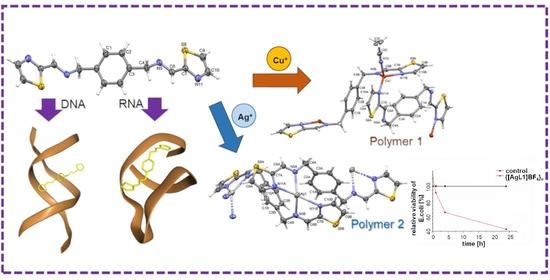
New N4-Donor Ligands as Supramolecular Guests for DNA and RNA: Synthesis, Structural Characterization, In Silico, Spectrophotometric and Antimicrobial Studies
Abstract
1. Introduction
2. Results
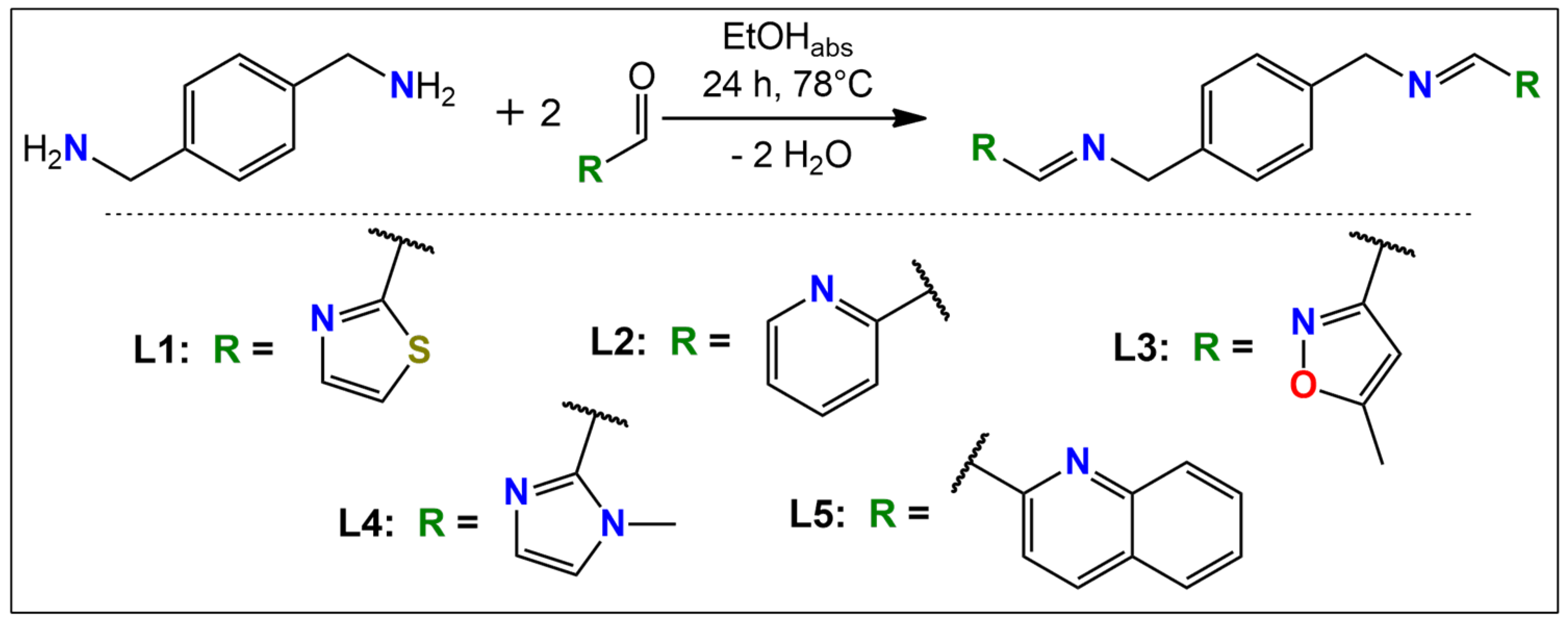
2.1. Design and Synthesis
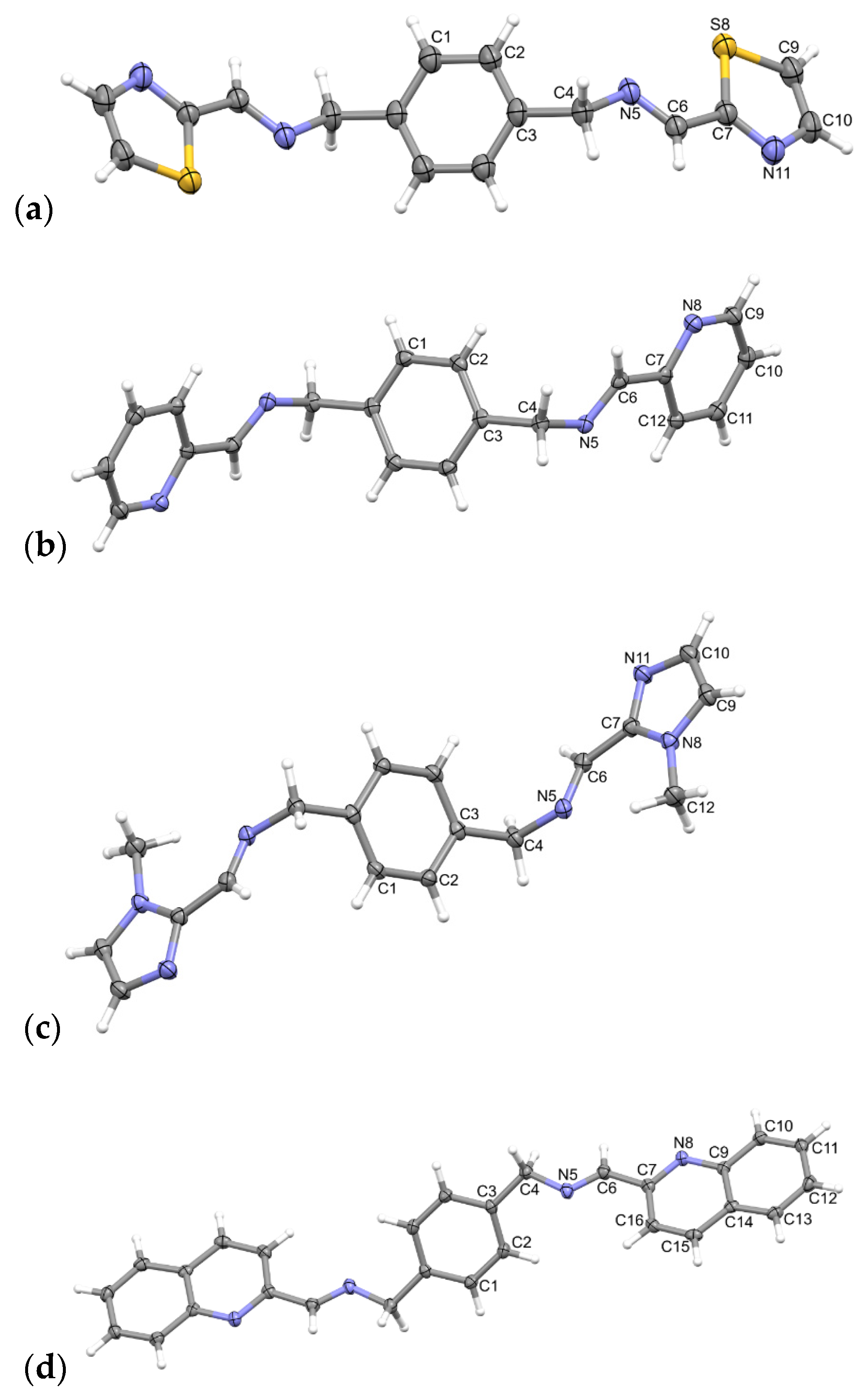
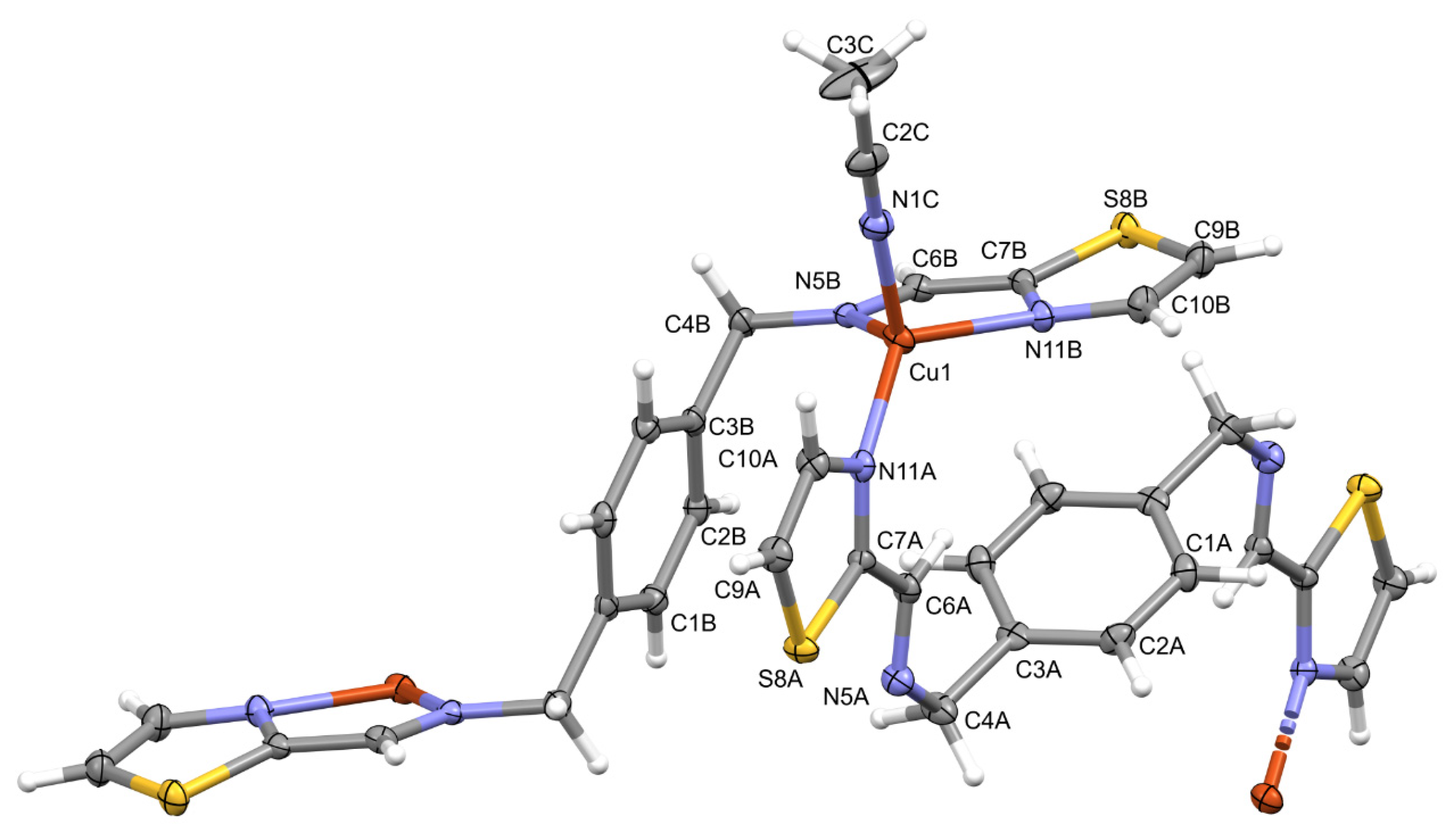
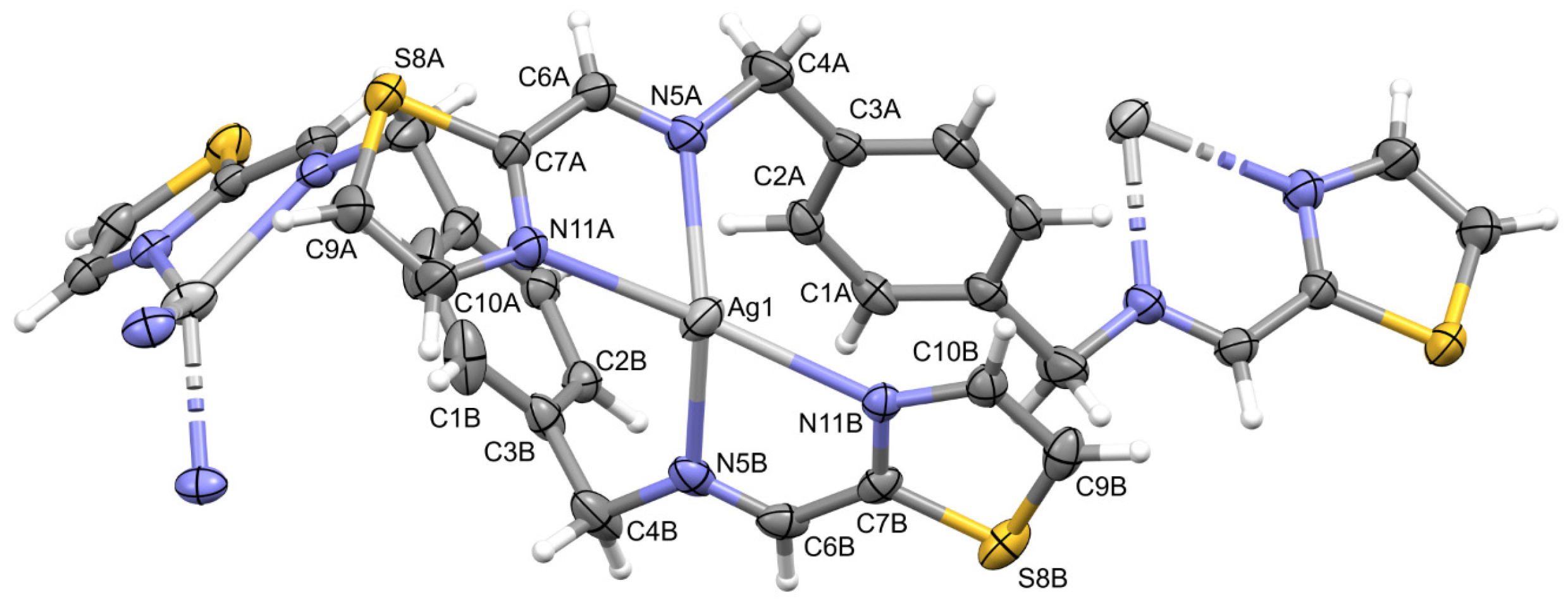
2.2. Description of Structures
| MW [g/mol] | cLogP | Num. of H-Bond Acceptors | Num. of H-Bond Donors | tPSA [Å]2 | WLogP | MR | Number of Atoms | Num. of Rotatable Bonds | XlogP3 | Number of Rings | Num. of Carbons | Number of Heteroatoms | Lipinski/Ghose/ Veber/Egan/ Muegge Violations | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 326.44 | 3.31 | 4 | 0 | 106.98 | 3.53 | 93.68 | 36 | 6 | 3.12 | 3 | 16 | 6 | 0/0/0/0/0 |
| L2 | 314.38 | 3.09 | 4 | 0 | 50.50 | 3.41 | 97.92 | 42 | 6 | 2.92 | 3 | 20 | 4 | 0/0/0/0/0 |
| L3 | 322.36 | 2.95 | 6 | 0 | 76.78 | 3.21 | 92.39 | 42 | 6 | 2.76 | 3 | 18 | 6 | 0/0/0/0/0 |
| L4 | 320.39 | 1.77 | 4 | 0 | 60.36 | 2.09 | 96.43 | 44 | 6 | 1.24 | 3 | 18 | 6 | 0/0/0/0/0 |
| L5 | 414.50 | 5.13 | 4 | 0 | 50.50 | 5.72 | 132.93 | 54 | 6 | 5.60 | 5 | 28 | 4 | 1/1/0/0/1 |
| Lipinski [29] | ≤500 | ≤5 | ≤10 | ≤5 | ≤140 | |||||||||
| Ghose [30] | 160 ≤ MW ≤ 480 | −0.4 ≤ Wlog P≤ 5.6 | 40 ≤ MR ≤ 130 | 20 ≤ atoms ≤ 70 | ||||||||||
| Veber [31] | ≤140 | ≤10 | ||||||||||||
| Egan [32] | ≤131.6 | ≤5.88 | ||||||||||||
| Muegge [33] | 200 ≤ MW ≤ 500 | ≤10 | ≤5 | ≤150 | ≤15 | −2 ≤ XlogP3 ≤ 5 | ≤7 | >4 | > 1 |
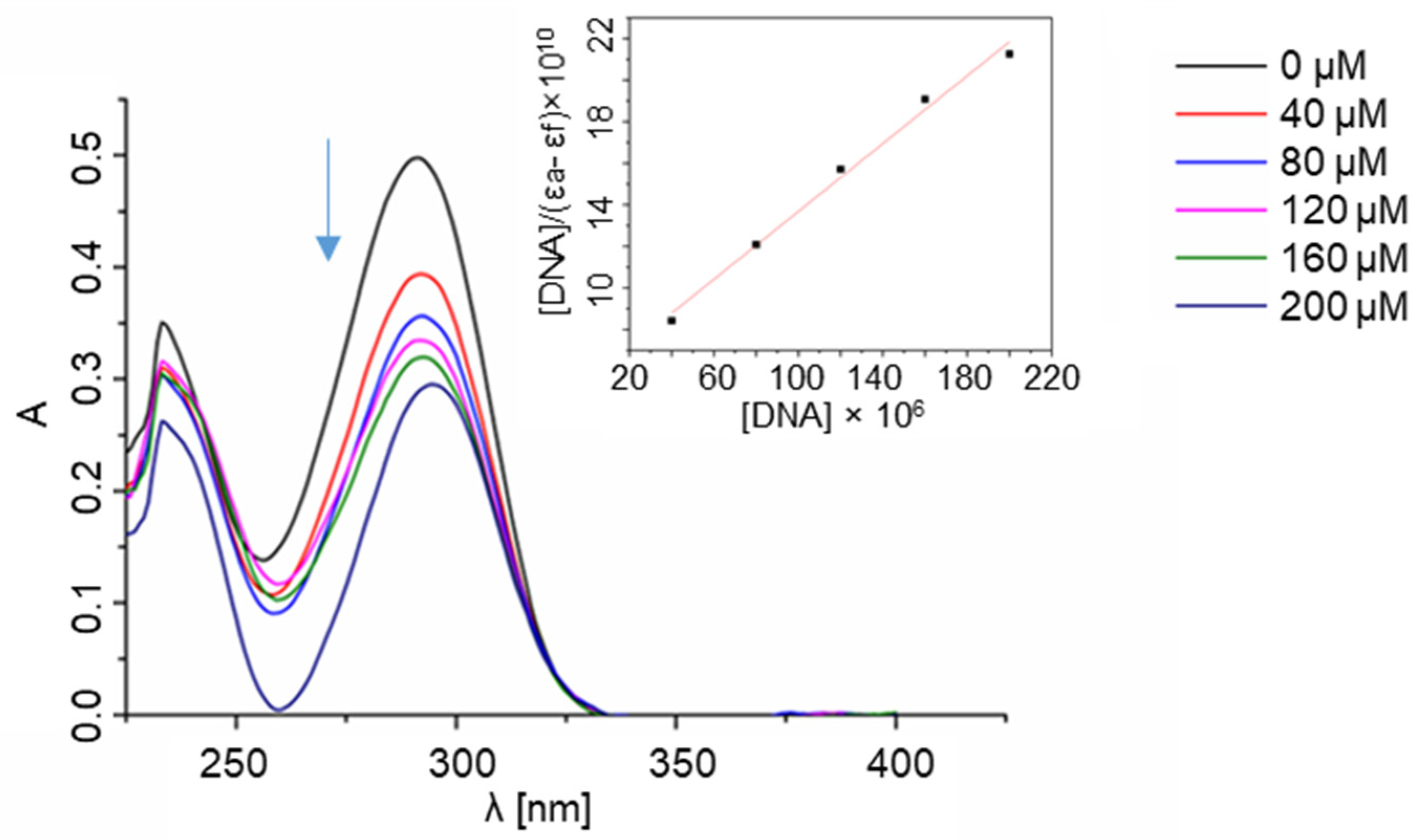
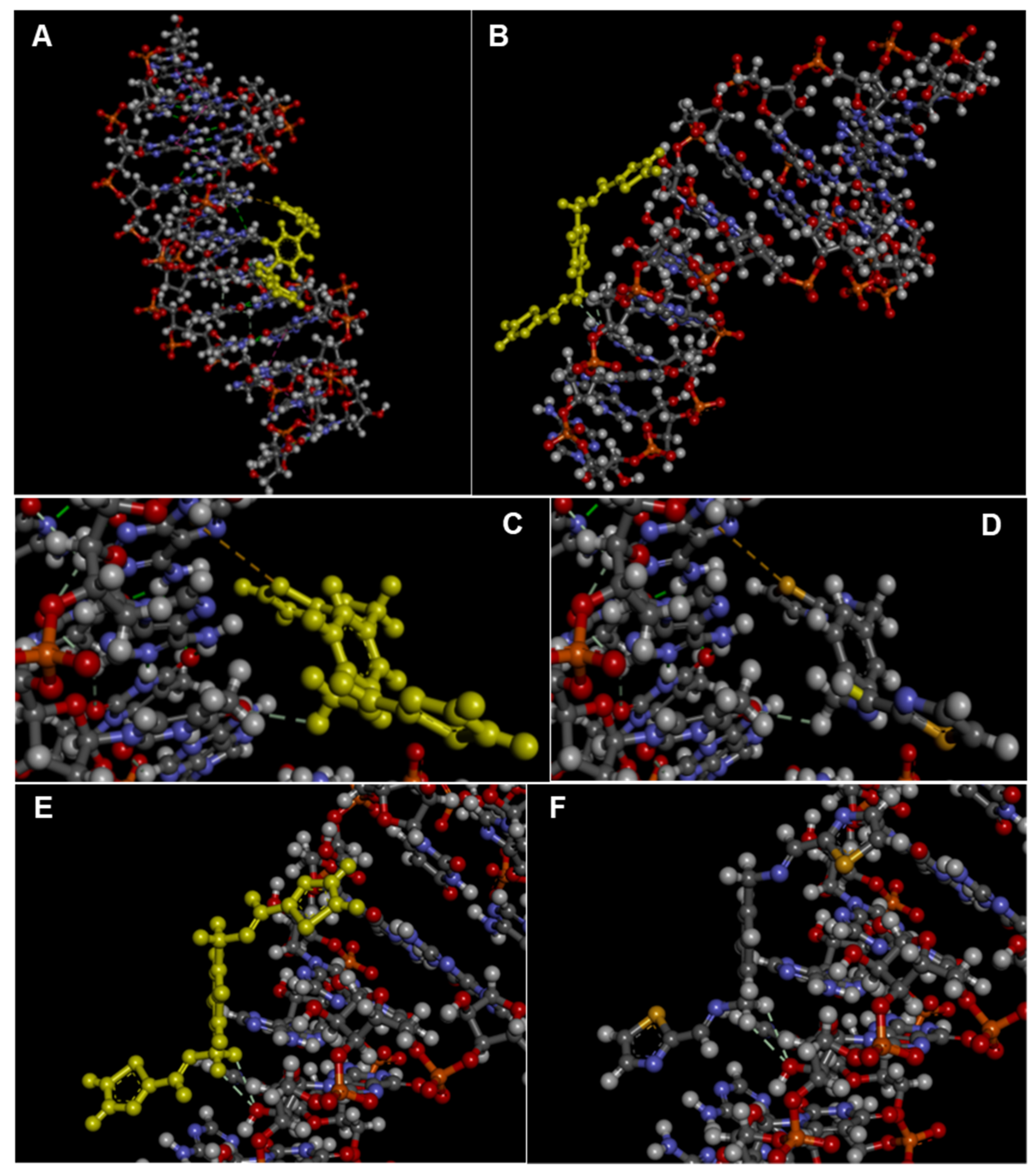
2.3. Interactions with Nucleic Acids


| HDOCK Score Top-1 Ranked Pose | HDOCK Score (Top-1–3 Poses) ± SD | HDOCK Score (Top-1–10 Poses) ± SD | Interface Residues | |
|---|---|---|---|---|
| DNA * | −70.22 | −69.45 ± 0.69 | −66.76 ± 2.37 | dA5, dA6, dT7, dT19 |
| RNA ** | −62.25 | −61.57 ± 0.64 | −57.69 ± 3.67 | U7, G8, U9, C12, A13, C15, U16 |
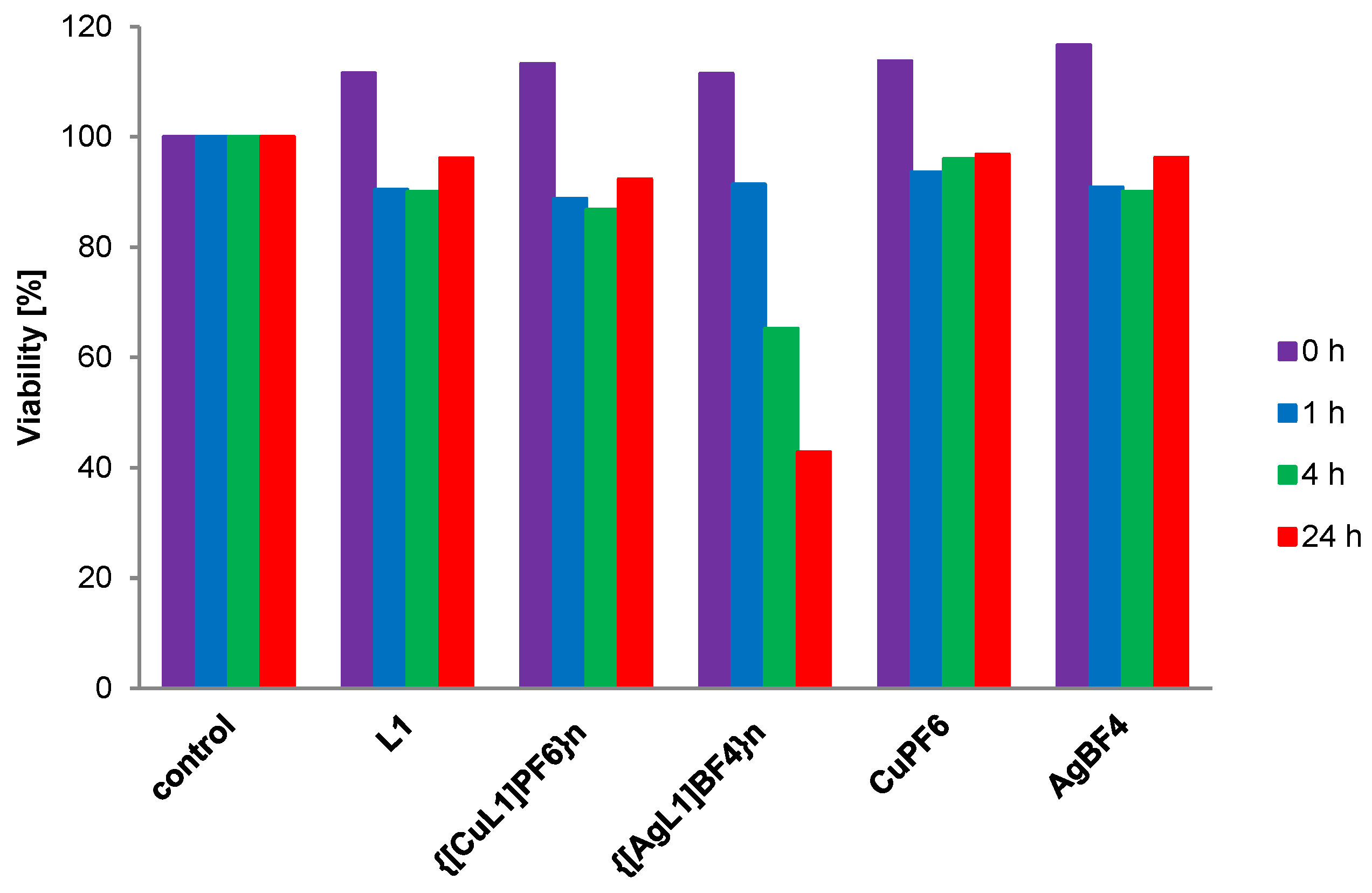
2.4. Effect of Selected Compounds on Bacterial Proliferation
3. Conclusions
4. Experimental
4.1. Materials and Methods
4.2. Synthesis of Ligands L1–L5
4.3. Synthesis of the Coordination Polymers
4.4. X-ray Crystallography
4.5. In Silico Pharmacokinetic Property Studies
