Stereoselective Synthesis of Flavonoids: A Brief Overview
Abstract
1. Introduction
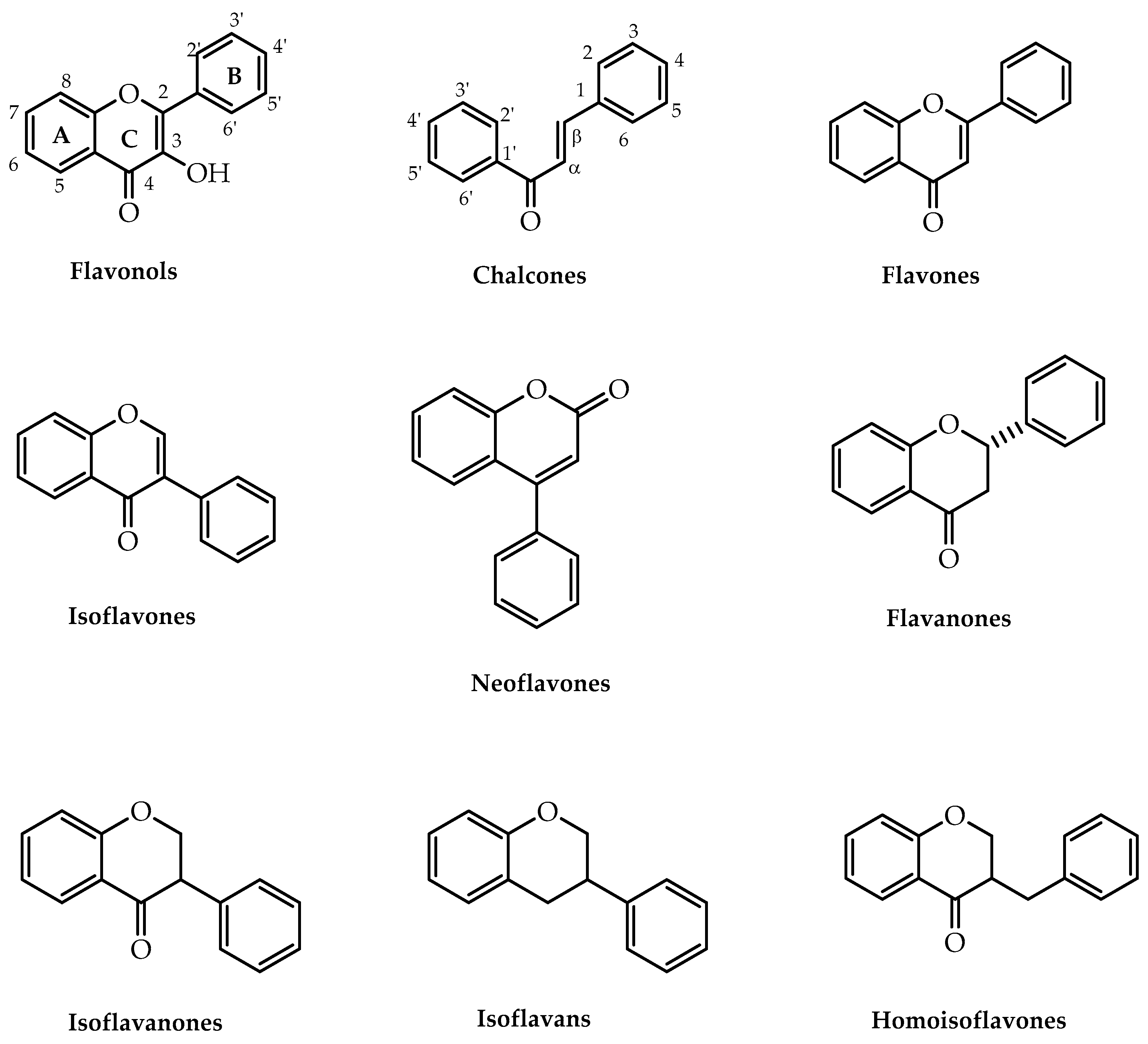
| Flavonoids | Synthetic Approaches | References |
|---|---|---|
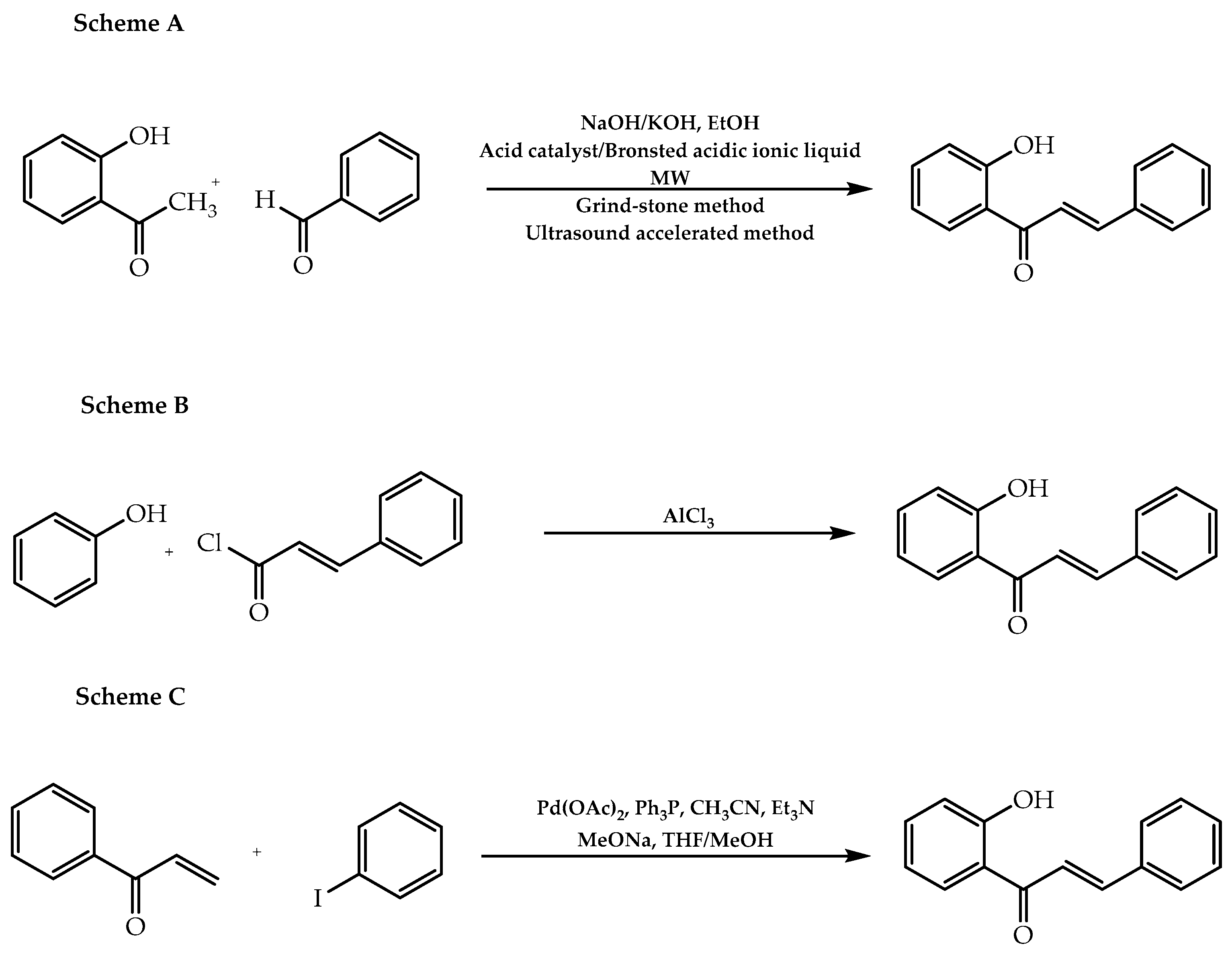
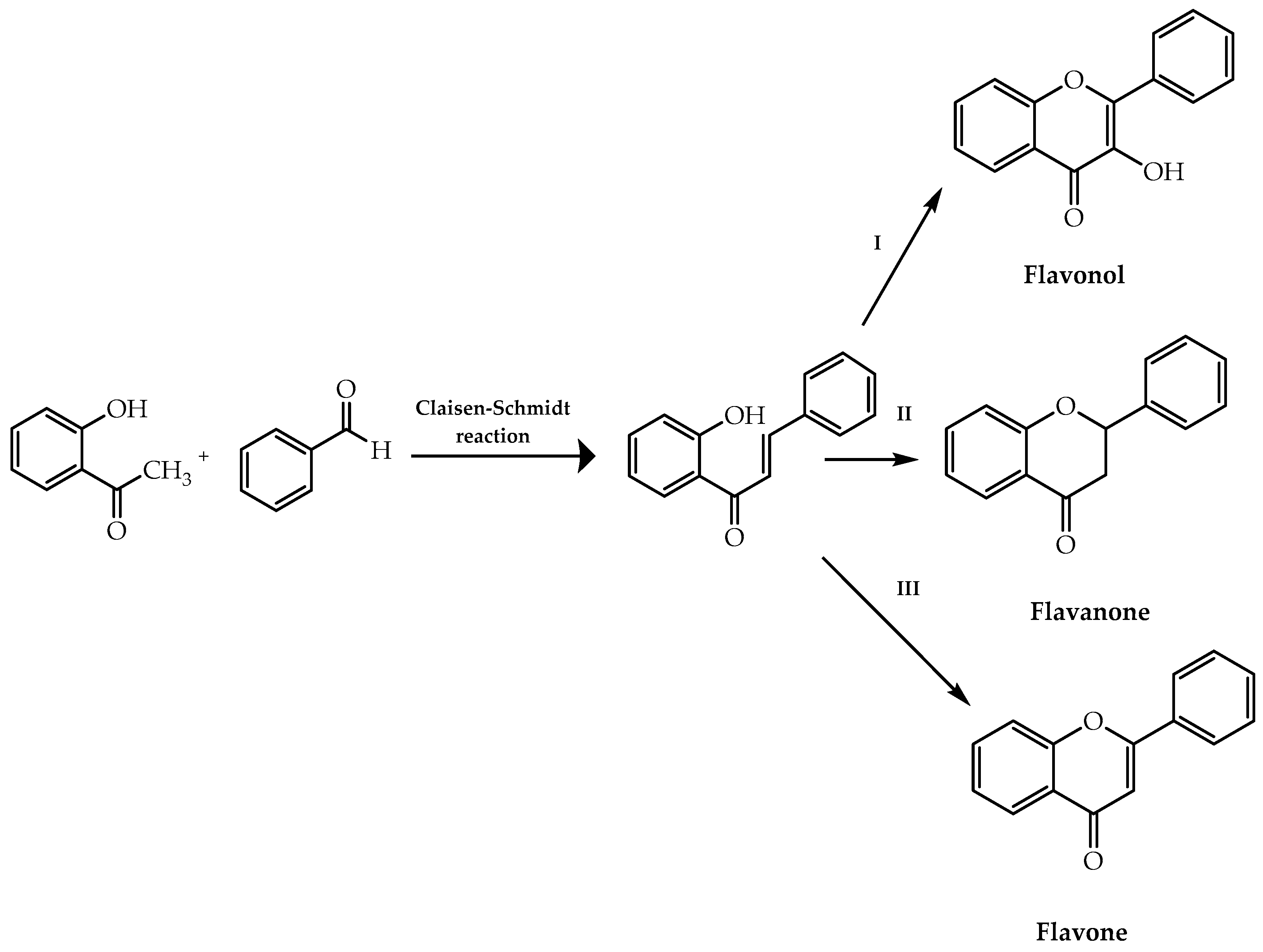
| Chalcones | Claisen–Schmidt reaction | [21,30,31] |
| Friedel–Crafts reaction | [21] | |
| Heck coupling | [32] | |
| Suzuki–Miyaura reaction | [62] | |
| Flavonols | Algar–Flynn–Oyamada reaction | [21,34] |
| Karl von Auwers reaction | [60] | |
| Kostanecki methodology | [29] | |
| Flavanones | Intramolecular cyclisation of 2′-hydroxychalcones | [36,37,38,39,40,41,42,43] |
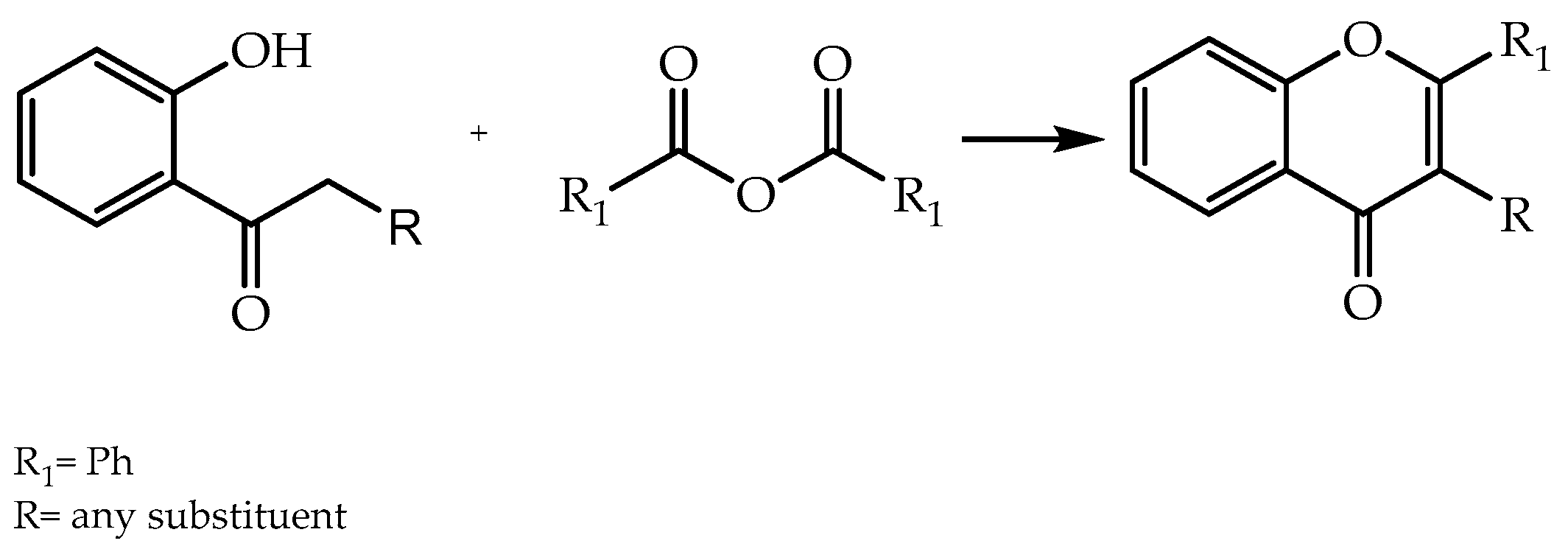
| Flavones | Oxidative cyclisation of 2′-hydroxychalcones | [44,45,46,47,48,49,50,51] |
| Allan–Robinson reaction | [21,52] | |
| Baker–Venkataraman reaction | [21,54] | |
| Kostanecki reaction | [55] | |
| Mentzer pyrone synthesis | [57] | |
| Suzuki–Miyaura reaction | [62] | |
| Isoflavones | Allan–Robinson reaction | [21,52] |
| Suzuki–Miyaura reaction | [62] | |
| Deoxybenzoin route | [22] | |
| Reductive cleavage of isoxazoles | [23] | |
| Intramolecular ketene cycloaddition followed by decarboxylation | ||
| Rearrangement and cyclisation of chalcone epoxides | [24] | |
| Rearrangement of flavanones | ||
| Wacker–Cook tandem conversion of α-methylene deoxybenzoins | [25] | |
| Cu(I)-mediated cyclisation of 3-(2-bromophenyl)-3-oxopropanol | [26] | |
| Neoflavones | Suzuki–Miyaura reaction | [62] |
| Pechmann reaction | [27] | |
| Perkin reaction | ||
| Wittig reaction of benzophenones | ||
| Metal-catalysed cross-coupling reactions such as Stille type | ||
| Direct arylation by the palladium-catalysed oxidative Heck coupling of arylboronic acids to coumarins | [28] |
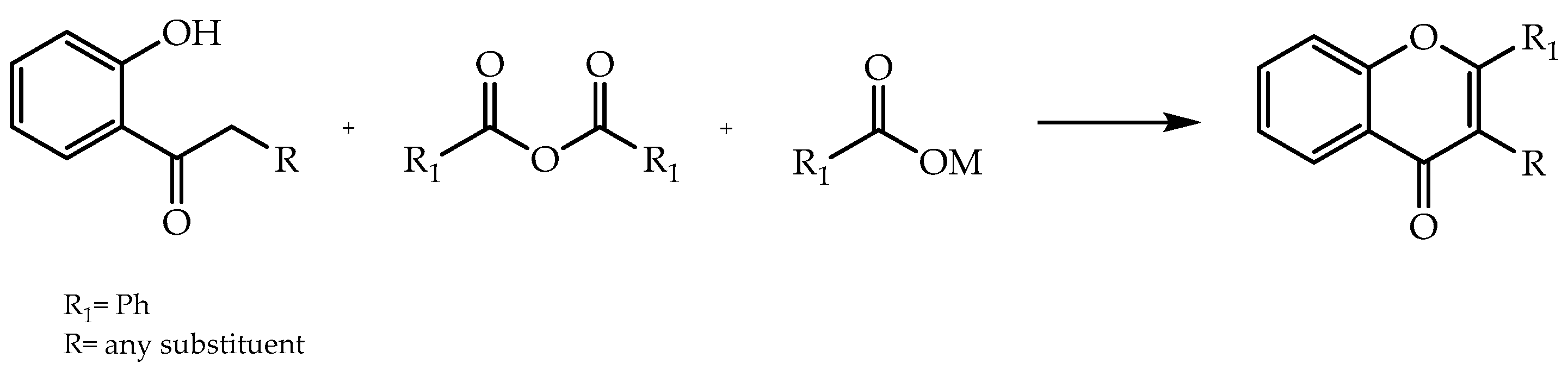
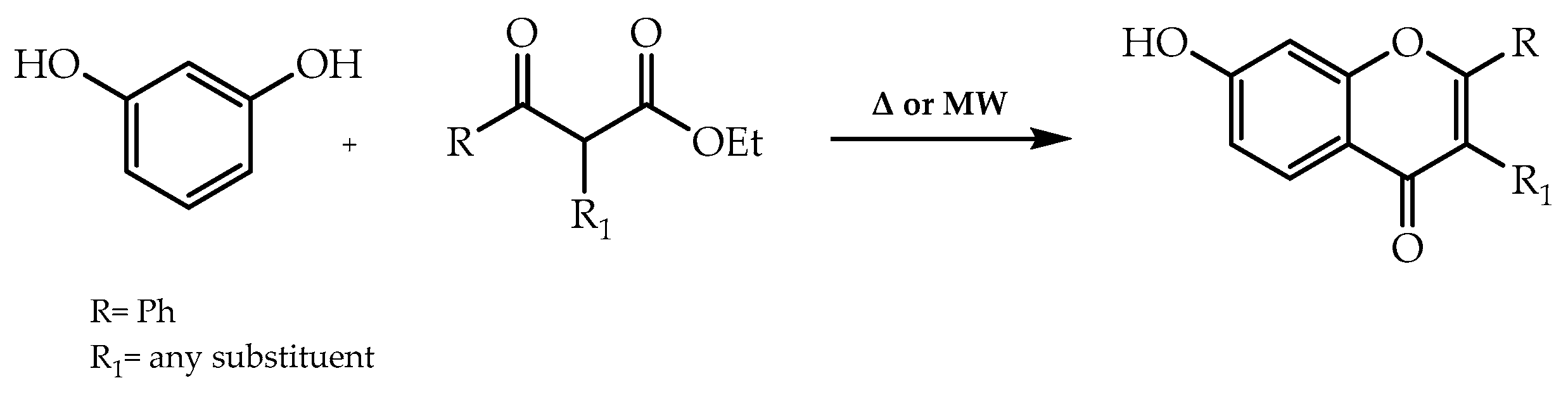
2. Stereoselective Synthesis of Flavonoids
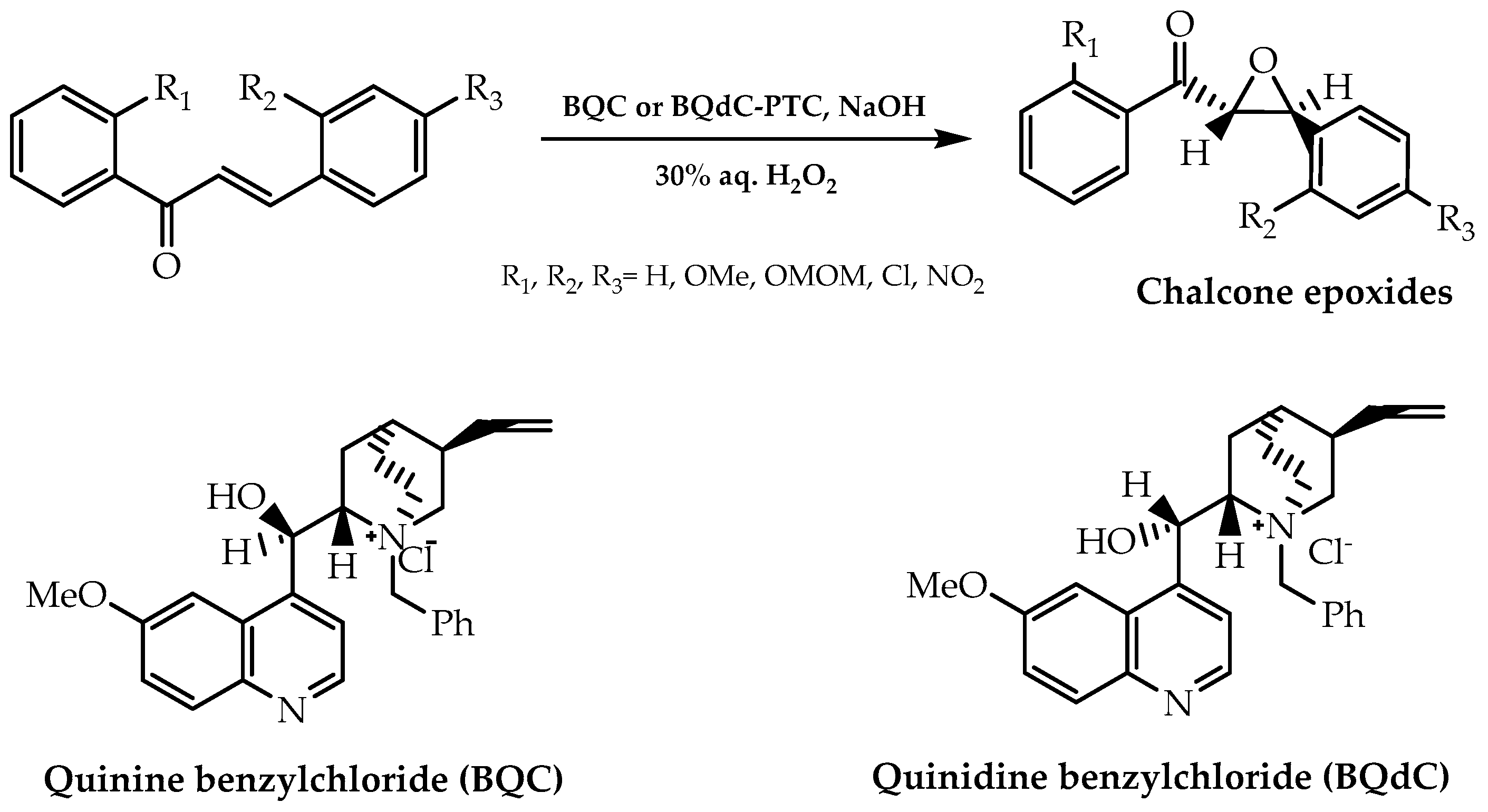
2.1. Stereoselective Chalcone Epoxidation Approach
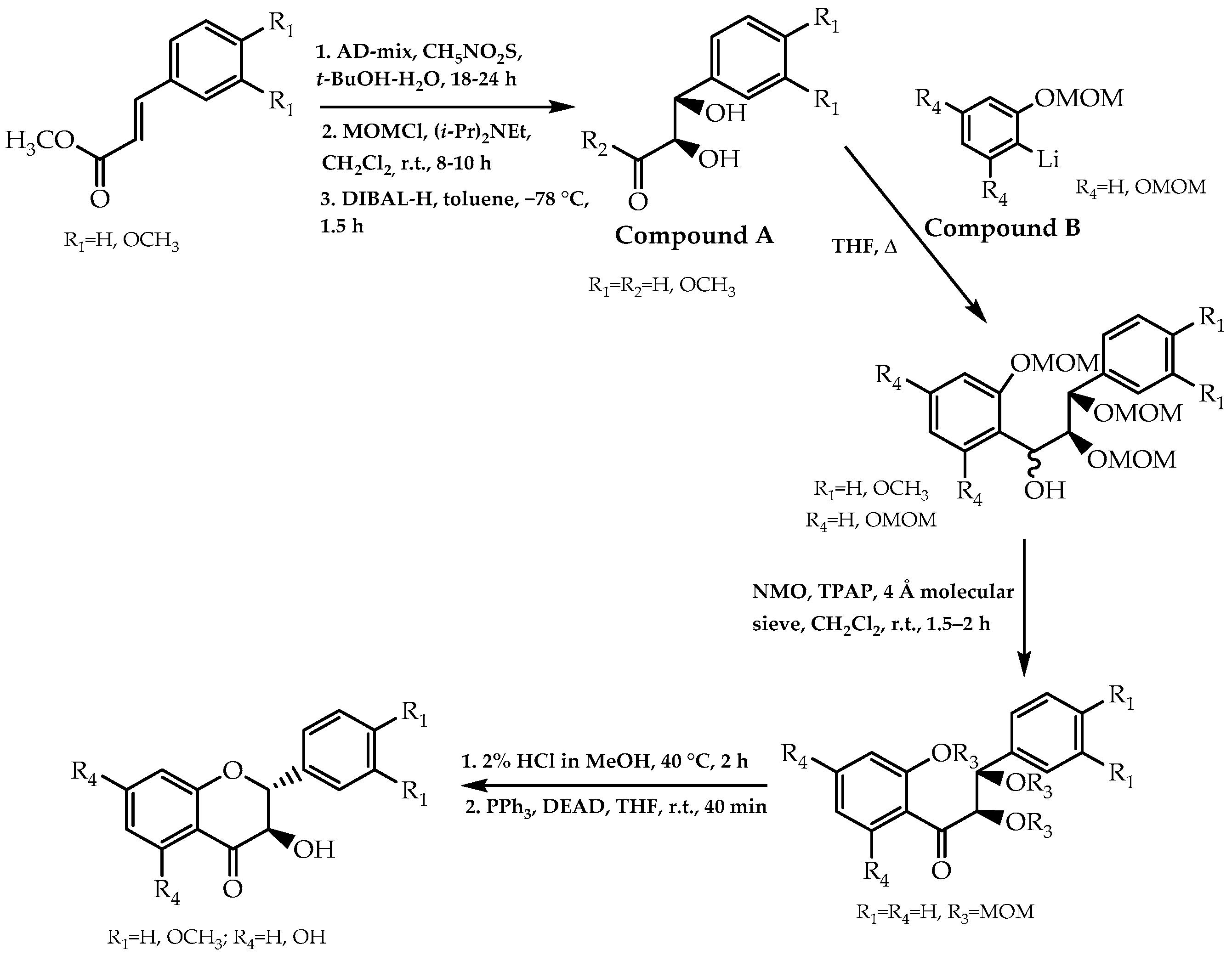
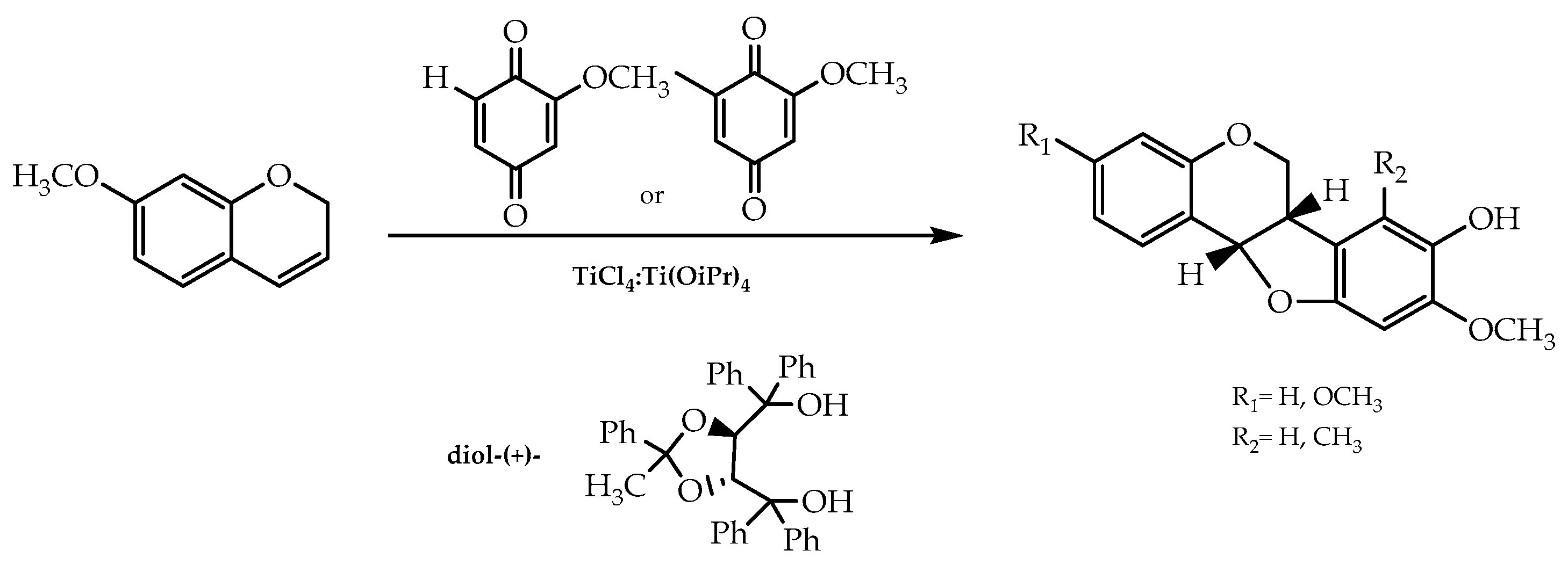
2.2. Sharpless Asymmetric Dihydroxylation, Mitsunobu Reaction, and Cycloaddition of 1,4-Benzoquinone
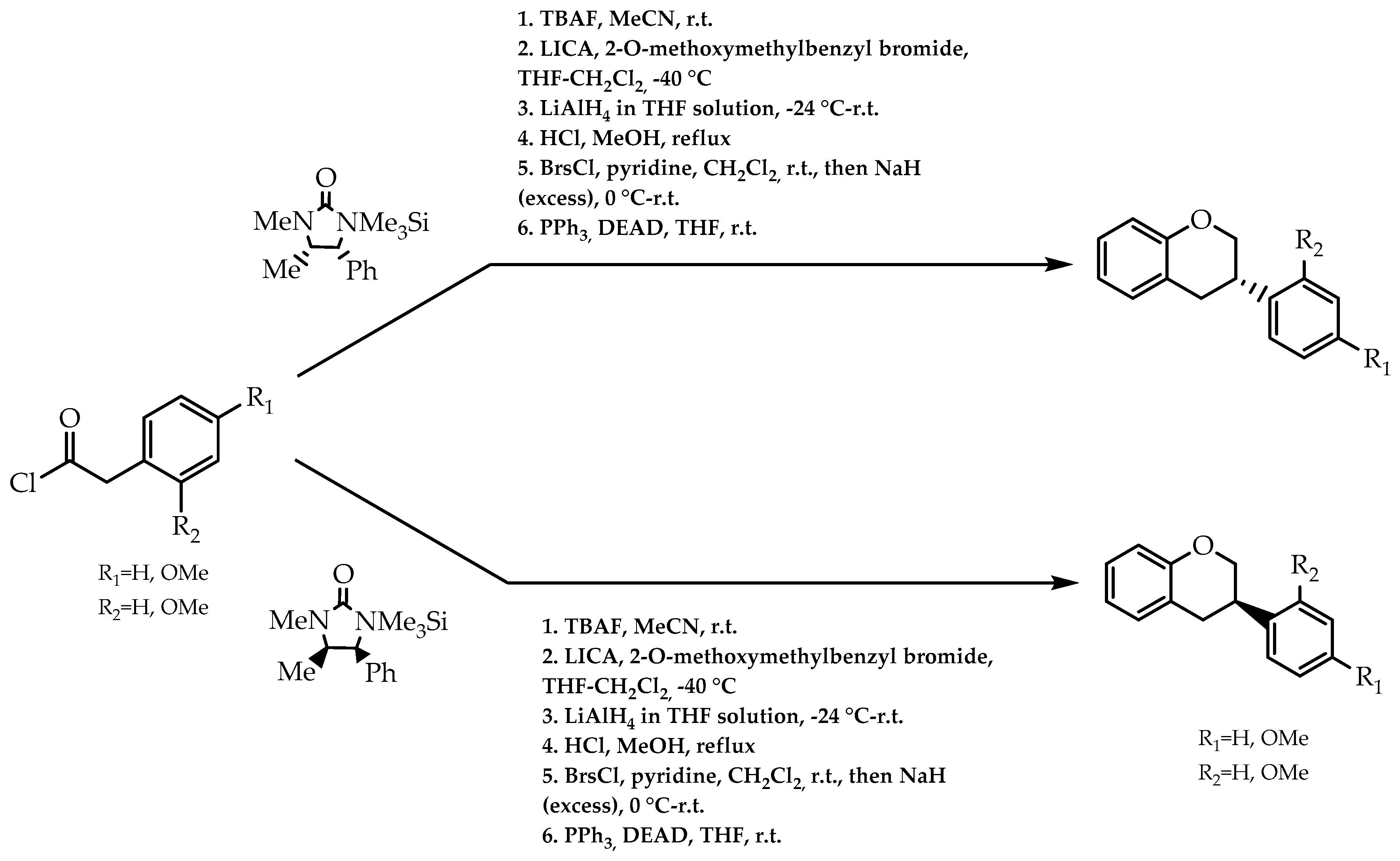
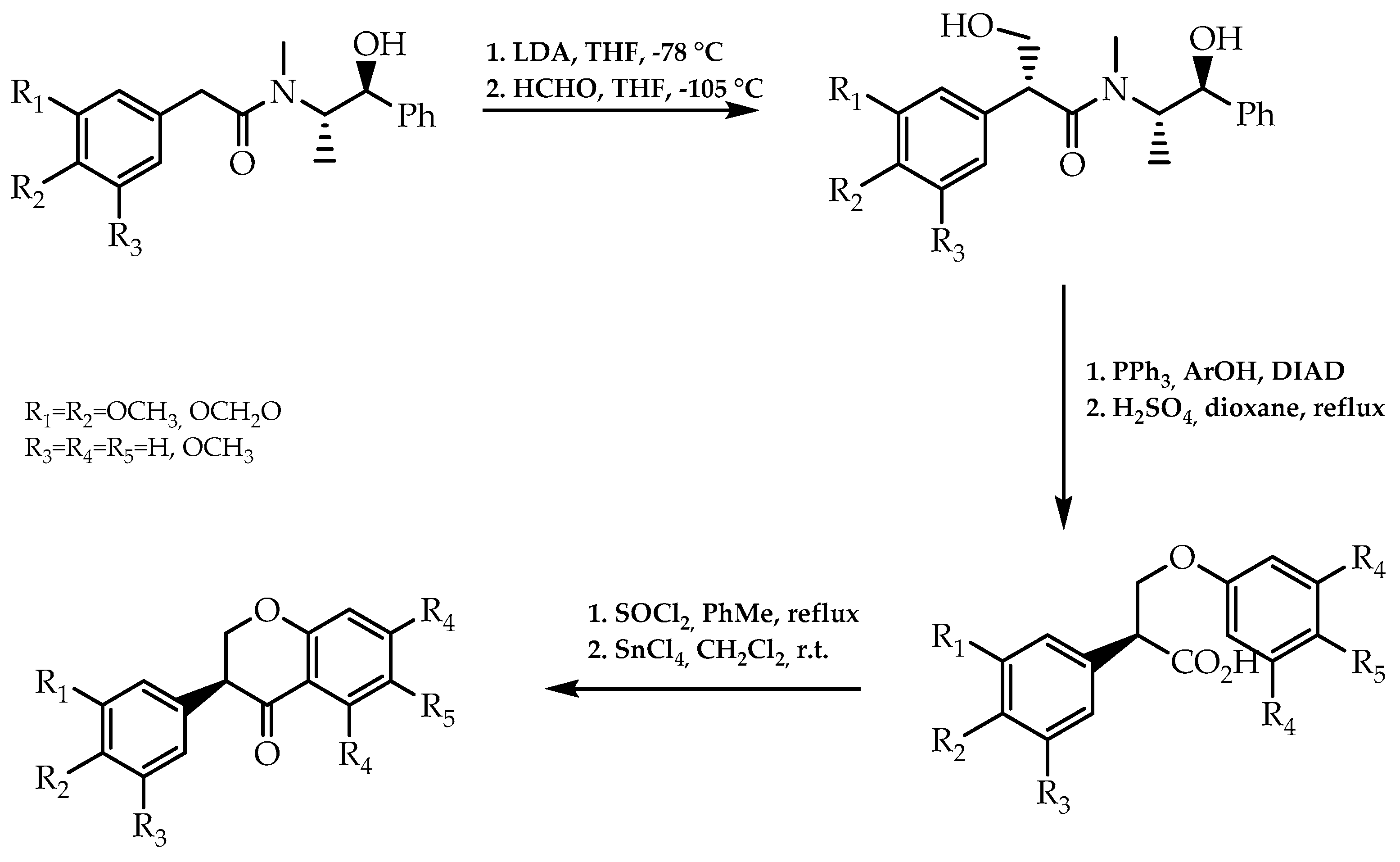
2.3. Chiral Auxliaries Approach
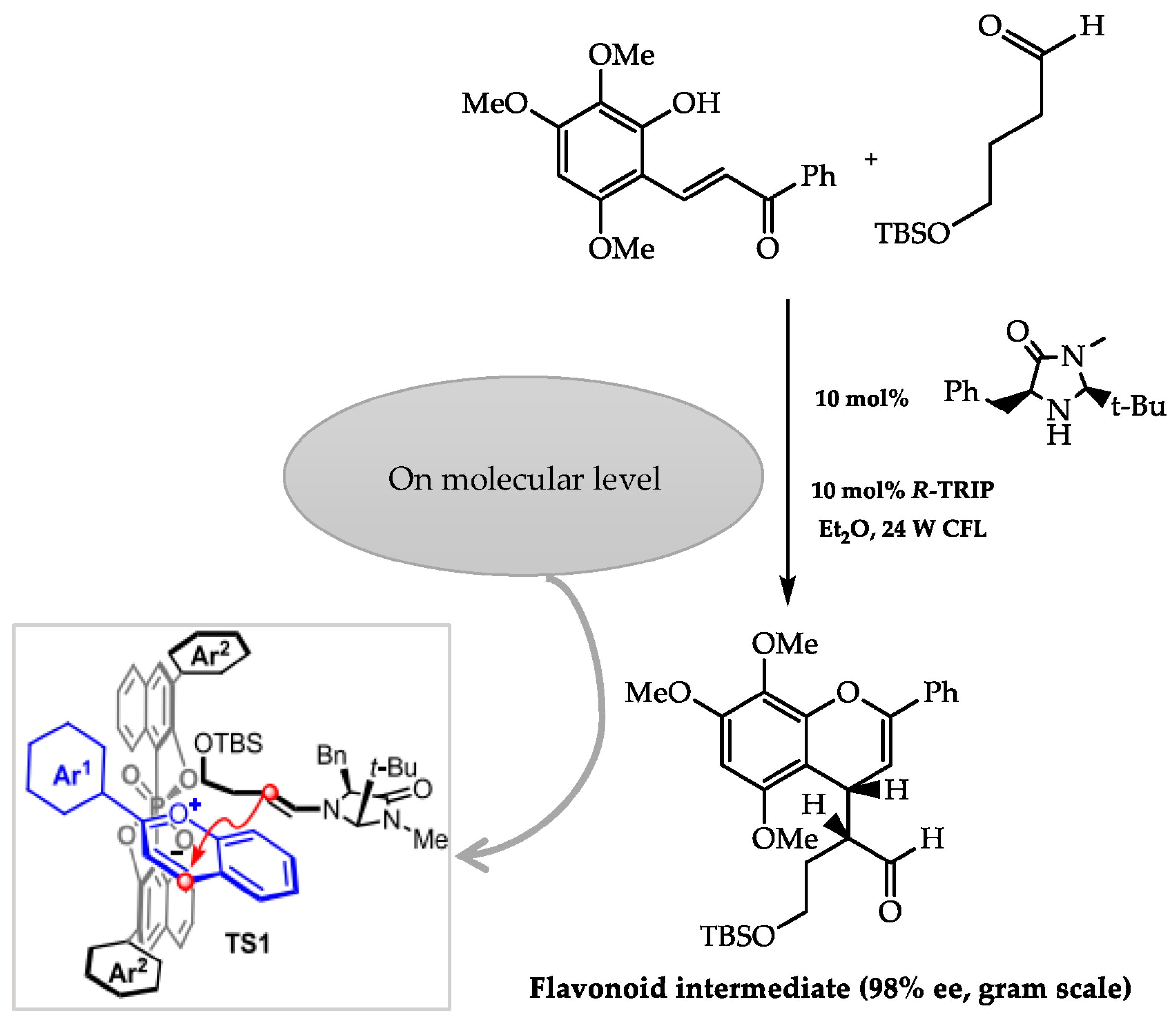
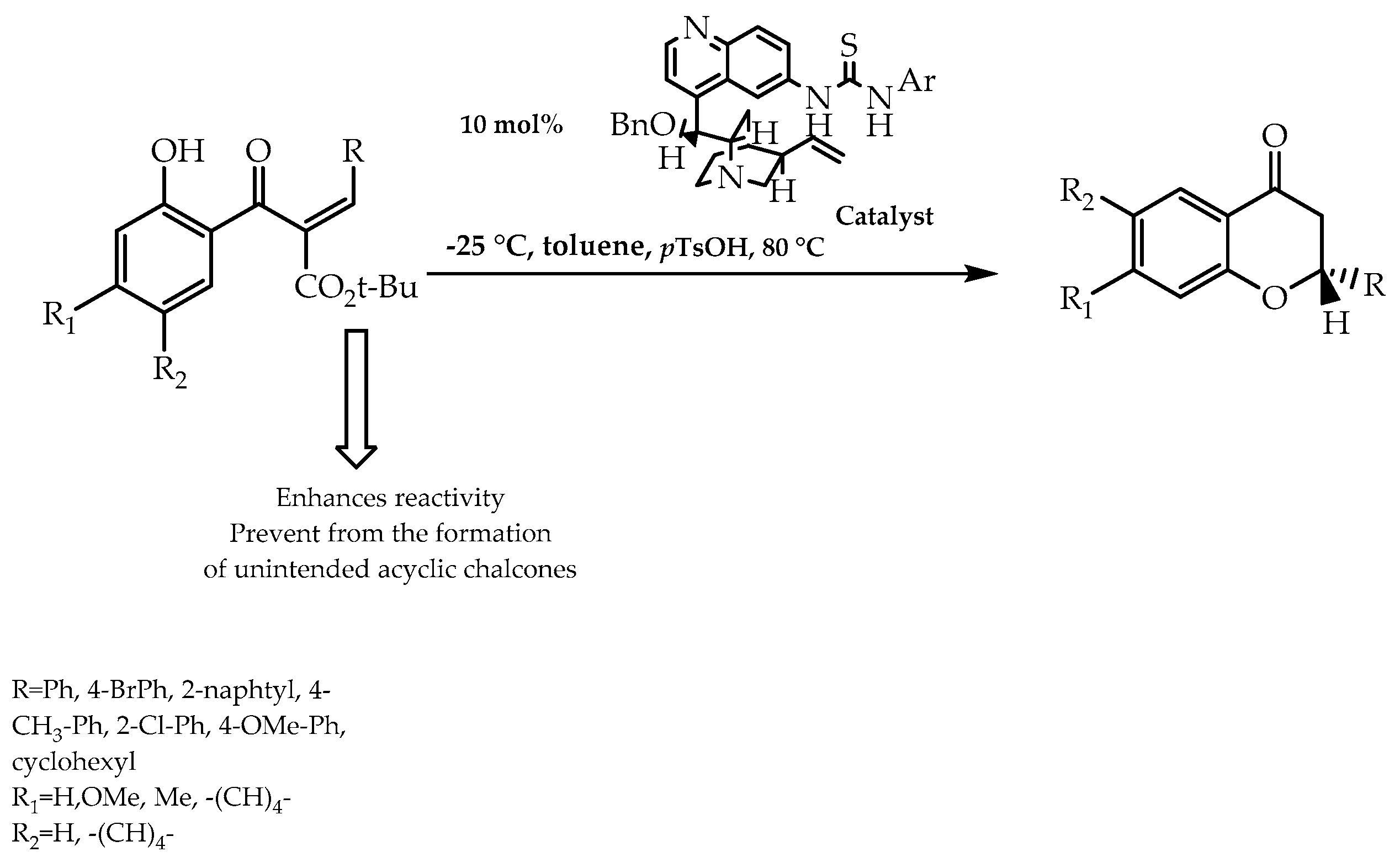
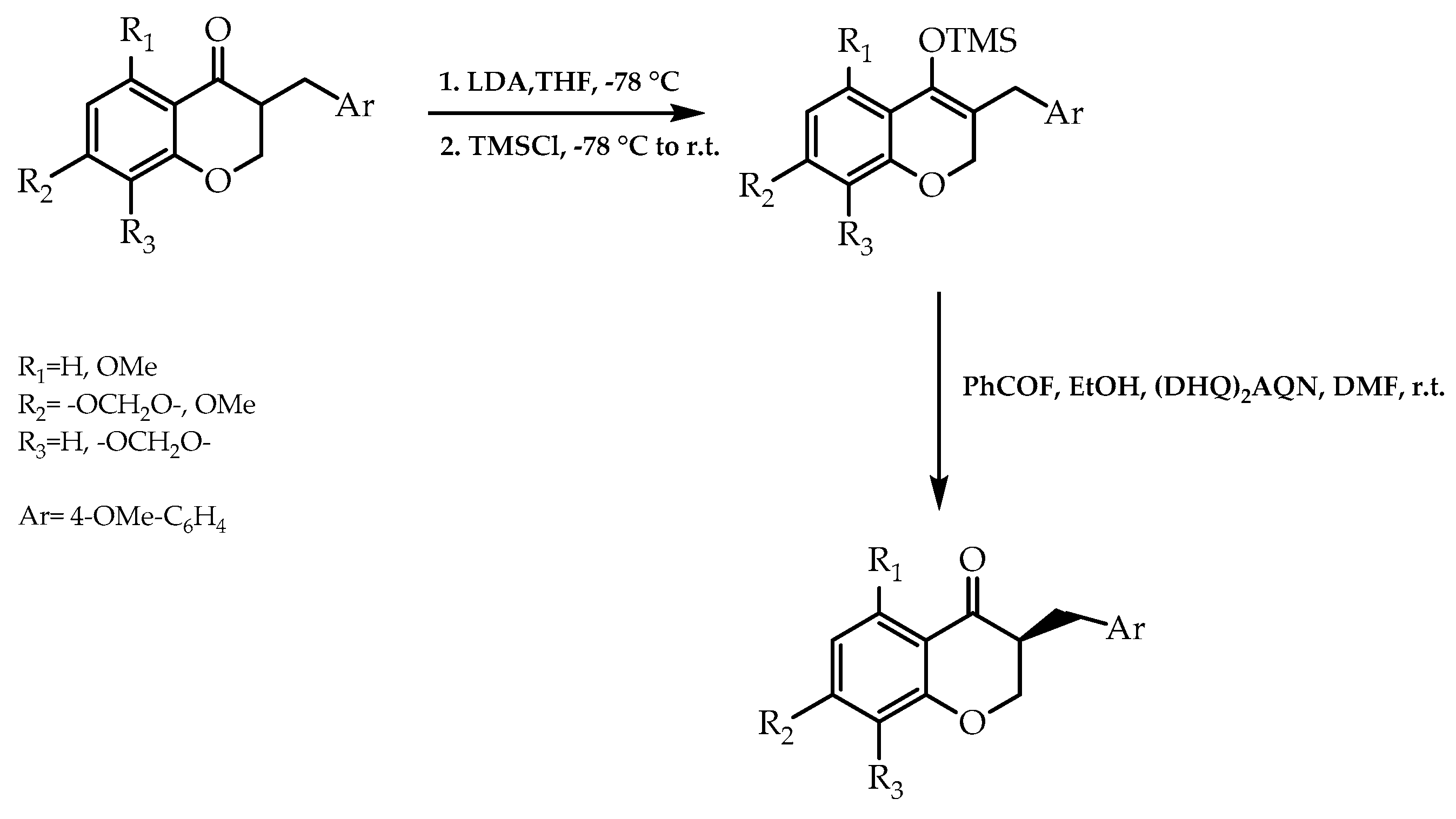
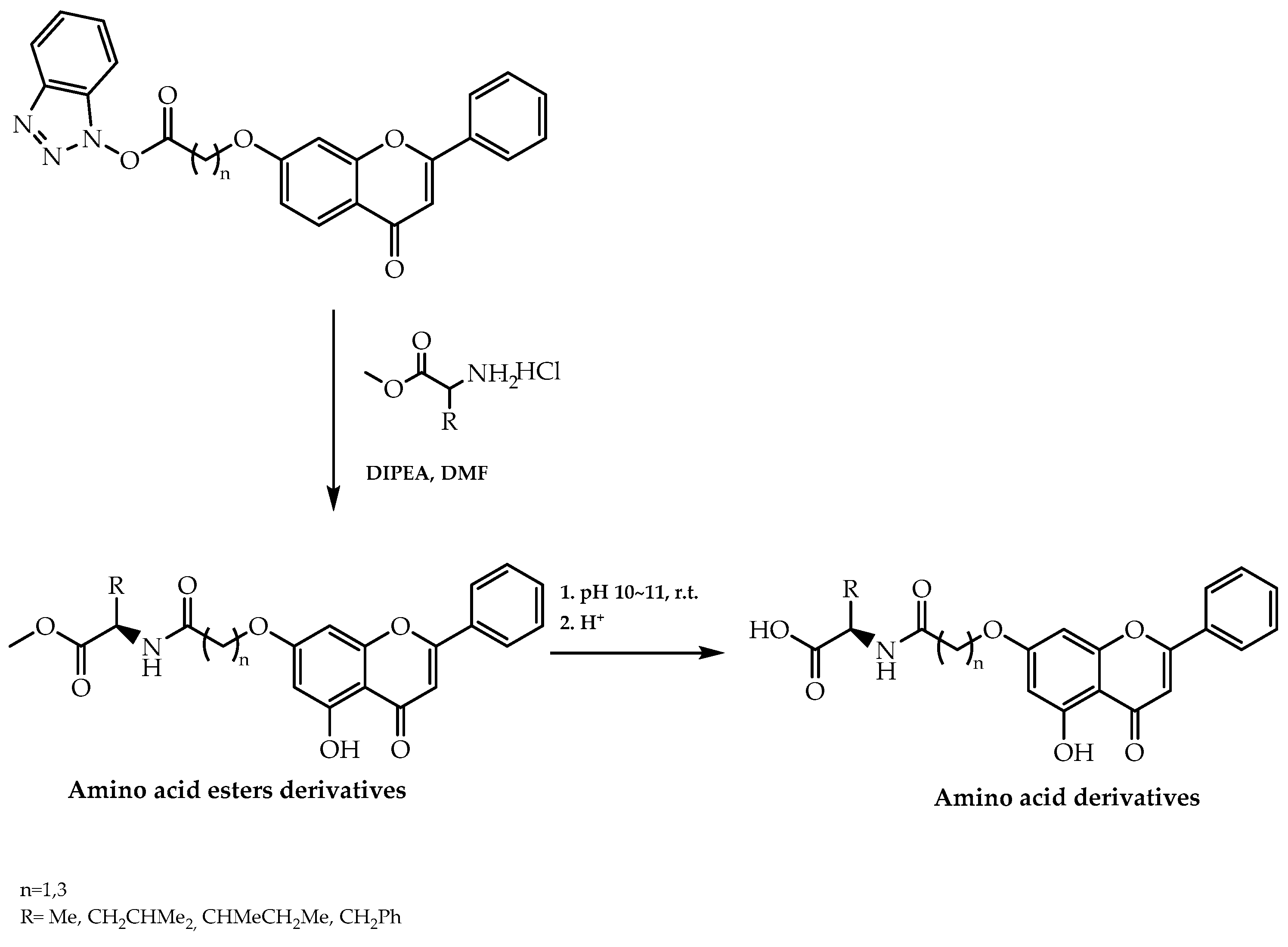
2.4. Organocatalysis
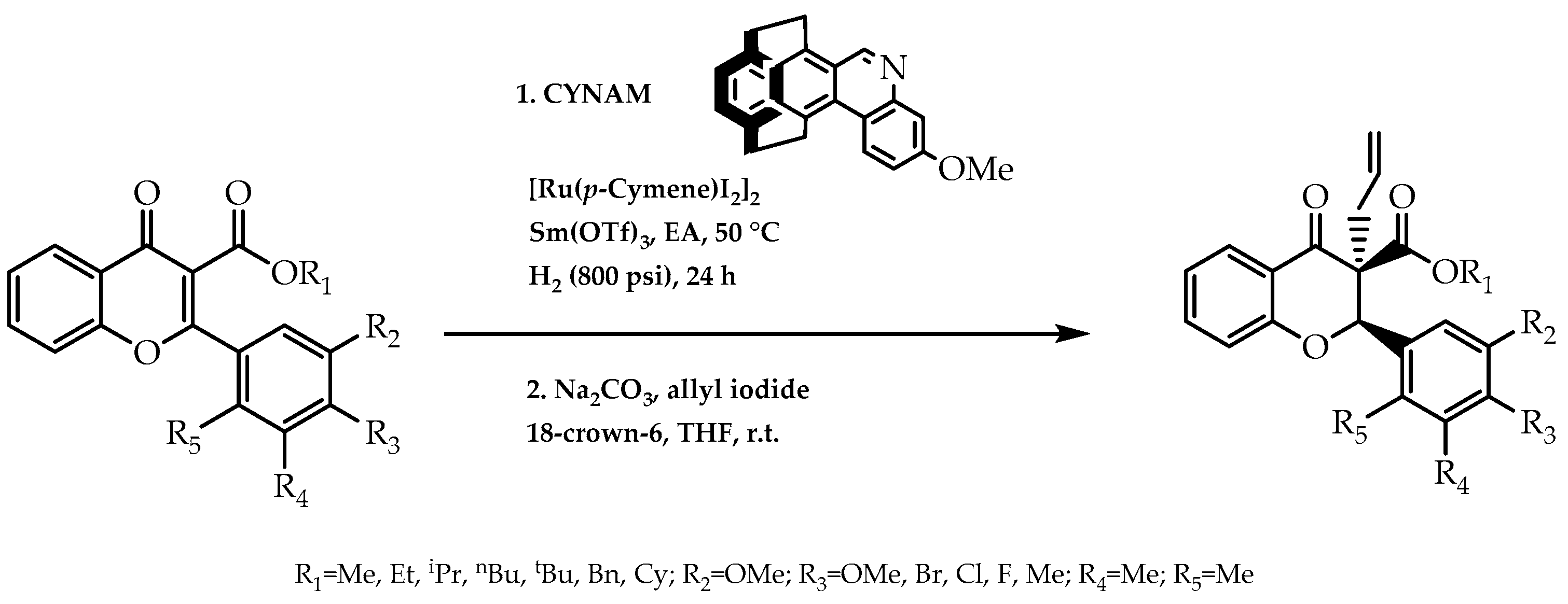
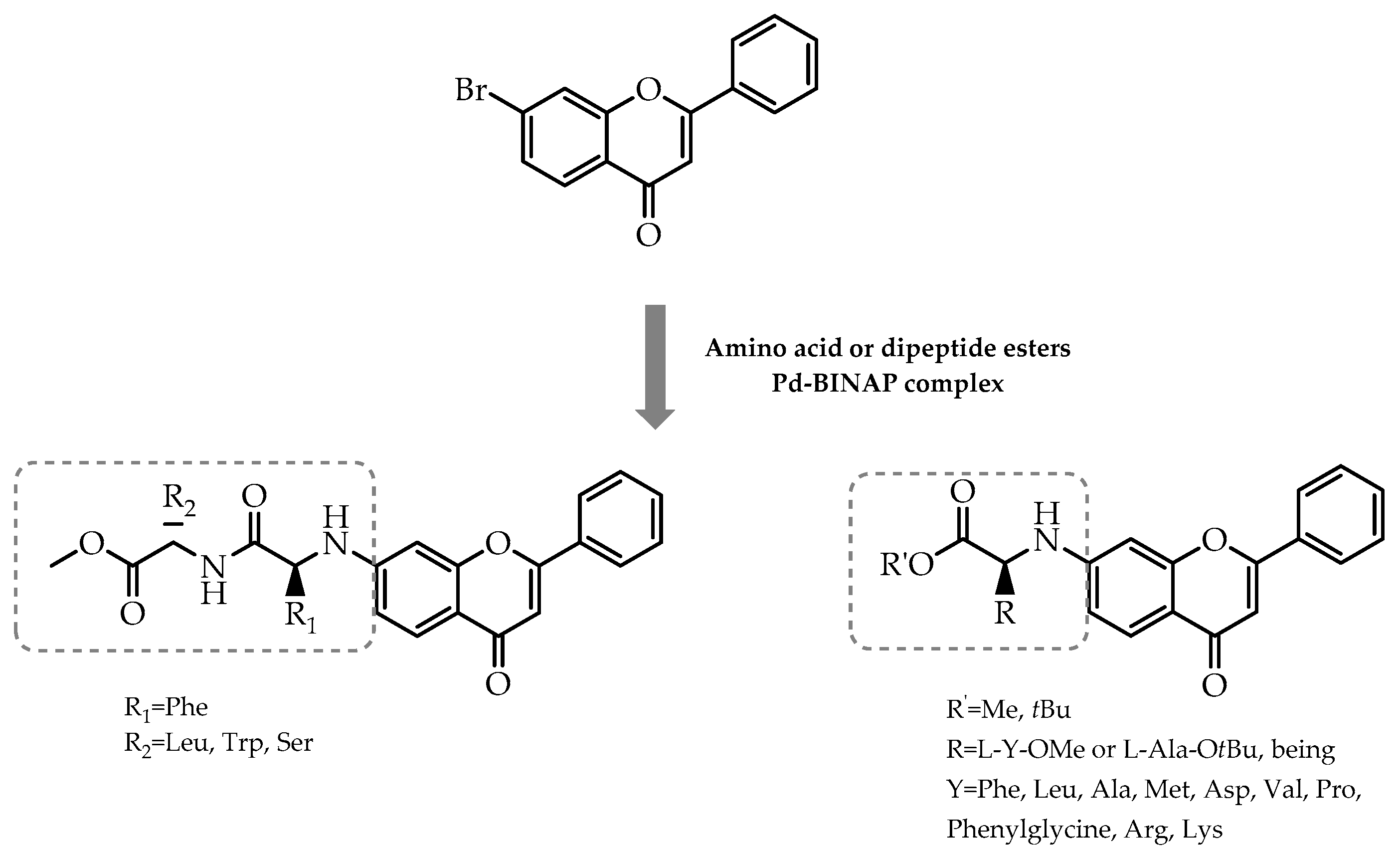
2.5. Organometallic Catalysis
2.6. Biocatalysis
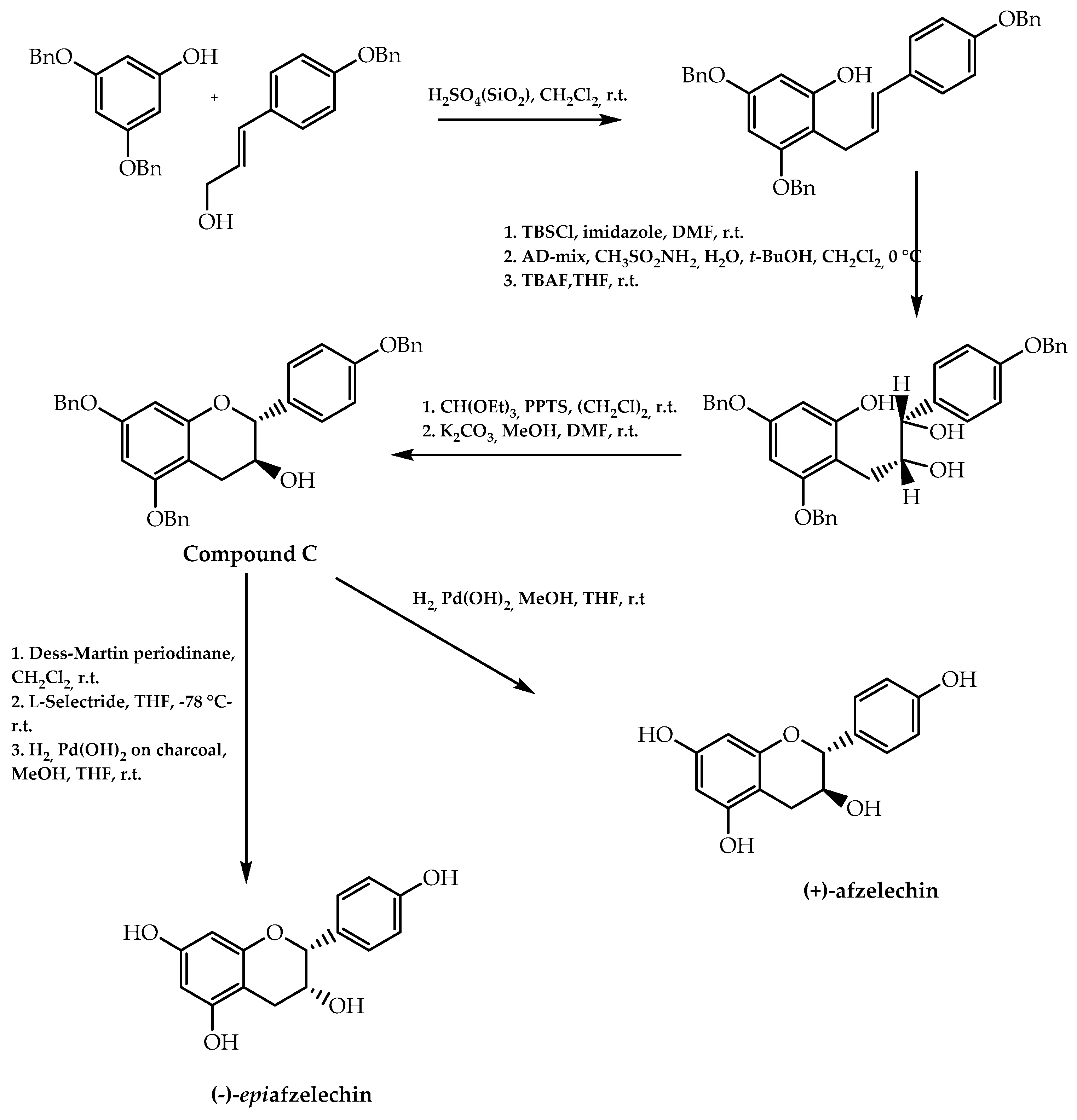
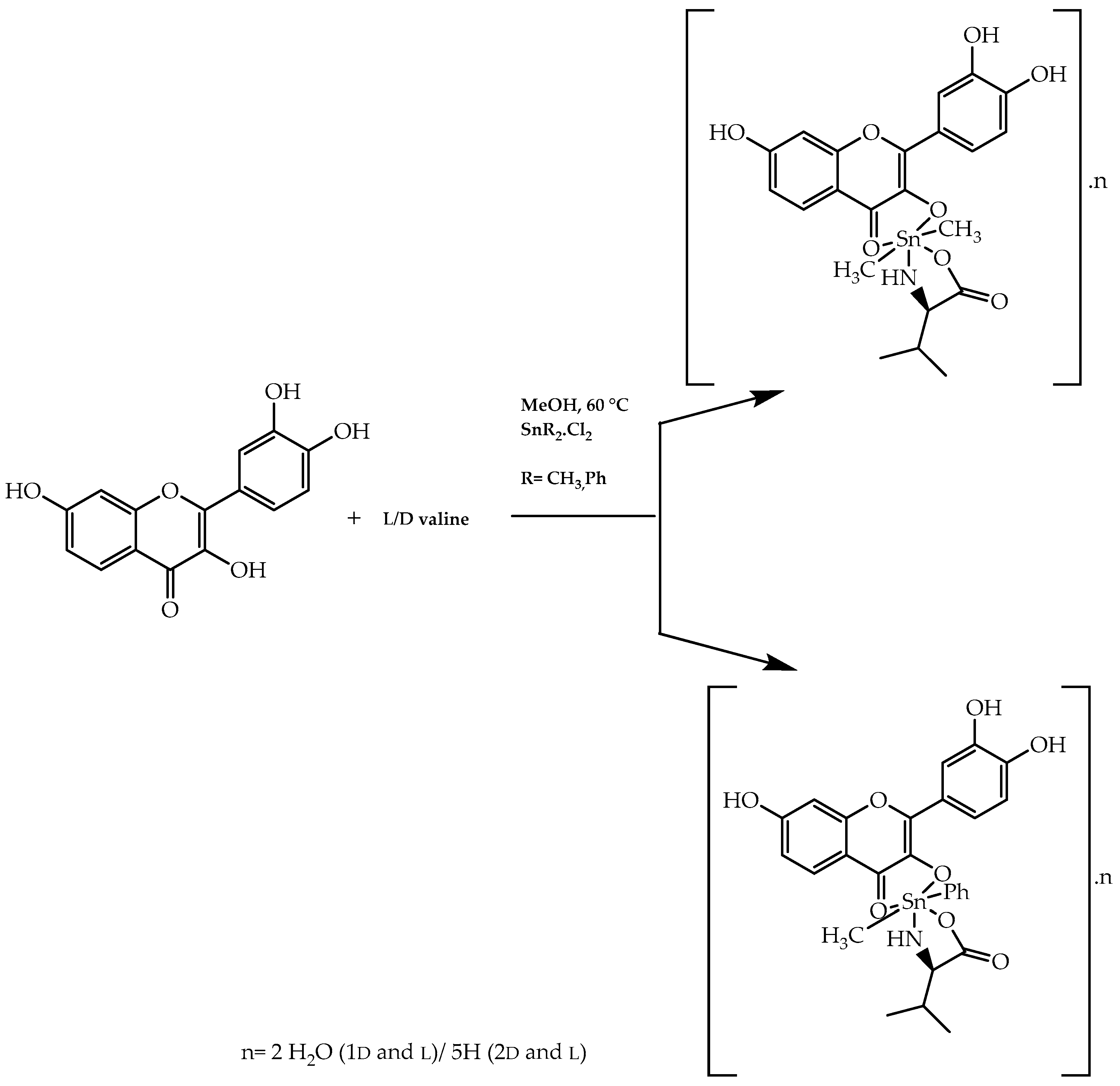
2.7. Chiral Pool Methodology
2.8. Other Synthetic Methologies
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally friendly methods for flavonoid extraction from plant material: Impact of their operating conditions on yield and antioxidant properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar] [CrossRef]
- Martins, B.T.; Correia da Silva, M.; Pinto, M.; Cidade, H.; Kijjoa, A. Marine natural flavonoids: Chemistry and biological activities. Nat. Prod. Res. 2019, 33, 3260–3272. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Zhang, Y.; Zhao, X.; Zhao, J.; Wang, X. The potential of functionalized dressing releasing flavonoids facilitates scar-free healing. Front. Med. 2022, 9, 978120. [Google Scholar] [CrossRef]
- Boniface, P.K.; Ferreira, E.I. Flavonoids as efficient scaffolds: Recent trends for malaria, leishmaniasis, Chagas disease, and dengue. Phytother. Res. 2019, 33, 2473–2517. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef]
