett. 2017, 196, 78–82. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Chen, Y.F.; Huang, W.X.; He, D.L.; Situ, Y.; Huang, H. Construction of heterostructured g-C3N4/Ag/TiO2 microspheres with enhanced photocatalysis performance under visible-light irradiation. ACS Appl. Mater. Inter. 2014, 6, 14405–14414. [Google Scholar] [CrossRef]
- Sui, Y.M.; Fu, W.Y.; Yang, H.B.; Zeng, Y.; Zhang, Y.Y.; Zhao, Q.; Li, Y.G.; Zhou, X.M.; Leng, Y.; Li, M.H.; et al. Low temperature synthesis of Cu2O crystals: Shape evolution and growth mechanism. Cryst. Growth Des. 2010, 10, 99–108. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Zhang, J.; Wang, B.S.; Zada, A.; Humayun, M. Biochemical synthesis of Ag/AgCl nanoparticles for visible-light-driven photocatalytic removal of colored dyes. Materials 2015, 8, 2043–2053. [Google Scholar] [CrossRef]
- Li, Y.P.; Wang, B.W.; Liu, S.H.; Duan, X.F.; Hu, Z.Y. Synthesis and characterization of Cu2O/TiO2 photocatalysts for H2 evolution from aqueous solution with different scavengers. Appl. Surf. Sci. 2015, 324, 736–744. [Google Scholar] [CrossRef]
- Wang, F.Z.; Li, W.J.; Gu, S.N.; Li, H.D.; Wu, X.; Ren, C.J.; Liu, X.T. Facile fabrication of direct Z-scheme MoS2/Bi2WO6 heterojunction photocatalyst with superior photocatalytic performance under visible light irradiation. J. Photochem. Photobiol. A 2017, 335, 140–148. [Google Scholar] [CrossRef]
- Purvis, K.L.; Lu, G.; Schwartz, J.; Bernasek, S.L. Surface characterization and modification of indium tin oxide in ultrahigh vacuum. J. Am. Chem. Soc. 2000, 122, 1808–1809. [Google Scholar] [CrossRef]
- Forget, A.; Limoges, B.; Balland, V. Efficient chemisorption of organophosphorous redox probes on indium tin oxide surfaces under mild conditions. Langmuir 2015, 31, 1931–1940. [Google Scholar] [CrossRef]
- Tohsophon, T.; Dabirian, A.; De Wolf, S.; Morales-Masis, M.; Ballif, C. Environmental stability of high-mobility indium-oxide based transparent electrodes. APL Mater. 2015, 3, 116105. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T.; Matralis, H. Influence of the preparation method on the performance of CuO-CeO2 catalysts for the selective oxidation of CO. Appl. Catal. B-Environ. 2005, 56, 87–93. [Google Scholar] [CrossRef]
- Lee, Y.H.; Leu, I.C.; Liao, C.L.; Chang, S.T.; Fung, K.Z. Fabrication and characterization of Cu2O nanorod arrays and their electrochemical performance in Li-ion batteries. Electrochem. Solid-State Lett. 2006, 9, A207. [Google Scholar] [CrossRef]
- Li, J.W.; Yang, X.Q.; Ma, C.R.; Lei, Y.; Cheng, Z.Y.; Rui, Z.B. Selectively recombining the photoinduced charges in bandgap-broken Ag3PO4/GdCrO3 with a plasmonic Ag bridge for efficient photothermocatalytic VOCs degradation and CO2 reduction. Appl. Catal. B-Environ. 2021, 291, 120053. [Google Scholar] [CrossRef]