4.6. Spectrophotometric Titration of the Ligands with Nucleic Acids
4.6.1. Ligand–DNA Interactions
4.6.2. Ligand-RNA Interactions
4.7. Effect of Compounds on Bacterial Proliferation
4.8. Molecular Docking Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.M.R.M.; Mitra, S.; Emran, T.B.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.K.C.; Ng, R.W.Y.; Leung, S.S.Y.; Hui, M.; Ip, M. Overcoming the rising incidence and evolving mechanisms of antibiotic resistance by novel drug delivery approaches—An overview. Adv. Drug Deliv. Rev. 2022, 181, 114078. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Ibrahim, H.; Bala, M.D.; Friedrich, H.B. Poly-functional imino-N-heterocyclic carbene ligands: Synthesis, complexation, and catalytic applications. Coord. Chem. Rev. 2022, 469, 214652. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J. Molecular Assembly of Schiff Base Interactions: Construction and Application. Chem. Rev. 2015, 115, 1597–1621. [Google Scholar] [CrossRef]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: A review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff Bases: A Short Survey on an Evergreen Chemistry Tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef]
- Szymańska, M.; Pospieszna-Markiewicz, I.; Mańka, M.; Insińska-Rak, M.; Dutkiewicz, G.; Patroniak, V.; Fik-Jaskółka, M.A. Synthesis and Spectroscopic Investigations of Schiff Base Ligand and Its Bimetallic Ag(I) Complex as DNA and BSA Binders. Biomolecules 2021, 11, 1449. [Google Scholar] [CrossRef] [PubMed]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Huang, Y.; Duan, Y.; Liu, Q.; Xu, Q.; Jia, J.; Wang, J.; Tong, Q.; Luo, P.; Wen, Y.; et al. Schiff-base silver nanocomplexes formation on natural biopolymer coated mesoporous silica contributed to the improved curative effect on infectious microbes. Nano Res. 2021, 14, 2735–2748. [Google Scholar] [CrossRef]
- Fik, M.A.; Gorczyński, A.; Kubicki, M.; Hnatejko, Z.; Fedoruk-Wyszomirska, A.; Wyszko, E.; Giel-Pietraszuk, M.; Patroniak, V. 6,6″-Dimethyl-2,2′:6′,2″-terpyridine revisited: New fluorescent silver(I) helicates with in vitro antiproliferative activity via selective nucleoli targeting. Eur. J. Med. Chem. 2014, 86, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, A.A.; Zamisa, S.J.; Islam, M.S.; Olofinsan, K.; Salau, V.F.; Mocktar, C.; Omondi, B. Quinoline Functionalized Schiff Base Silver (I) Complexes: Interactions with Biomolecules and In Vitro Cytotoxicity, Antioxidant and Antimicrobial Activities. Molecules 2021, 26, 1205. [Google Scholar] [CrossRef]