- Shamsudin, N.F.; Ahmed, Q.U.; Mahmood, S.; Shah, S.A.A.; Sarian, M.N.; Khattak, M.M.A.K.; Khatib, A.; Sabere, A.S.M.; Yusoff, Y.M.; Latip, J. Flavonoids as antidiabetic and anti-inflammatory agents: A review on structural activity relationship-based studies and meta-analysis. Int. J. Mol. Sci. 2022, 23, 12605. [Google Scholar] [CrossRef]
- Xing, N.; Meng, X.; Wang, S. Isobavachalcone: A comprehensive review of its plant sources, pharmacokinetics, toxicity, pharmacological activities and related molecular mechanisms. Phytother. Res. 2022, 36, 3120–3142. [Google Scholar] [CrossRef]
- Kashyap, P.; Thakur, M.; Singh, N.; Shikha, D.; Kumar, S.; Baniwal, P.; Yadav, Y.S.; Sharma, M.; Sridhar, K.; Inbaraj, B.S. In silico evaluation of natural flavonoids as a potential inhibitor of coronavirus disease. Molecules 2022, 27, 6374. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics applications and chemistry of chalcone derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef]
- Luo, Y.; Jian, Y.; Liu, Y.; Jiang, S.; Muhammad, D.; Wang, W. Flavanols from nature: A phytochemistry and biological activity review. Molecules 2022, 27, 719. [Google Scholar] [CrossRef]
- Gupta, D.; Guliani, E. Flavonoids: Molecular mechanism behind natural chemoprotective behavior-a mini review. Biointerface Res. Appl. Chem. 2022, 12, 5983–5995. [Google Scholar] [CrossRef]
- Pereira, D.; Pinto, M.; Correia-da-Silva, M.; Cidade, H. Recent advances in bioactive flavonoid hybrids linked by 1,2,3-triazole ring obtained by click chemistry. Molecules 2022, 27, 230. [Google Scholar] [CrossRef]
- Moreira, J.; Almeida, J.; Saraiva, L.; Cidade, H.; Pinto, M. Chalcones as promising antitumor agents by targeting the p53 pathway: An overview and new insights in drug-likeness. Molecules 2021, 26, 3737. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary flavonoids: Cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological applications and health-promoting properties of flavonols: An updated view. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef]
- Addi, M.; Elbouzidi, A.; Abid, M.; Tungmunnithum, D.; Elamrani, A.; Hano, C. An overview of bioactive flavonoids from citrus fruits. Appl. Sci. 2022, 12, 29. [Google Scholar] [CrossRef]
- Sithuba, T.; Masia, N.D.; Moema, J.; Murulana, L.C.; Masuku, G.; Bahadur, I.; Kabanda, M.M. Corrosion inhibitory potential of selected flavonoid derivatives: Electrochemical, molecular Zn surface interactions and quantum chemical approaches. Results Eng. 2022, 16, 100694. [Google Scholar] [CrossRef]
- Sharma, A.; Singh Tuli, H.; Sharma, A.K. Chemistry and Synthetic Overview of Flavonoids. In Current Aspects of Flavonoids: Their Role in Cancer Treatment; Singh Tuli, H., Ed.; Springer: Singapore, 2019; pp. 23–38. [Google Scholar]
- Baker, W.; Chadderton, J.; Harborne, J.B.; Ollis, W.D. A new synthesis of isoflavones. Part I. J. Chem. Soc. (Resumed) 1953, 381, 1852–1860. [Google Scholar] [CrossRef]
- Harborne, J.B. The flavonoids: Advances in research since 1986. J. Chem. Educ. 1995, 72, A73. [Google Scholar] [CrossRef]
- Dixon, R.A.; Ferreira, D. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Granados-Covarrubias, E.H.; Maldonado, L.A. A Wacker–Cook synthesis of isoflavones: Formononetine. Tetrahedron Lett. 2009, 50, 1542–1545. [Google Scholar] [CrossRef]
- Li, Q.-L.; Liu, Q.-L.; Ge, Z.-Y.; Zhu, Y.-M. A novel synthesis of isoflavones via copper(I)-catalyzed intramolecular cyclization reaction. Helv. Chim. Acta 2011, 94, 1304–1309. [Google Scholar] [CrossRef]