- Li, J.W.; Chen, J.Y.; Fang, H.L.; Guo, X.M.; Rui, Z.B. Plasmonic metal bridge leading type III heterojunctions to robust type B photothermocatalysts. Ind. Eng. Chem. Res. 2021, 60, 8420–8429. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Xu, L.F.; Cao, J.J.; Ho, W.K.; Lee, S.C. Visible-light-active plasmonic Ag-SrTiO3 nanocomposites for the degradation of NO in air with high selectivity. ACS Appl. Mater. Inter. 2016, 8, 4165–4174. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Catalá, J.; Navlani-García, M.; Berenguer-Murcia, Á.; Cazorla-Amorós, D. Exploring CuxO-doped TiO2 modified with carbon nanotubes for CO2 photoreduction in a 2D-flow reactor. J. CO2 Util. 2021, 54, 101796. [Google Scholar] [CrossRef]
- Yang, X.Q.; Liu, S.H.; Li, J.W.; Chen, J.Y.; Rui, Z.B. Promotion effect of strong metal-support interaction to thermocatalytic, photocatalytic, and photothermocatalytic oxidation of toluene on Pt/SrTiO3. Chemosphere 2020, 249, 126096. [Google Scholar] [CrossRef]
- Jing, J.P.; Chen, Z.Y.; Feng, C. Dramatically enhanced photoelectrochemical properties and transformed p/n type of g-C3N4 caused by K and I co-doping. Electrochim. Acta 2019, 297, 488–496. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, J.; Zhu, Y.F.; Liu, Q.; Zhang, H.; Du, R.G.; Lin, C.J. Fabrication of CdTe/ZnS core/shell quantum dots sensitized TiO2 nanotube films for photocathodic protection of stainless steel. Corros. Sci. 2015, 99, 118–124. [Google Scholar] [CrossRef]
- Li, W.F.; Wei, L.C.; Shen, T.; Wei, Y.N.; Li, K.J.; Liu, F.Q.; Li, W.H. Ingenious preparation of “layered-closed” TiO2-BiVO4-CdS film and its highly stable and sensitive photoelectrochemical cathodic protection performance. Chem. Eng. J. 2022, 429, 132511. [Google Scholar] [CrossRef]
- Ding, J.; Liu, P.; Zhou, M.; Yu, H.B. Nafion-endowed graphene super-anticorrosion performance. ACS Sustain. Chem. Eng. 2020, 8, 15344–15353. [Google Scholar] [CrossRef]



| Rs (Ω) | Rd (Ω) | Rc (Ω) | |
|---|---|---|---|
| P25 | 9.24 | 31.68 | 2.47 × 104 |
| CuOx/P25 | 10.12 | 239.7 | 1.22 × 104 |
| Ag/P25 | 11.50 | 2.10 × 104 | 300.5 |
| CuOx/Ag/P25 | 8.51 | 1.21 × 104 | 1.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Pan, G.; Fang, L.; Liu, Y.; Rui, Z. Z-Scheme CuOx/Ag/TiO2 Heterojunction as Promising Photoinduced Anticorrosion and Antifouling Integrated Coating in Seawater. Molecules 2023, 28, 456. https://doi.org/10.3390/molecules28010456
Guo X, Pan G, Fang L, Liu Y, Rui Z. Z-Scheme CuOx/Ag/TiO2 Heterojunction as Promising Photoinduced Anticorrosion and Antifouling Integrated Coating in Seawater. Molecules. 2023; 28(1):456. https://doi.org/10.3390/molecules28010456
Chicago/Turabian StyleGuo, Xiaomin, Guotao Pan, Lining Fang, Yan Liu, and Zebao Rui. 2023. "Z-Scheme CuOx/Ag/TiO2 Heterojunction as Promising Photoinduced Anticorrosion and Antifouling Integrated Coating in Seawater" Molecules 28, no. 1: 456. https://doi.org/10.3390/molecules28010456
APA StyleGuo, X., Pan, G., Fang, L., Liu, Y., & Rui, Z. (2023). Z-Scheme CuOx/Ag/TiO2 Heterojunction as Promising Photoinduced Anticorrosion and Antifouling Integrated Coating in Seawater. Molecules, 28(1), 456. https://doi.org/10.3390/molecules28010456