- Starosta, R.; Brzuszkiewicz, A.; Bykowska, A.; Komarnicka, U.K.; Bażanów, B.; Florek, M.; Gadzała, Ł.; Jackulak, N.; Król, J.; Marycz, K. A novel copper(I) complex, [CuI(2,2′-biquinoline)P(CH2N(CH2CH2)2O)3]—Synthesis, characterisation and comparative studies on biological activity. Polyhedron 2013, 50, 481–489. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Starosta, R.; Płotek, M.; de Almeida, R.F.M.; Jeżowska-Bojczuk, M.; Kyzioł, A. Copper(i) complexes with phosphine derived from sparfloxacin. Part II: A first insight into the cytotoxic action mode. Dalton Trans. 2016, 45, 5052–5063. [Google Scholar] [CrossRef]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Niorettini, A.; Kozieł, S.; Pucelik, B.; Barzowska, A.; Wojtala, D.; Ziółkowska, A.; Lesiów, M.; Kyzioł, A.; Caramori, S.; et al. Two out of Three Musketeers Fight against Cancer: Synthesis, Physicochemical, and Biological Properties of Phosphino CuI, RuII, IrIII Complexes. Pharmaceuticals 2022, 15, 169. [Google Scholar] [CrossRef]
- Sathyadevi, P.; Krishnamoorthy, P.; Butorac, R.R.; Cowley, A.H.; Dharmaraj, N. Synthesis of novel heterobimetallic copper(i) hydrazone Schiff base complexes: A comparative study on the effect of heterocyclic hydrazides towards interaction with DNA/protein, free radical scavenging and cytotoxicity. Metallomics 2012, 4, 498–511. [Google Scholar] [CrossRef]
- Villarreal, W.; Castro, W.; González, S.; Madamet, M.; Amalvict, R.; Pradines, B.; Navarro, M. Copper (I)-Chloroquine Complexes: Interactions with DNA and Ferriprotoporphyrin, Inhibition of β-Hematin Formation and Relation to Antimalarial Activity. Pharmaceuticals 2022, 15, 921. [Google Scholar]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yang, Y.-W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, C. Synthesis, functionalization, and applications of metal–organic frameworks in biomedicine. Dalton Trans. 2018, 47, 2114–2133. [Google Scholar] [CrossRef]
- Han, D.; Liu, X.; Wu, S. Metal organic framework-based antibacterial agents and their underlying mechanisms. Chem. Soc. Rev. 2022, 51, 7138–7169. [Google Scholar] [CrossRef] [PubMed]