- Garazd, M.M.; Garazd, Y.L.; Khilya, V.P. Neoflavones. 2. Methods for synthesizing and modifying 4-arylcoumarins. Chem. Nat. Compd. 2005, 41, 245–271. [Google Scholar] [CrossRef]
- Li, Y.; Qi, Z.; Wang, H.; Fu, X.; Duan, C. Palladium-catalyzed oxidative Heck coupling reaction for direct synthesis of 4-arylcoumarins using coumarins and arylboronic acids. J. Org. Chem. 2012, 77, 2053–2057. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, P.; Singh Tuli, H.; Sharma, A.K. Phytochemical and Pharmacological Properties of Flavonols. In eLS; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1–12. [Google Scholar]
- Suma, A.T.; Wahyuningsih, T. Efficient synthesis of chloro chalcones under ultrasound irradiation, their anticancer activities and molecular docking studies. Rasayan J. Chem. 2019, 12, 502–510. [Google Scholar] [CrossRef]
- Kristanti, A.; Suwito, H.; Aminah, N.S.; Haq, K.; Hardiyanti, H.D.; Anggraeni, H.; Faiza, N.; Anto, R.; Muharromah, S. Synthesis of some chalcone derivatives, in vitro and in silico toxicity evaluation. Rasayan J. Chem. 2020, 13, 654–662. [Google Scholar] [CrossRef]
- Bianco, A.; Cavarischia, C.; Farina, A.; Guiso, M.; Marra, C. A new synthesis of flavonoids via Heck reaction. Tetrahedron Lett. 2003, 44, 9107–9109. [Google Scholar] [CrossRef]
- Ekanayake, U.G.M.; Weerathunga, H.; Weerasinghe, J.; Waclawik, E.R.; Sun, Z.; MacLeod, J.M.; O’Mullane, A.P.; Ostrikov, K. Sustainable Claisen-Schmidt chalcone synthesis catalysed by plasma-recovered MgO nanosheets from seawater. Sustain. Mater. Technol. 2022, 32, e00394. [Google Scholar] [CrossRef]
- Taichiro, O. A new general method for the synthesis of the derivates of flavonol. Bull. Chem. Soc. Jpn. 1935, 10, 182–186. [Google Scholar] [CrossRef]
- Nhu, D.; Hawkins, B.C.; Burns, C.J. Phase transfer catalysis extends the scope of the Algar–Flynn–Oyamada synthesis of 3-hydroxyflavones. Aust. J. Chem. 2015, 68, 1102–1107. [Google Scholar] [CrossRef]
- Brennan, C.M.; Hunt, I.; Jarvis, T.C.; Johnson, C.D.; McDonnell, P.D. Stereoelectronic effects in ring closure reactions: The 2′-hydroxychalcone—Flavanone equilibrium, and related systems. Can. J. Chem. 1990, 68, 1780–1785. [Google Scholar] [CrossRef]
- Tanaka, K.; Sugino, T. Efficient conversion of 2′-hydroxychalcones into flavanones and flavanols in a water suspension medium. Green Chem. 2001, 3, 133–134. [Google Scholar] [CrossRef]
- Goud, B.S.; Panneerselvam, K.; Zacharias, D.E.; Desirajua, G.R. Intramolecular Michael-type addition in the solid state. J. Chem. Soc. Perkin Trans. 2 1995, 325–330. [Google Scholar] [CrossRef]
- Sanicanin, Z.; Tabakovic, I. Electrochemical transformations of 2′-hydroxychalcones into flavanoids. Tetrahedron Lett. 1986, 27, 407–408. [Google Scholar] [CrossRef]
- Maki, Y.; Shimada, K.; Sako, M.; Hirota, K. Photo-oxidative cyclisation of 2′-hydroxychalcones leading to flavones induced by heterocycle n-oxides: High efficiency of pybimido[54-g]pteridine n-oxide for the photochemical dehydrogenation. Tetrahedron 1988, 44, 3187–3194. [Google Scholar] [CrossRef]
- Kumar, D.; Patel, G.; Kumar, A.K.; Roy, R. Ionic liquid catalyzed expeditious synthesis of 2-aryl-2,3-dihydroquinolin-4(1H)-ones and 2-aryl-2,3-dihydro-4H-chromen-4-ones under microwave irradiation. J. Heterocycl. Chem. 2009, 46, 791–795. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, X.; Yin, Z.; Xie, J. An efficient catalytic synthesis of flavanones under green conditions. J. Chem. Res. 2011, 35, 220–221. [Google Scholar] [CrossRef]
- Son, S.H.; Cho, Y.Y.; Yoo, H.-S.; Lee, S.J.; Kim, Y.M.; Jang, H.J.; Kim, D.H.; Shin, J.-W.; Kim, N.-J. Divergent synthesis of flavones and flavanones from 2′-hydroxydihydrochalcones via palladium(ii)-catalyzed oxidative cyclization. RSC Adv. 2021, 11, 14000–14006. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, M.; Simoens, M.; Falchi, G.; Lavaggi, M.L.; Piro, O.E.; Castellano, E.E.; Vidal, A.; Azqueta, A.; Monge, A.; de Ceráin, A.L.; et al. Synthetic chalcones, flavanones, and flavones as antitumoral agents: Biological evaluation and structure–activity relationships. Bioorg. Med. Chem. 2007, 15, 3356–3367. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.S.; Kondhare, D.D.; Varala, R.; Zubaidha, P.K. Cyclization of 2′-hydroxychalcones to flavones using ammonium iodide as an iodine source: An eco-friendly approach. J. Serb. Chem. Soc. 2013, 78, 909–916. [Google Scholar] [CrossRef]
- Gula´csi, K.; Litkeia, G.R.; Antus, S.N.; Gunda, T.S.E. A short and facile synthetic route to prenylated flavones. Cyclodehydrogenation of prenylated 2′-hydroxychalcones by a hypervalent iodine reagent. Tetrahedron 1998, 54, 13867–13876. [Google Scholar] [CrossRef]
- Lamba, M.; Makrandi, J.K. Sodium selenite-dimethylsulfoxide: A highly efficient reagent for dehydrogenation. J. Chem. Res. 2008, 2008, 225–226. [Google Scholar] [CrossRef]
- Ahmed, N.; Ali, H.; van Lier, J.E. Silica gel supported InBr3 and InCl3: New catalysts for the facile and rapid oxidation of 2′-hydroxychalcones and flavanones to their corresponding flavones under solvent free conditions. Tetrahedron Lett. 2005, 46, 253–256. [Google Scholar] [CrossRef]
- Du, Z.; Ng, H.; Zhang, K.; Zeng, H.; Wang, J. Ionic liquid mediated Cu-catalyzed cascade oxa-Michael-oxidation: Efficient synthesis of flavones under mild reaction conditions. Org. Biomol. Chem. 2011, 9, 6930–6933. [Google Scholar] [CrossRef]
- Yukio, H.; Toshinori, O.; Noboru, T. The direct preparation of flavones from 2′-hydroxychalcones using disulfides. Bull. Chem. Soc. Jpn. 1986, 59, 2351–2352. [Google Scholar] [CrossRef]
- Zambare, A.S.; Sangshetti, J.N.; Kokare, N.D.; Shinde, D.B. Development of mild and efficient method for synthesis of substituted flavones using oxalic acid catalyst. Chin. Chem. Lett. 2009, 20, 171–174. [Google Scholar] [CrossRef]
- Wang, Z. Allan-Robinson Condensation. In Comprehensive Organic Name Reactions; Wiley: Hoboken, NJ, USA, 2010; pp. 64–67. [Google Scholar]
- Horie, T.; Kawamura, Y.; Tsukayama, M.; Yoshikazi, S. Studies of the selective O-alkylation and dealkylation of flavonoids. XII.: A new, convenient method for synthesizing 3, 5-dihydroxy-6, 7-dimethoxyflavones from 3, 5, 6, 7-tetramethoxyflavones. Chem. Pharm. Bull. 1989, 37, 1216–1220. [Google Scholar] [CrossRef]
- Li, J.J. Baker-Venkataraman rearrangement. In Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 14–15. [Google Scholar]
- Limberakis, C. Other Six-Membered Heterocycles. In Name Reactions in Heterocyclic Chemistry; Li, J.J., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 521–533. [Google Scholar]
- DeMeyer, N.H.A.; Mishra, L.; Pandey, H.-K.; Pieters, L.A.C.; Vanden Berghe, D.A.; Vlietinick, A.J. 4′-Hydroxy-3-methoxyflavones with potent antipicornavirus activity. J. Med. Chem. 1991, 34, 736–746. [Google Scholar] [CrossRef]
- Wang, Z. Mentzer pyrone synthesis. In Comprehensive Organic Name Reactions; Wang, Z., Ed.; Wiley: Hoboken, NJ, USA, 2010; pp. 1901–1904. [Google Scholar]