- Maranescu, B.; Visa, A. Applications of Metal-Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef]
- Chakraborty, B.; Halder, P.; Paine, T.K. Conformational supramolecular isomerism in one-dimensional silver(i) coordination polymer of a flexible bis(bidentate)N,N-donor ligand with p-xylyl spacer. Dalton Trans. 2011, 40, 3647–3654. [Google Scholar] [CrossRef]
- Wende, C.; Lüdtke, C.; Kulak, N. Copper Complexes of N-Donor Ligands as Artificial Nucleases. Eur. J. Inorg. Chem. 2014, 2014, 2597–2612. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods. 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-Based Approach in Designing Combinatorial or Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of Drug Absorption Using Multivariate Statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple Selection Criteria for Drug-like Chemical Matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Kosar, N.; Mahmood, T.; Muhammad, M.; Ullah, F.; Tahir, M.N.; Ribeiro, A.I.; Khan, E. Schiff Bases Derived from 2-Amino-6-methylbenzothiazole, 2-Amino-5-chloropyridine and 4-Chlorobenzaldehyde: Structure, Computational Studies and Evaluation of Biological Activity. ChemistrySelect 2022, 7, e202203386. [Google Scholar] [CrossRef]
- Fik-Jaskółka, M.A.; Pospieszna-Markiewicz, I.; Roviello, G.N.; Kubicki, M.; Radecka-Paryzek, W.; Patroniak, V. Synthesis and Spectroscopic Investigation of a Hexaaza Lanthanum(III) Macrocycle with a Hybrid-Type G4 DNA Stabilizing Effect. Inorg. Chem. 2021, 60, 2122–2126. [Google Scholar] [CrossRef]
- Bocian, A.; Szymańska, M.; Brykczyńska, D.; Kubicki, M.; Wałęsa-Chorab, M.; Roviello, G.N.; Fik-Jaskółka, M.A.; Gorczyński, A.; Patroniak, V. New Artificial Biomimetic Enzyme Analogues Based on Iron(II/III) Schiff Base Complexes: An Effect of (Benz)imidazole Organic Moieties on Phenoxazinone Synthase and DNA Recognition. Molecules 2019, 24, 3173. [Google Scholar] [CrossRef] [PubMed]
- Pospieszna-Markiewicz, I.; Fik-Jaskółka, M.A.; Hnatejko, Z.; Patroniak, V.; Kubicki, M. Synthesis and Characterization of Lanthanide Metal Ion Complexes of New Polydentate Hydrazone Schiff Base Ligand. Molecules 2022, 27, 8390. [Google Scholar] [CrossRef]
- Marcon, G.; Carotti, S.; Coronnello, M.; Messori, L.; Mini, E.; Orioli, P.; Mazzei, T.; Cinellu, M.A.; Minghetti, G. Gold(III) Complexes with Bipyridyl Ligands: Solution Chemistry, Cytotoxicity, and DNA Binding Properties. J. Med. Chem. 2002, 45, 1672–1677. [Google Scholar] [CrossRef]