- Seijas, J.A.; Vázquez-Tato, M.P.; Carballido-Reboredo, R. Solvent-free synthesis of functionalized flavones under microwave irradiation. J. Org. Chem. 2005, 70, 2855–2858. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Gonçalves, C.; Martins, B.T.; Palmeira, A.; Vasconcelos, V.; Pinto, M.; Almeida, J.R.; Correia-da-Silva, M.; Cidade, H. Flavonoid glycosides with a triazole moiety for marine antifouling applications: Synthesis and biological activity evaluation. Mar. Drugs 2021, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Kshatriya, R.; Jejurkar, V.P.; Saha, S. In memory of Prof. Venkataraman: Recent advances in the synthetic methodologies of flavones. Tetrahedron 2018, 74, 811–833. [Google Scholar] [CrossRef]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.R. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. Biomed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef]
- Selepe, M.A.; Van Heerden, F.R. Application of the Suzuki-Miyaura reaction in the synthesis of flavonoids. Molecules 2013, 18, 4739–4765. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Hurtová, M.; Biedermann, D.; Osifová, Z.; Cvačka, J.; Valentová, K.; Křen, V. Preparation of synthetic and natural derivatives of flavonoids using Suzuki-Miyaura cross-coupling reaction. Molecules 2022, 27, 967. [Google Scholar] [CrossRef]
- Liu, Z.-Q. What about the progress in the synthesis of flavonoid from 2020? Eur. J. Med. Chem. 2022, 243, 114671. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Cidade, H.; Pinto, M.; Tiritan, M.E. Chiral flavonoids as antitumor agents. Pharmaceuticals 2021, 14, 1267. [Google Scholar] [CrossRef]
- Marais, J.P.; Ferreira, D.; Slade, D. Stereoselective synthesis of monomeric flavonoids. Phytochemistry 2005, 66, 2145–2176. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-F.; Sun, Q.; Wang, D.; Liu, S.; Lin, B.; Liu, C.-T.; Li, L.-Z.; Huang, X.-X.; Song, S.-J. Chiral separation of cytotoxic flavan derivatives from Daphne giraldii. J. Nat. Prod. 2016, 79, 2236–2242. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Yao, G.-D.; Song, X.-Y.; Qi, X.-L.; Xi, Y.-F.; Li, L.-Z.; Huang, X.-X.; Song, S.-J. Autophagy antagonizes apoptosis induced by flavan enantiomers from Daphne giraldii in hepatic carcinoma cells in vitro. Eur. J. Med. Chem. 2017, 133, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.-D.; Sun, Q.; Song, X.-Y.; Huang, X.-X.; Song, S.-J. Flavan enantiomers from Daphne giraldii selectively induce apoptotic cell death in p53-null hepatocarcinoma cells in vitro. Chem. Biol. Interact. 2018, 289, 1–8. [Google Scholar] [CrossRef]
- Xu, L.; Huang, T.; Huang, C.; Wu, C.; Jia, A.; Hu, X. Chiral separation, absolute configuration, and bioactivity of two pairs of flavonoid enantiomers from Morus nigra. Phytochemistry 2019, 163, 33–37. [Google Scholar] [CrossRef]
- Hofmann, J.; Fayez, S.; Scheiner, M.; Hoffmann, M.; Oerter, S.; Appelt-Menzel, A.; Maher, P.; Maurice, T.; Bringmann, G.; Decker, M. Sterubin: Enantioresolution and configurational stability, enantiomeric purity in nature, and neuroprotective activity in vitro and in vivo. Chem. Eur. J. 2020, 26, 7299–7308. [Google Scholar] [CrossRef]
- Marais, J.P.J.; Deavours, B.; Dixon, R.A.; Ferreira, D. The Stereochemistry of Flavonoids. In The Science of Flavonoids; Grotewold, E., Ed.; Springer: New York, NY, USA, 2006; pp. 1–46. [Google Scholar]
- Helder, R.; Hummelen, J.C.; Laane, R.W.P.M.; Wiering, J.S.; Wynberg, H. Catalytic asymmetric induction in oxidation reactions. The synthesis of optically active epoxides. Tetrahedron Lett. 1976, 17, 1831–1834. [Google Scholar] [CrossRef]
- Juliá, S.; Guixer, J.; Masana, J.; Rocas, J.; Colonna, S.; Annuziata, R.; Molinari, H. Synthetic enzymes. Part 2. Catalytic asymmetric epoxidation by means of polyamino-acids in a triphase system. J. Chem. Soc. Perkin Trans. 1982, 1, 1317–1324. [Google Scholar] [CrossRef]