- Kiran, T.; Pathak, M.; Chanda, K.; Balamurali, M.M. DNA and Protein Interaction Studies of Heteroleptic Copper (II) Derivatives of Benzothiazole-Based Schiff Base and N,N-Donor Ligands. ChemistrySelect 2020, 5, 6792–6799. [Google Scholar] [CrossRef]
- Natale, D.; Mareque-Rivas, J.C. The combination of transition metal ions and hydrogen-bonding interactions. Chem. Commun. 2008, 4, 425–437. [Google Scholar] [CrossRef]
- Cerasino, L.; Hannon, M.J.; Sletten, E. DNA Three-Way Junction with a Dinuclear Iron(II) Supramolecular Helicate at the Center: A NMR Structural Study. Inorg. Chem. 2007, 46, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.-L.; Xu, W.-S.; Gao, W.-L.; Zhang, J.; Zhao, Y.; Long, J.-Y.; Anson, C.E.; Powell, A.K. DNA binding and cytotoxicity activity of a chiral iron(III) triangle complex based on a natural rosin product. J. Photochem. Photobiol. B 2015, 142, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Hermann, T.; Tor, Y. RNA as a target for small-molecule therapeutics. Expert. Opin. Therap. Patents 2005, 15, 49–62. [Google Scholar] [CrossRef]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef]
- Stallings, S.C.; Moore, P.B. The structure of an essential splicing element: Stem loop IIa from yeast U2 snRNA. Structure 1997, 5, 1173–1185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mihiretu, A.; Ayana, M.; Yibeltal, A. Synthesis, characterization and antibacterial activity of metalloporphyrins: Role of central metal ion. Results Chem. 2020, 2, 100073. [Google Scholar]
- Reichmann, M.E.; Rice, S.A.; Thomas, C.A.; Doty, P. A Further Examination of the Molecular Weight and Size of Desoxypentose Nucleic Acid. J. Am. Chem. Soc. 1954, 76, 3047–3053. [Google Scholar] [CrossRef]
- Sheet, S.K.; Sen, B.; Aguan, K.; Khatua, S. A cationic organoiridium(iii) complex-based AIEgen for selective light-up detection of rRNA and nucleolar staining. Dalton Trans. 2018, 47, 11477–11490. [Google Scholar] [CrossRef]
- Agilent Technologies Ltd. CrysAlis PRO (Version 1.171.39.46); Agilent Technologies Ltd.: Santa Clara, CA, USA, 2018. [Google Scholar]
- Sheldrick, G. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A Simple, Robust, and Efficient Description of n-Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Wildman, S.A.; Crippen, G.M. Prediction of Physicochemical Parameters by Atomic Contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Liu, Q.; Nakagome, I.; Matsushita, Y. Simple Method of Calculating Octanol/Water Partition Coefficient. Chem. Pharm. Bull. 1992, 40, 127–130. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Nakagome, I.; Hirano, H. Comparison of Reliability of log P Values for Drugs Calculated by Several Methods. Chem. Pharm. Bull. 1994, 42, 976–978. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings1PII of original article: S0169-409X(96)00423-1. The article was originally published in Advanced Drug Delivery Reviews 23 (1997) 3–25.1. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar]
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Shivakumar, L.; Shivaprasad, K.; Revanasiddappa, H.D. Synthesis, spectroscopic characterization, antimicrobial, DNA binding and oxidative-induced DNA cleavage activities: New oxovanadium(IV) complexes of 2-(2-hydroxybenzylideneamino)isoindoline-1,3-dione. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 659–666. [Google Scholar] [CrossRef]
- Fan, J.; Fan, A.; Zhang, L. Progress in molecular docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Roviello, V.; Scognamiglio, P.L.; Caruso, U.; Vicidomini, C.; Roviello, G.N. Evaluating In Silico the Potential Health and Environmental Benefits of Houseplant Volatile Organic Compounds for an Emerging ‘Indoor Forest Bathing’ Approach. Int. J. Environ. Res. Public Health 2022, 19, 273. [Google Scholar]
- Greco, F.; Falanga, A.P.; Terracciano, M.; D’Ambrosio, C.; Piccialli, G.; Oliviero, G.; Roviello, G.N.; Borbone, N. CD, UV, and In Silico Insights on the Effect of 1,3-Bis(1’-uracilyl)-2-propanone on Serum Albumin Structure. Biomolecules 2022, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Roviello, V.; Musumeci, D.; Mokhir, A.; Roviello, N.G. Evidence of Protein Binding by a Nucleopeptide Based on a Thyminedecorated L-Diaminopropanoic Acid through CD and In Silico Studies. Curr. Med. Chem. 2021, 28, 5004–5015. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, D.; Zhou, P.; Li, B.; Huang, S.-Y. HDOCK: A web server for protein–protein and protein–DNA/RNA docking based on a hybrid strategy. Nucleic Acids Res. 2017, 45, W365–W373. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Tao, H.; He, J.; Huang, S.-Y. The HDOCK server for integrated protein–protein docking. Nat. Protoc. 2020, 15, 1829–1852. [Google Scholar] [CrossRef]