- Bentley, P.A.; Bergeron, S.; Cappi, M.W.; Hibbs, D.E.; Hursthouse, M.B.; Nugent, T.C.; Pulido, R.; Roberts, S.M.; Wu, L.E. Asymmetric epoxidation of enones employing polymeric α-amino acids in non-aqueous media. Chem. Commun. 1997, 8, 739–740. [Google Scholar] [CrossRef]
- Nel, R.J.J.; van Rensburg, H.; van Heerden, P.S.; Coetzee, J.; Ferreira, D. Stereoselective synthesis of flavonoids. Part 7. Poly-oxygenated β-hydroxydihydrochalcone derivatives. Tetrahedron 1999, 55, 9727–9736. [Google Scholar] [CrossRef]
- Jew, S.-S.; Kim, H.-A.; Bae, S.-Y.; Kim, J.-H.; Park, H.-G. Enantioselective synthetic method for 3-hydroxyflavanones: An approach to (2R,3R)-3′,4′-O-dimethyltaxifolin. Tetrahedron Lett. 2000, 41, 7925–7928. [Google Scholar] [CrossRef]
- van Rensburg, H.S.; van Heerden, P.; Ferreira, D. Enantioselective synthesis of flavonoids. Part 3.1trans- and cis-Flavan-3-ol methyl ether acetates. J. Chem. Soc. Perkin Trans. 1 1997, 1997, 3415–3422. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Condensed tannins, a viable solution to meet the need for sustainable and effective multifunctionality in food packaging: Structure, sources, and properties. J. Agric. Food Chem. 2022, 70, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.B.; Chan, T.H. Enantioselective synthesis of afzelechin and epiafzelechin. Tetrahedron 2004, 60, 8207–8211. [Google Scholar] [CrossRef]
- Pinard, E.; Gaudry, M.; Hénot, F.; Thellend, A. Asymmetric total synthesis of (+)-pisatin, a phytoalexin from garden peas (Pisum sativum L.). Tetrahedron Lett. 1998, 39, 2739–2742. [Google Scholar] [CrossRef]
- Engler, T.A.; Letavic, M.A.; Iyengar, R.; LaTessa, K.O.; Reddy, J.P. Asymmetric reactions of 2-methoxy-1,4-benzoquinones with styrenyl systems: enantioselective syntheses of 8-aryl-3-methoxybicyclo[4.2.0]oct-3-en-2,5-diones, 7-aryl-3-hydroxybicyclo[3.2.1]oct-3-en-2,8-diones, 2-aryl-6-methoxy-2,3-dihydrobenzofuran-5-ols, and pterocarpans. J. Org. Chem. 1999, 64, 2391–2405. [Google Scholar] [CrossRef]
- Chinnabattigalla, S.; Dakoju, R.K.; Gedu, S. Recent advances on the synthesis of flavans, isoflavans, and neoflavans. J. Heterocycl. Chem. 2021, 58, 415–441. [Google Scholar] [CrossRef]
- Versteeg, M.; Bezuidenhoudt, B.C.B.; Ferreira, D.; Swart, K.J. The first enantioselective synthesis of isoflavonoids: (R)- and (S)-isoflavans. J. Chem. Soc. Chem. Commun. 1995, 1317–1318. [Google Scholar] [CrossRef]
- Xu, D.; Hu, J.; Chen, L.; Chen, L.; Su, J.; Yang, J.; Deng, S.; Zhang, H.; Xie, W. Asymmetric synthesis of ent-fissistigmatin C. Org. Lett. 2021, 23, 93–96. [Google Scholar] [CrossRef]
- Vicario, J.L.; Badía, D.; Domínguez, E.; Rodríguez, M.; Carrillo, L. The first stereocontrolled synthesis of isoflavanones. Tetrahedron Lett. 2000, 41, 8297–8300. [Google Scholar] [CrossRef]
- Biddle, M.M.; Lin, M.; Scheidt, K.A. Catalytic enantioselective synthesis of flavanones and chromanones. J. Am. Chem. Soc. 2007, 129, 3830–3831. [Google Scholar] [CrossRef] [PubMed]
- Poisson, T.; Gembus, V.; Dalla, V.; Oudeyer, S.; Levacher, V. Organocatalyzed enantioselective protonation of silyl enol ethers: Scope, limitations and application to the preparation of enantioenriched homoisoflavones. J. Org. Chem. 2010, 75, 7704–7716. [Google Scholar] [CrossRef] [PubMed]
- Lestini, E.; Blackman, L.D.; Zammit, C.M.; Chen, T.; Williams, R.J.; Inam, M.; Couturaud, B.; O’Reilly, R.K. Palladium-polymer nanoreactors for the aqueous asymmetric synthesis of therapeutic flavonoids. Polym. Chem. 2018, 9, 820–823. [Google Scholar] [CrossRef]