- Majumder, A.; Kanti Mondal, S.; Mukhoty, S.; Bag, S.; Mondal, A.; Begum, Y.; Sharma, K.; Banik, A. Virtual screening and docking analysis of novel ligands for selective enhancement of tea (Camellia sinensis) flavonoids. Food Chem. X 2022, 13, 100212. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Zou, X. An iterative knowledge-based scoring function for protein–protein recognition. Proteins Struct. Funct. Genet. 2008, 72, 557–579. [Google Scholar] [CrossRef]

| Compound | L1 | L2 | L4 |
|---|---|---|---|
| Formula | C16H14N4S2 | C20H18N4 | C18H20N6 |
| Formula weight | 326.43 /c | 314.38 /c | 320.40 /c |
| Crystal system | monoclinic | Triclinic | triclinic |
| Space group | P21/c | P-1 | P-1 |
| a (Å) | 9.0685 (4) | 4.4796 (3) | 6.0318 (5) |
| b (Å) | 7.5193 (2) | 9.9845 (12) | 7.7536 (7) |
| c (Å) | 11.6105 (4) | 10.2941 (11) | 9.1295 (10) |
| α (°) | 90 | 61.700 (11) | 79.190 (8) |
| β (°) | 108.245 (4) | 84.554 (7) | 80.956 (8) |
| γ (°) | 90 | 81.318 (8) | 78.176 (7) |
| V (Å3) | 751.90 (5) | 400.60 (8) | 407.35 (7) |
| Z | 2 | 1 | 1 |
| Dx(g cm−3) | 1.442 | 1.303 | 1.306 |
| F(000) | 340 | 166 | 170 |
| μ(mm−1) | 3.213 | 0.080 | 0.083 |
| Reflections: | |||
| collected | 2967 | 2434 | 2598 |
| unique (Rint) | 1526 (0.0318) | 1547 (0.0063) | 1565 (0.0144) |
| with I > 2σ(I) | 1395 | 1441 | 1344 |
| R(F) [I > 2σ(I)] | 0.0539 | 0.0317 | 0.0429 |
| wR(F2) [I > 2σ(I)] | 0.1397 | 0.0783 | 0.1128 |
| R(F) [all data] | 0.0567 | 0.0338 | 0.0509 |
| wR(F2) [all data] | 0.1429 | 0.0800 | 0.1184 |
| Goodness of fit | 1.037 | 1.068 | 1.041 |
| max/min Δρ (e·Å−3) | 0.62/−0.49 | 0.20/−0.18 | 0.27/−0.22 |
| CCDC number | 2209859 | 2209860 | 2209861 |
| Compound | L5 | Polymer1 | Polymer2 |
| Formula | C28H22N4 | C36H34Cu2N10S4· 2PF6·2H2O | C32H28Ag2N8S4· 2BF4 |
| Formula weight | 414.49 /c | 1188.02 /c | 1042.22 /c |
| Crystal system | triclinic | triclinic | monoclinic |
| Space group | P-1 | P-1 | C2/c |
| a (Å) | 5.9704 (6) | 8.6731 (2) | 17.21206 (16) |
| b (Å) | 9.3939 (11) | 12.2325 (3) | 11.57724 (11) |
| c (Å) | 10.3962 (11) | 12.4167 (3) | 18.9418 (2) |
| α (°) | 102.733 (10) | 105.634 (2) | 90 |
| β (°) | 101.646 (9) | 105.729 (2) | 98.0626 (9) |
| γ (°) | 107.981 (10) | 98.175 (2) | 90 |
| V(Å3) | 517.56 (10) | 1187.71 (5) | 3737.19 (6) |
| Z | 1 | 1 | 4 |
| Dx(g cm−3) | 1.330 | 1.661 | 1.852 |
| F(000) | 218 | 600 | 2064 |
| μ(mm−1) | 0.080 | 1.231 | 11.198 |
| Reflections: | |||
| collected | 3561 | 23396 | 31659 |
| unique (Rint) | 2066 (0.0196) | 4907 (0.0152) | 3908 (0.0576) |
| with I > 2σ(I) | 1603 | 4768 | 3819 |
| R(F) [I > 2σ(I)] | 0.0472 | 0.0325 | 0.0464 |
| wR(F2) [I > 2σ(I)] | 0.1007 | 0.0755 | 0.1502 |
| R(F) [all data] | 0.0633 | 0.0333 | 0.0469 |
| wR(F2) [all data] | 0.1099 | 0.0759 | 0.1510 |
| Goodness of fit | 1.059 | 1.015 | 1.084 |
| max/min Δρ (e·Å−3) | 0.19/−0.22 | 0.65/−0.50 | 1.36/−1.35 |
| CCDC number | 2209862 | 2209863 | 2209864 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ewert, E.; Pospieszna-Markiewicz, I.; Szymańska, M.; Kurkiewicz, A.; Belter, A.; Kubicki, M.; Patroniak, V.; Fik-Jaskółka, M.A.; Roviello, G.N. New N4-Donor Ligands as Supramolecular Guests for DNA and RNA: Synthesis, Structural Characterization, In Silico, Spectrophotometric and Antimicrobial Studies. Molecules 2023, 28, 400. https://doi.org/10.3390/molecules28010400
Ewert E, Pospieszna-Markiewicz I, Szymańska M, Kurkiewicz A, Belter A, Kubicki M, Patroniak V, Fik-Jaskółka MA, Roviello GN. New N4-Donor Ligands as Supramolecular Guests for DNA and RNA: Synthesis, Structural Characterization, In Silico, Spectrophotometric and Antimicrobial Studies. Molecules. 2023; 28(1):400. https://doi.org/10.3390/molecules28010400
Chicago/Turabian StyleEwert, Ernest, Izabela Pospieszna-Markiewicz, Martyna Szymańska, Adrianna Kurkiewicz, Agnieszka Belter, Maciej Kubicki, Violetta Patroniak, Marta A. Fik-Jaskółka, and Giovanni N. Roviello. 2023. "New N4-Donor Ligands as Supramolecular Guests for DNA and RNA: Synthesis, Structural Characterization, In Silico, Spectrophotometric and Antimicrobial Studies" Molecules 28, no. 1: 400. https://doi.org/10.3390/molecules28010400
APA StyleEwert, E., Pospieszna-Markiewicz, I., Szymańska, M., Kurkiewicz, A., Belter, A., Kubicki, M., Patroniak, V., Fik-Jaskółka, M. A., & Roviello, G. N. (2023). New N4-Donor Ligands as Supramolecular Guests for DNA and RNA: Synthesis, Structural Characterization, In Silico, Spectrophotometric and Antimicrobial Studies. Molecules, 28(1), 400. https://doi.org/10.3390/molecules28010400