- Holder, J.C.; Marziale, A.N.; Gatti, M.; Mao, B.; Stoltz, B.M. Palladium-catalyzed asymmetric conjugate addition of arylboronic acids to heterocyclic acceptors. Chemistry 2013, 19, 74–77. [Google Scholar] [CrossRef]
- Timmerman, J.C.; Sims, N.J.; Wood, J.L. Total synthesis of caesalpinnone A and caesalpinflavan B: Evolution of a concise strategy. J. Am. Chem. Soc. 2019, 141, 10082–10090. [Google Scholar] [CrossRef]
- Iwasaki, K.; Wan, K.K.; Oppedisano, A.; Crossley, S.W.M.; Shenvi, R.A. Simple, chemoselective hydrogenation with thermodynamic stereocontrol. J. Am. Chem. Soc. 2014, 136, 1300–1303. [Google Scholar] [CrossRef]
- Yang, J.; Lai, J.; Kong, W.; Li, S. Asymmetric synthesis of sakuranetin-relevant flavanones for the identification of new chiral antifungal leads. J. Agric. Food Chem. 2022, 70, 3409–3419. [Google Scholar] [CrossRef]
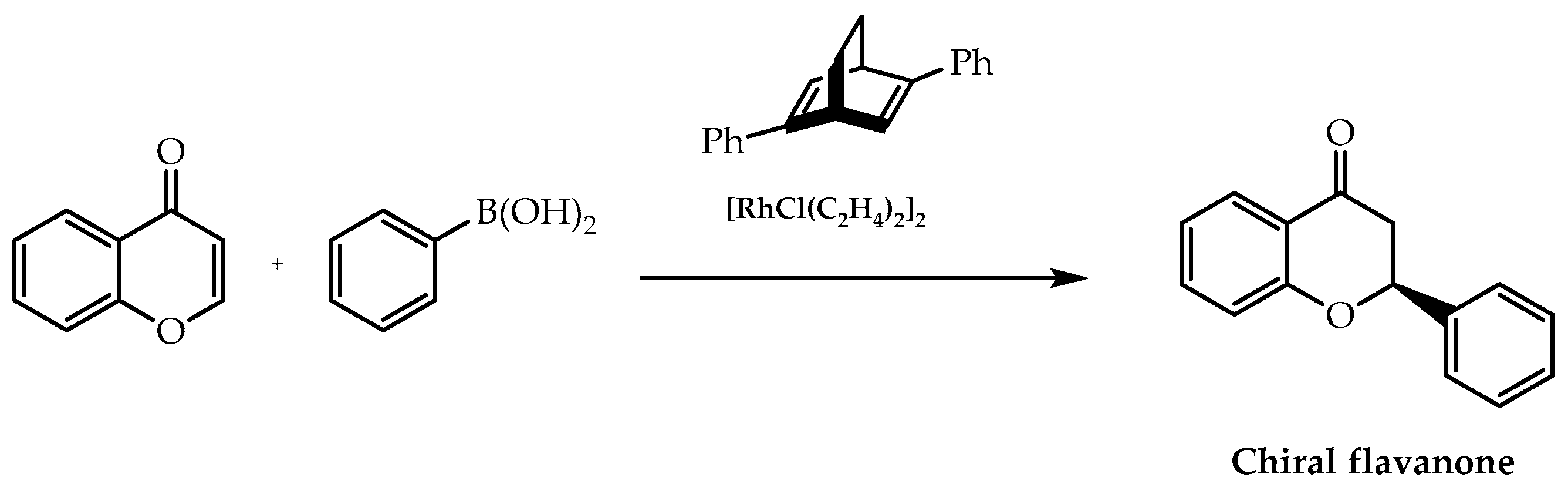
- He, Q.; So, C.M.; Bian, Z.; Hayashi, T.; Wang, J. Rhodium/chiral diene-catalyzed asymmetric 1,4-addition of arylboronic acids to chromones: A highly enantioselective pathway for accessing chiral flavanones. Chem. Asian J. 2015, 10, 540–543. [Google Scholar] [CrossRef]
- Chubatsu Nunes, H.H.; Nguyen, T.-D.; Dang, T.-T.T. Chemoenzymatic synthesis of natural products using plant biocatalysts. Curr. Opin. Green Sustain. Chem. 2022, 35, 100627. [Google Scholar] [CrossRef]
- Janeczko, T.; Dymarska, M.; Siepka, M.; Gniłka, R.; Leśniak, A.; Popłoński, J.; Kostrzewa-Susłow, E. Enantioselective reduction of flavanone and oxidation of cis- and trans-flavan-4-ol by selected yeast cultures. J. Mol. Catal. B Enzym. 2014, 109, 47–52. [Google Scholar] [CrossRef]
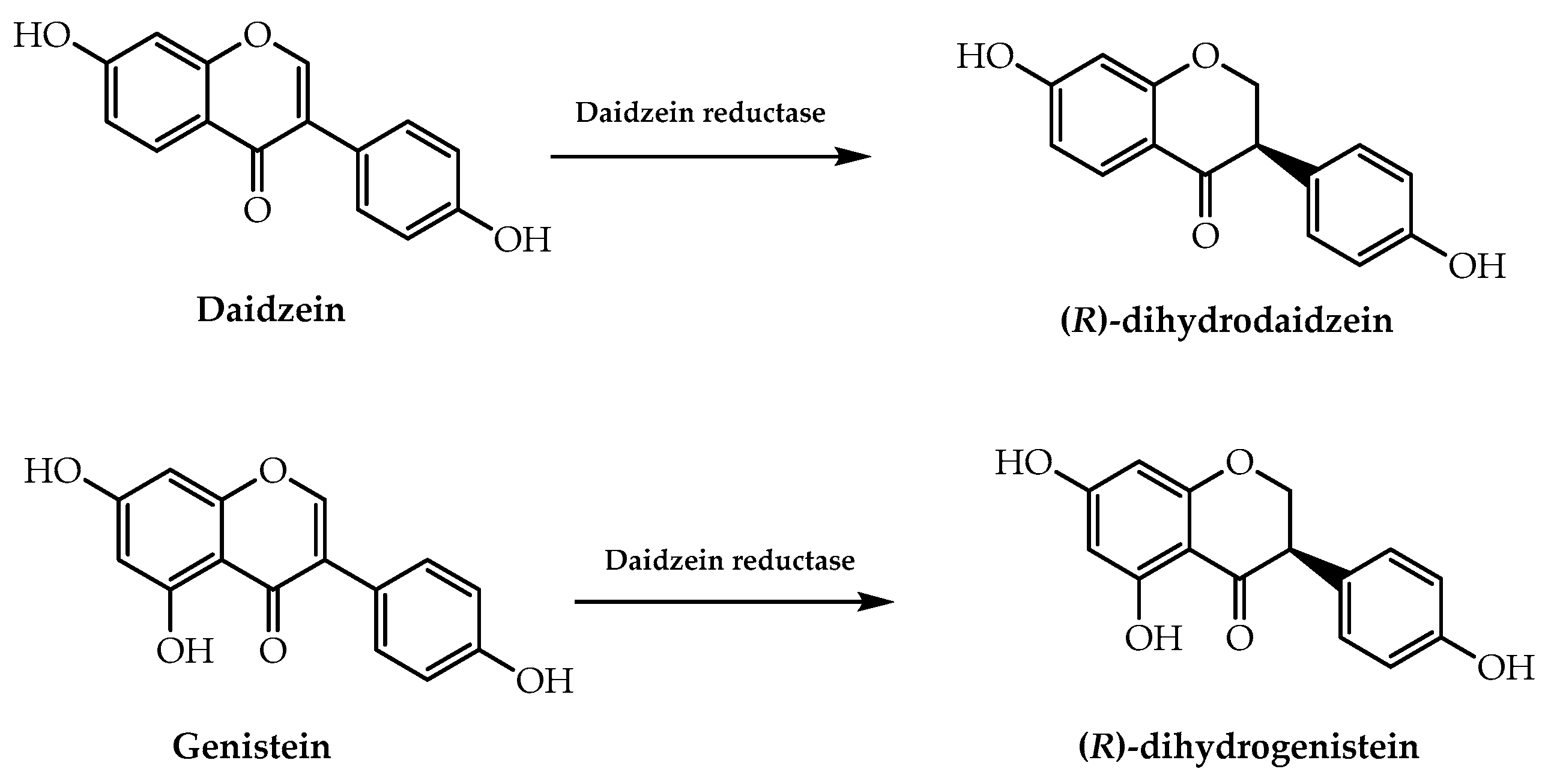
- Kawada, Y.; Goshima, T.; Sawamura, R.; Yokoyama, S.-I.; Yanase, E.; Niwa, T.; Ebihara, A.; Inagaki, M.; Yamaguchi, K.; Kuwata, K.; et al. Daidzein reductase of Eggerthella sp. YY7918, its octameric subunit structure containing FMN/FAD/4Fe-4S, and its enantioselective production of R-dihydroisoflavones. J. Biosci. Bioeng. 2018, 126, 301–309. [Google Scholar] [CrossRef] [PubMed]
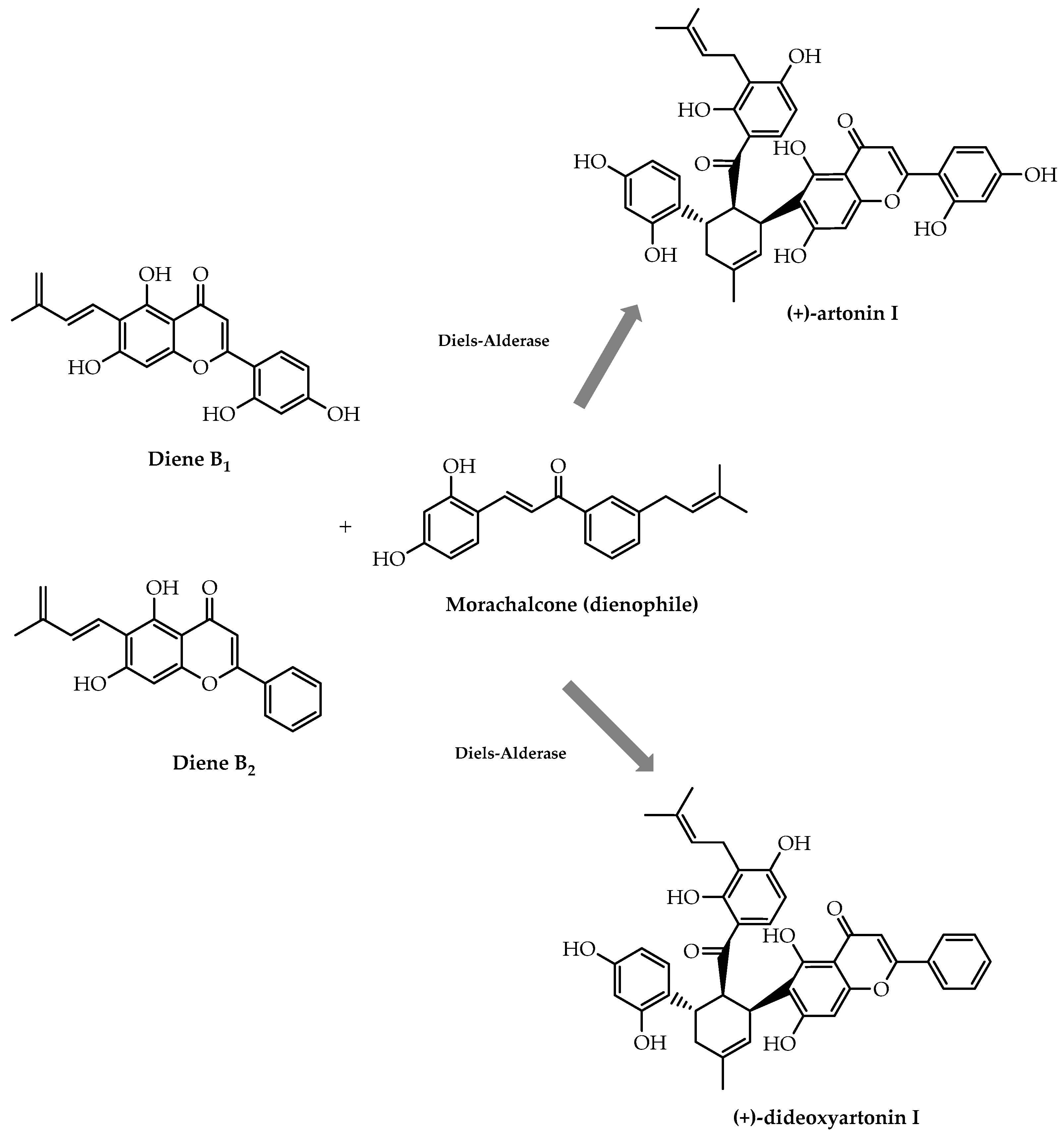
- Liu, X.; Yang, J.; Gao, L.; Zhang, L.; Lei, X. Chemoenzymatic total syntheses of artonin I with an intermolecular Diels–Alderase. Biotechnol. J. 2020, 15, 2000119. [Google Scholar] [CrossRef]
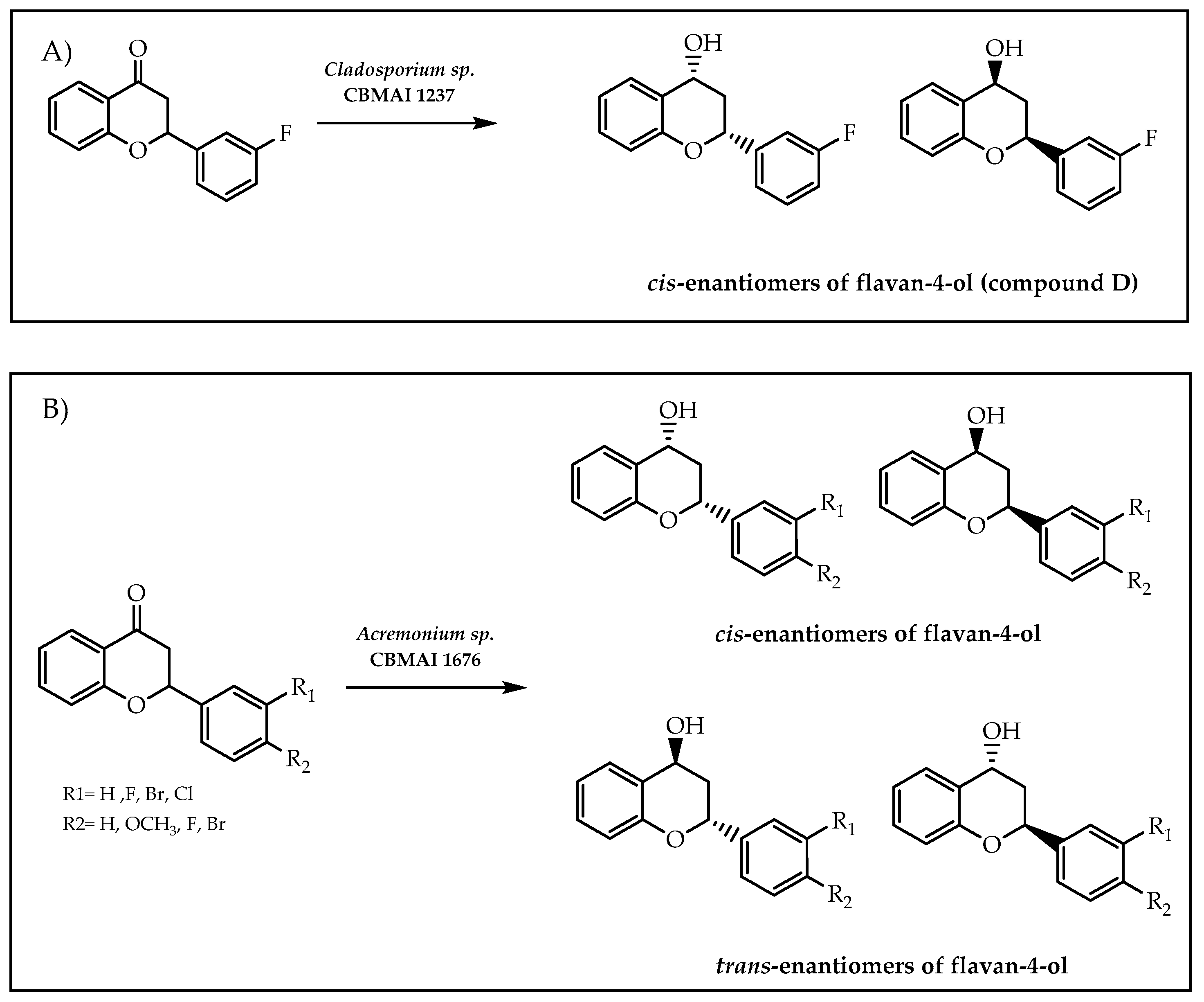
- de Matos, I.L.; Birolli, W.G.; Santos, D.D.A.; Nitschke, M.; Porto, A.L.M. Stereoselective reduction of flavanones by marine-derived fungi. Mol. Catal. 2021, 513, 111734. [Google Scholar] [CrossRef]
- Zhu, Z.-H.; Ding, Y.-X.; Wu, B.; Zhou, Y.-G. Design and synthesis of chiral and regenerable [2.2]paracyclophane-based NAD(P)H models and application in biomimetic reduction of flavonoids. Chem. Sci. 2020, 11, 10220–10224. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, Y.; Ma, J.; He, J.; Zheng, X.; Lei, X.; Jiang, G.; Zhang, L. Synthesis of novel amino acid derivatives containing chrysin as anti-tumor agents against human gastric carcinoma MGC-803 cells. Med. Chem. Res. 2015, 24, 1789–1798. [Google Scholar] [CrossRef]
- Parveen, S.; Tabassum, S.; Arjmand, F. Human topoisomerase I mediated cytotoxicity profile of l-valine-quercetin diorganotin(IV) antitumor drug entities. J. Organomet. Chem. 2016, 823, 23–33. [Google Scholar] [CrossRef]
- Pajtás, D.; Kónya, K.; Kiss-Szikszai, A.; Džubák, P.; Pethő, Z.; Varga, Z.; Panyi, G.; Patonay, T. Optimization of the synthesis of flavone–amino acid and flavone–dipeptide hybrids via Buchwald–Hartwig reaction. J. Org. Chem. 2017, 82, 4578–4587. [Google Scholar] [CrossRef]
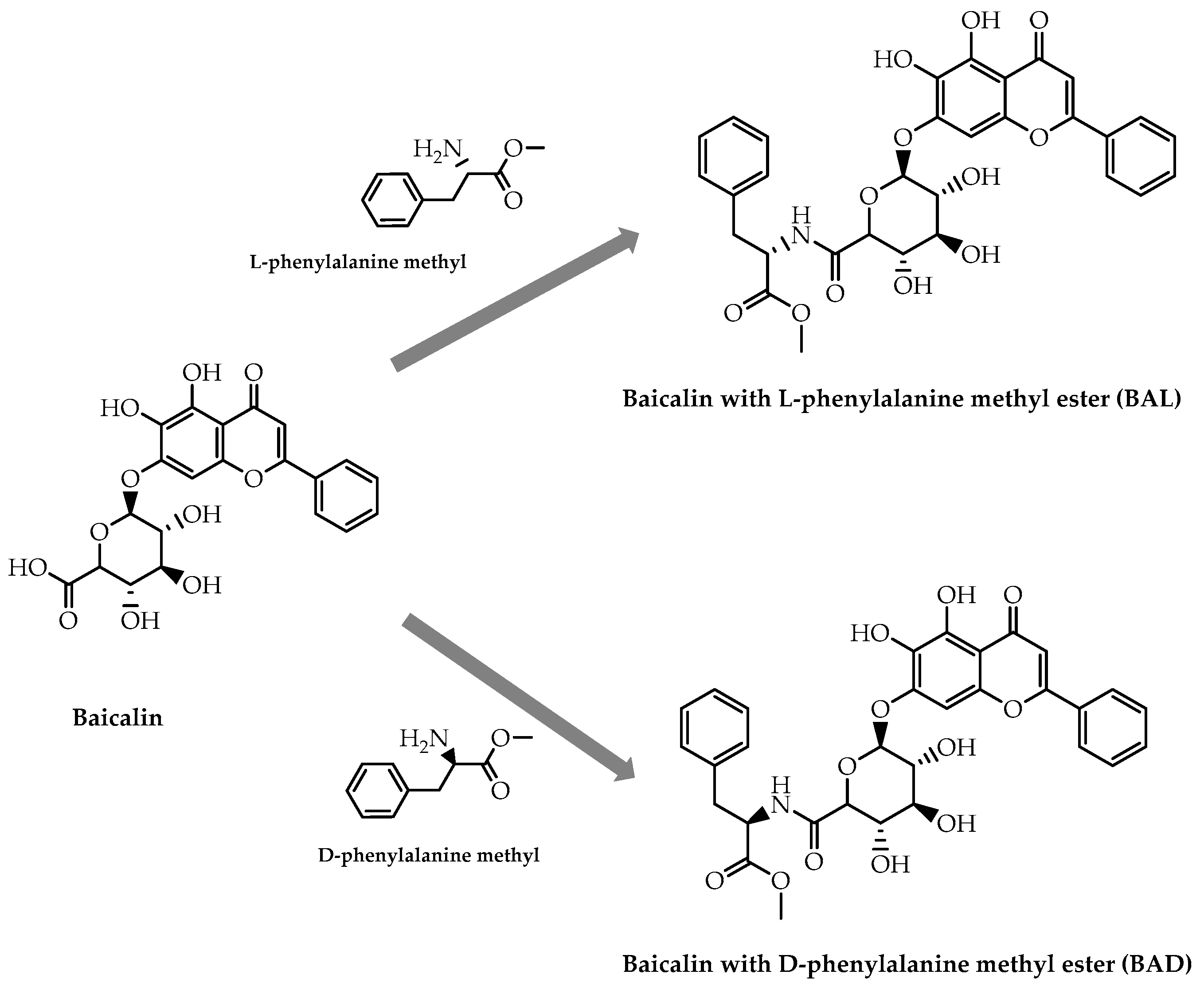
- Hou, Y.; Pi, C.; Feng, X.; Wang, Y.; Fu, S.; Zhang, X.; Zhao, L.; Wei, Y. Antitumor activity in vivo and vitro of new chiral derivatives of baicalin and induced apoptosis via the PI3K/Akt signaling pathway. Mol. Ther. Oncolytics 2020, 19, 67–78. [Google Scholar] [CrossRef]
- Shiraishi, N.; Kumazoe, M.; Fuse, S.; Tachibana, H.; Tanaka, H. The synthesis of trans-flavan-3-ol gallates by regioselective oxidative etherification and their cytotoxicity mediated by 67 LR. Chemistry 2016, 22, 13050–13053. [Google Scholar] [CrossRef]
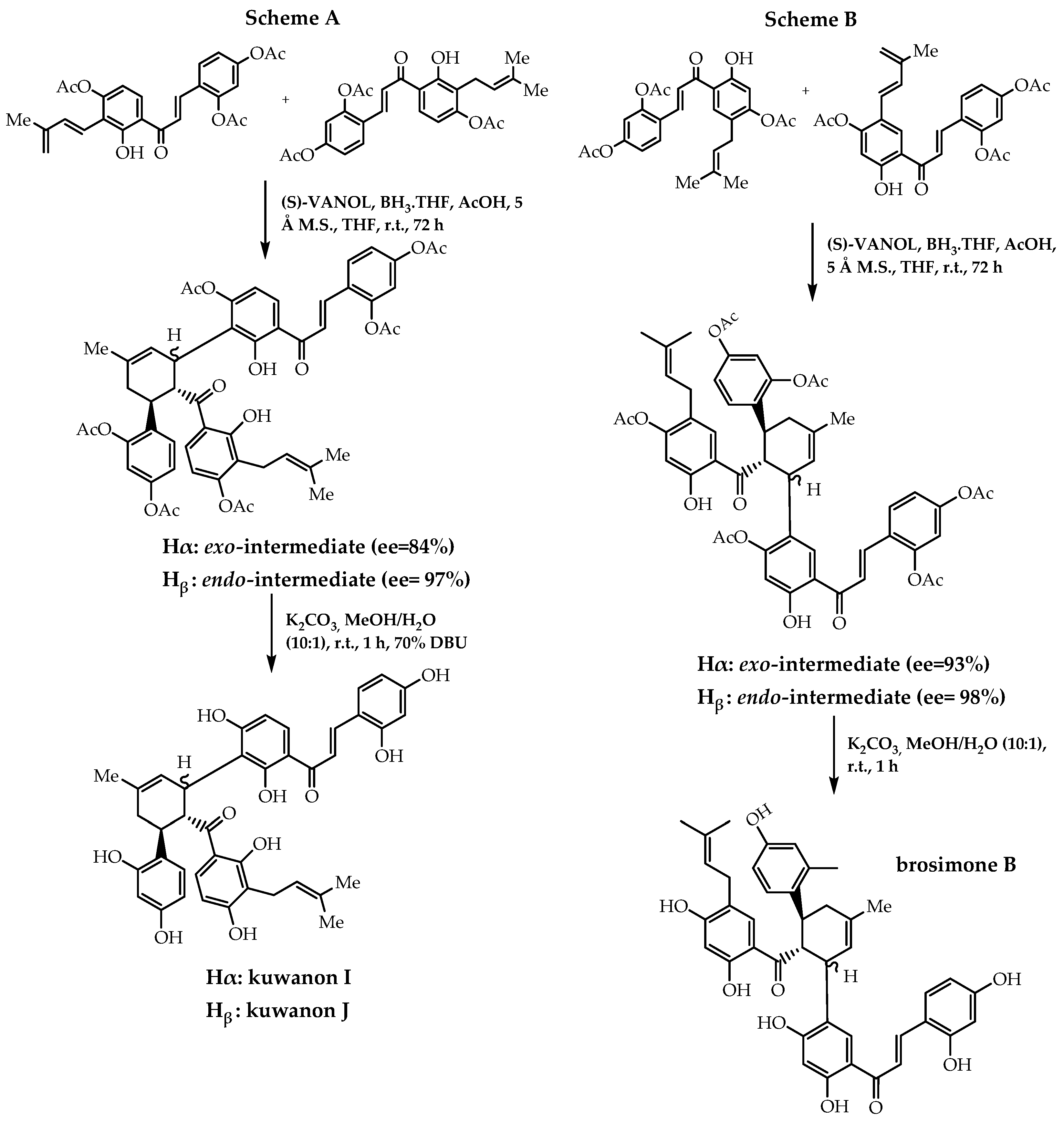
- Han, J.; Li, X.; Guan, Y.; Zhao, W.; Wulff, W.D.; Lei, X. Enantioselective biomimetic total syntheses of kuwanons I and J and brosimones A and B. Angew. Chem. Int. Ed. 2014, 53, 9257–9261. [Google Scholar] [CrossRef]
- Li, X.; Han, J.; Jones, A.X.; Lei, X. Chiral boron complex-promoted asymmetric Diels–Alder cycloaddition and its application in natural product synthesis. J. Org. Chem. 2016, 81, 458–468. [Google Scholar] [CrossRef] [PubMed]
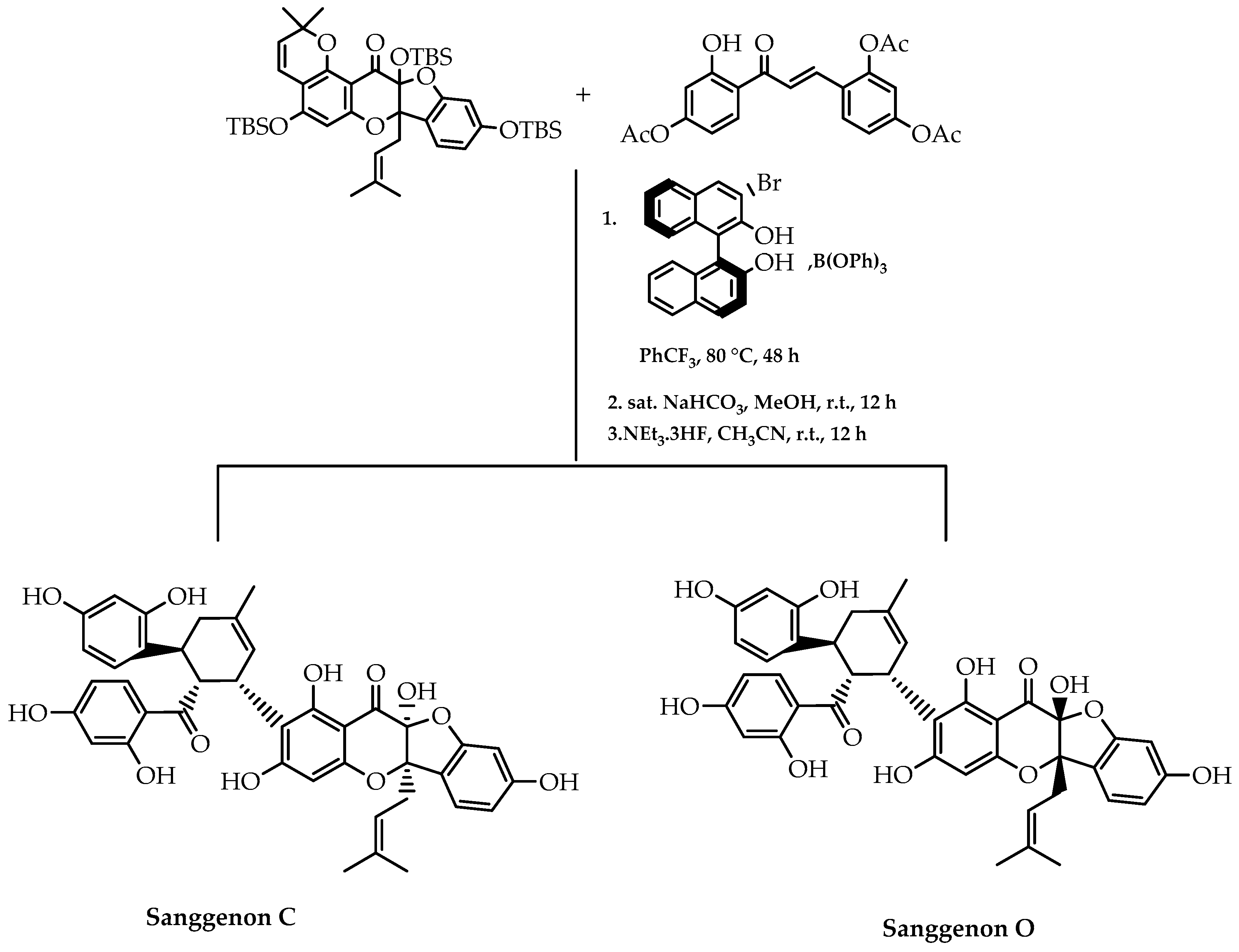
- Qi, C.; Xiong, Y.; Eschenbrenner-Lux, V.; Cong, H.; Porco, J.A., Jr. Asymmetric syntheses of the flavonoid Diels-Alder natural products sanggenons C and O. J. Am. Chem. Soc. 2016, 138, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.-Q.; Hou, Y.; Zhu, L.; Chen, G.; Xu, D.; Zhang, S.-Y.; He, Y.; Xie, W. A bio-inspired synthesis of hybrid flavonoids from 2-hydroxychalcone driven by visible light. RSC Adv. 2019, 9, 29005–29009. [Google Scholar] [CrossRef]
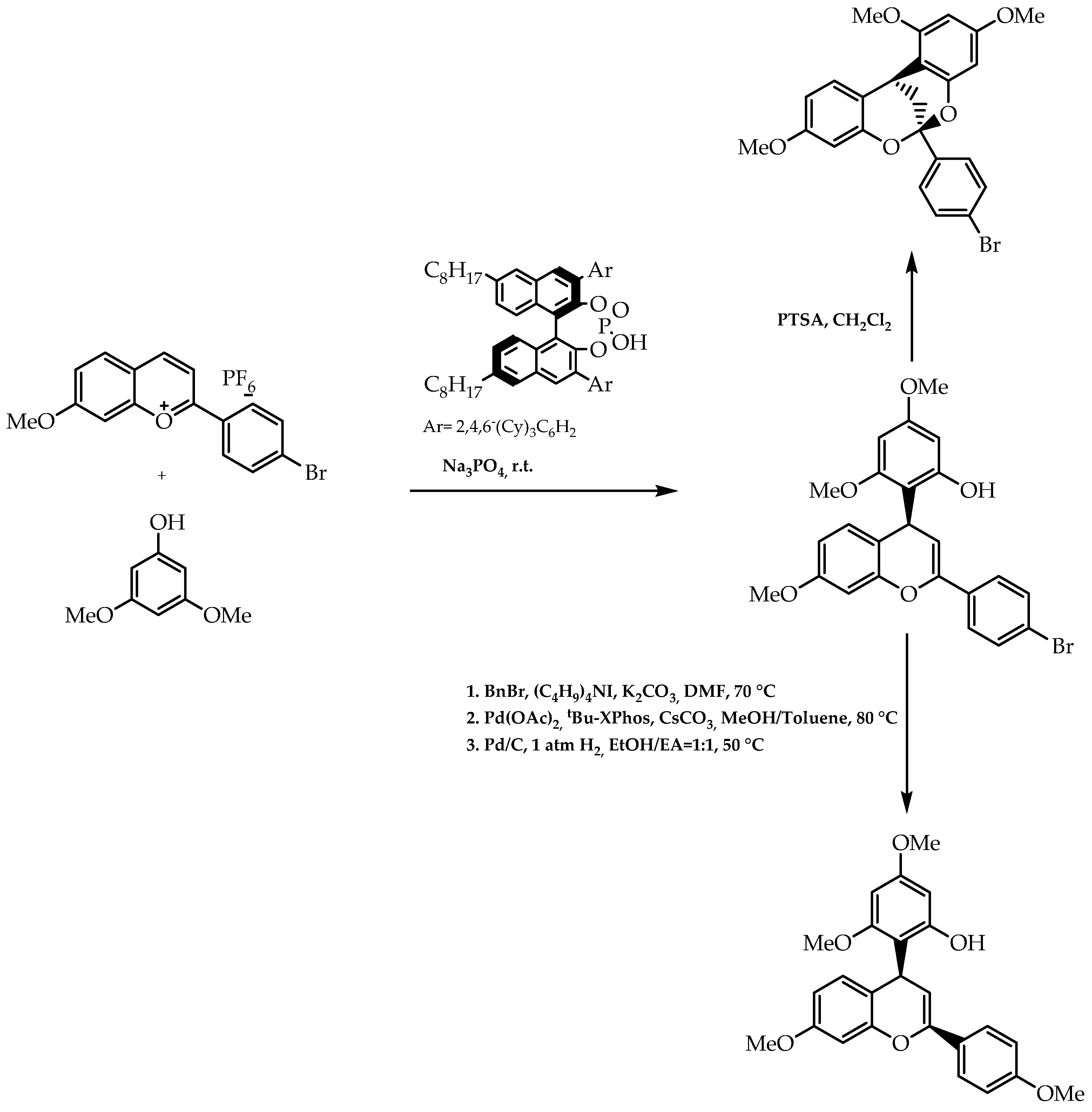
- Yang, Z.; He, Y.; Toste, F.D. Biomimetic approach to the catalytic enantioselective synthesis of flavonoids. J. Am. Chem. Soc. 2016, 138, 9775–9778. [Google Scholar] [CrossRef]
- Qin, T.; Metz, P. Enantioselective synthesis of isoflavanones by catalytic dynamic kinetic resolution. Org. Lett. 2017, 19, 2981–2984. [Google Scholar] [CrossRef]
- Ciesielski, P.; Metz, P. Asymmetric one-pot transformation of isoflavones to pterocarpans and its application in phytoalexin synthesis. Nat. Commun. 2020, 11, 3091. [Google Scholar] [CrossRef]
- Caleffi, G.S.; Brum, J.D.O.C.; Costa, A.T.; Domingos, J.L.O.; Costa, P.R.R. Asymmetric transfer hydrogenation of arylidene-substituted chromanones and tetralones catalyzed by Noyori–Ikariya Ru(II) complexes: One-pot reduction of C=C and C=O bonds. J. Org. Chem. 2021, 86, 4849–4858. [Google Scholar] [CrossRef] [PubMed]
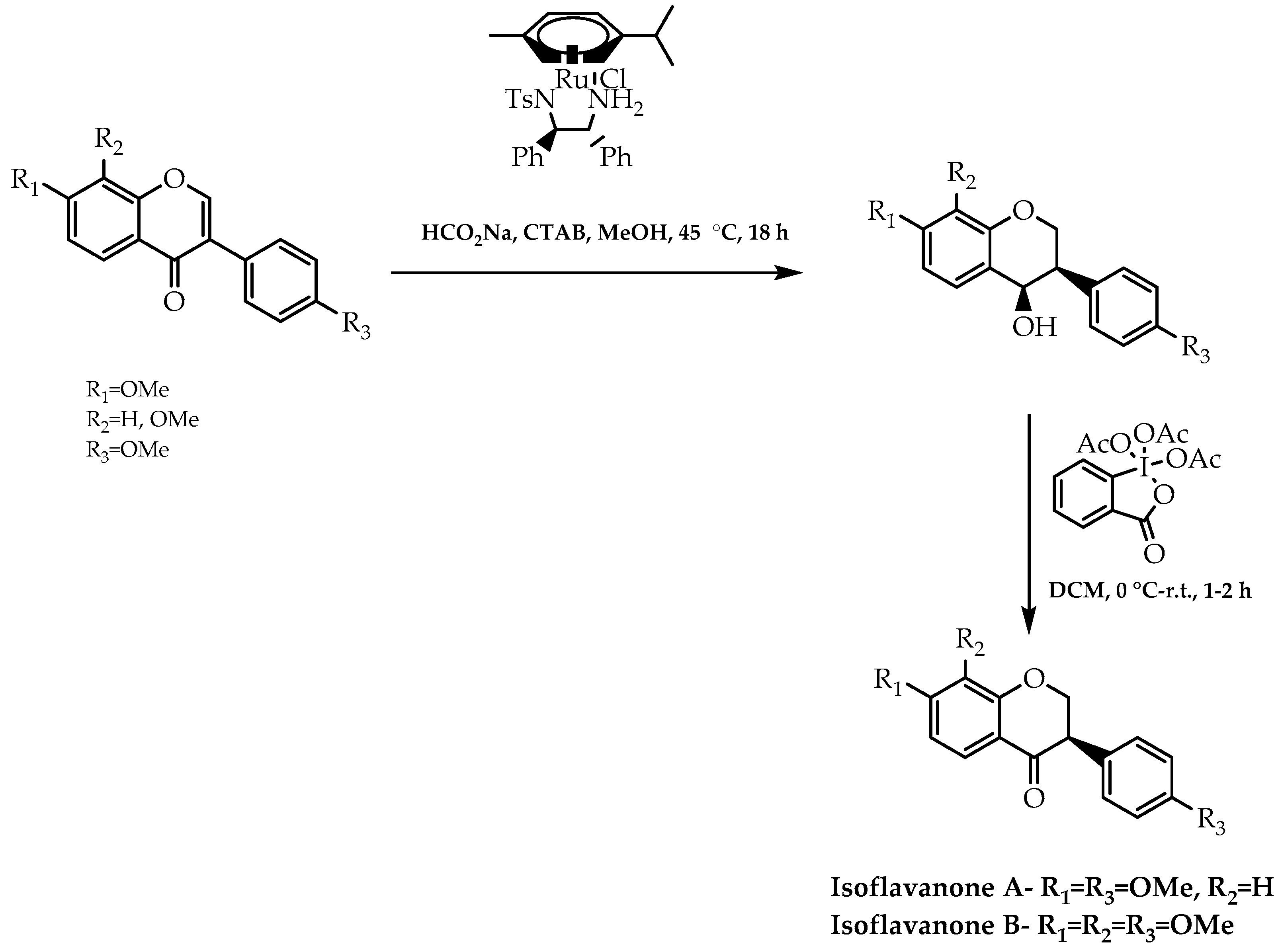
- Gaspar, F.V.; Caleffi, G.S.; Costa-Júnior, P.C.T.; Costa, P.R.R. Enantioselective synthesis of isoflavanones and pterocarpans through a RuII-catalyzed ATH-DKR of isoflavones. ChemCatChem 2021, 13, 5097–5108. [Google Scholar] [CrossRef]
- Al-Maharik, N. Isolation of naturally occurring novel isoflavonoids: An update. Nat. Prod. Rep. 2019, 36, 1156–1195. [Google Scholar] [CrossRef]





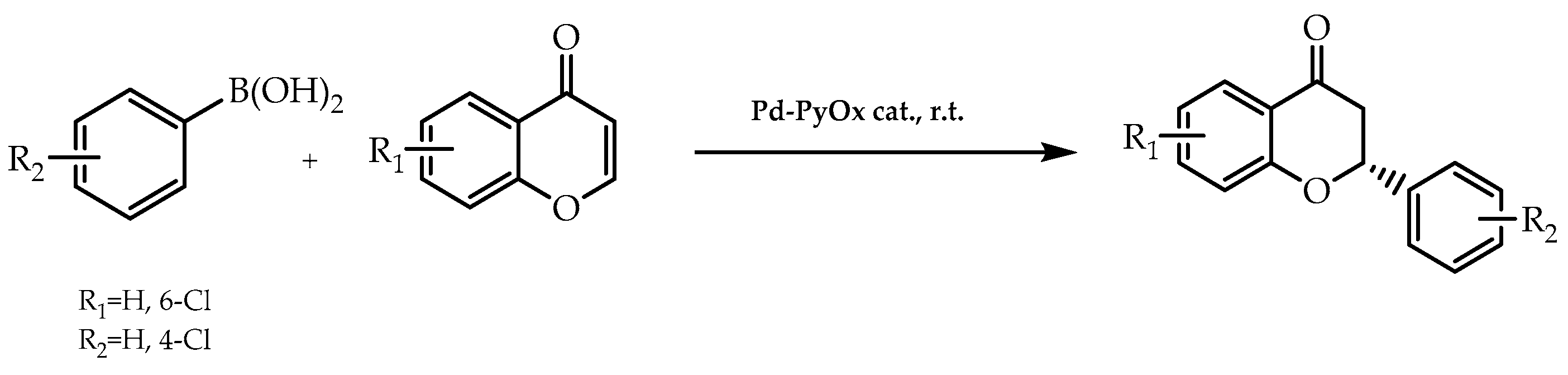






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.M.; Cidade, H.; Tiritan, M.E. Stereoselective Synthesis of Flavonoids: A Brief Overview. Molecules 2023, 28, 426. https://doi.org/10.3390/molecules28010426
Pereira AM, Cidade H, Tiritan ME. Stereoselective Synthesis of Flavonoids: A Brief Overview. Molecules. 2023; 28(1):426. https://doi.org/10.3390/molecules28010426
Chicago/Turabian StylePereira, Ana Margarida, Honorina Cidade, and Maria Elizabeth Tiritan. 2023. "Stereoselective Synthesis of Flavonoids: A Brief Overview" Molecules 28, no. 1: 426. https://doi.org/10.3390/molecules28010426
APA StylePereira, A. M., Cidade, H., & Tiritan, M. E. (2023). Stereoselective Synthesis of Flavonoids: A Brief Overview. Molecules, 28(1), 426. https://doi.org/10.3390/molecules28010426








