Saffron Characterization by a Multidisciplinary Approach
Abstract
1. Introduction
2. Results and Discussion
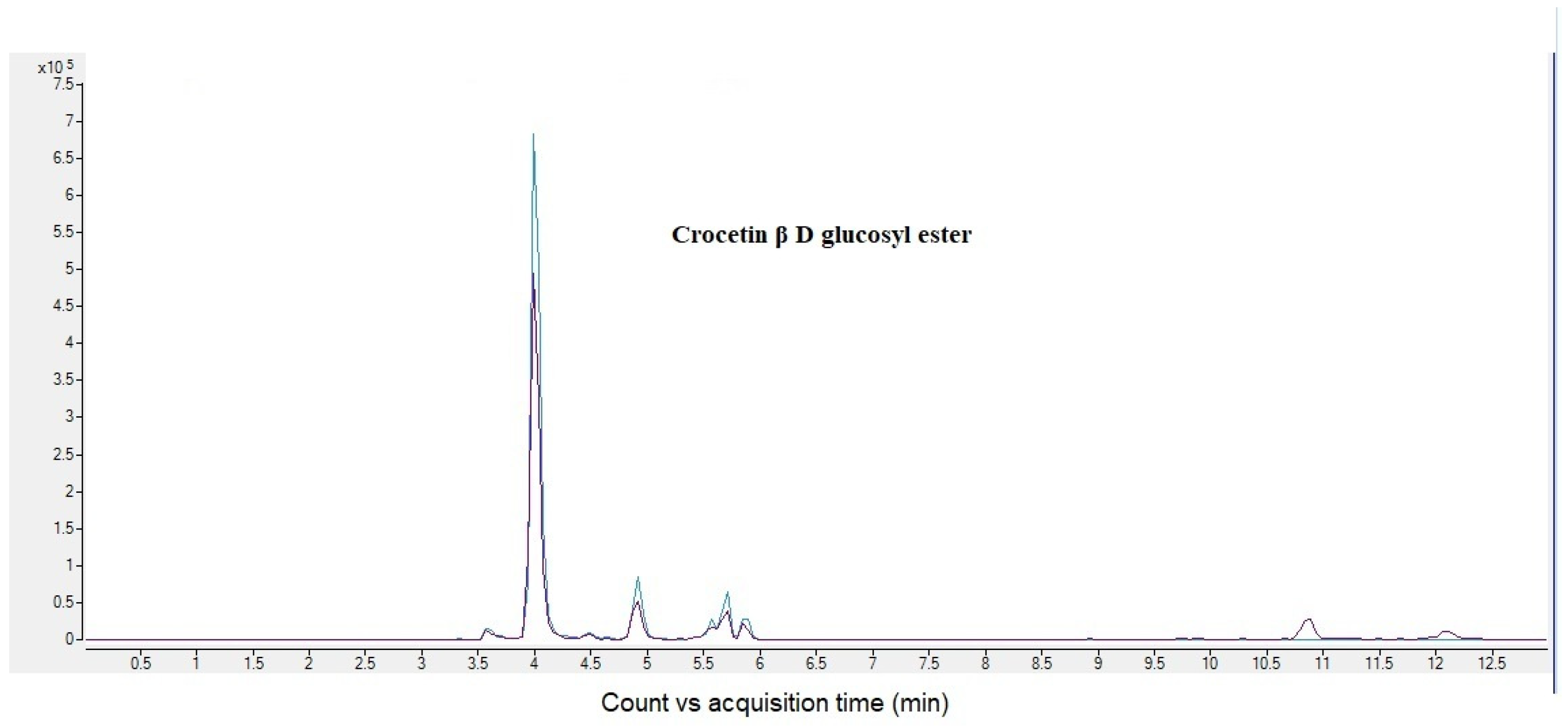
2.1. MRM Analysis
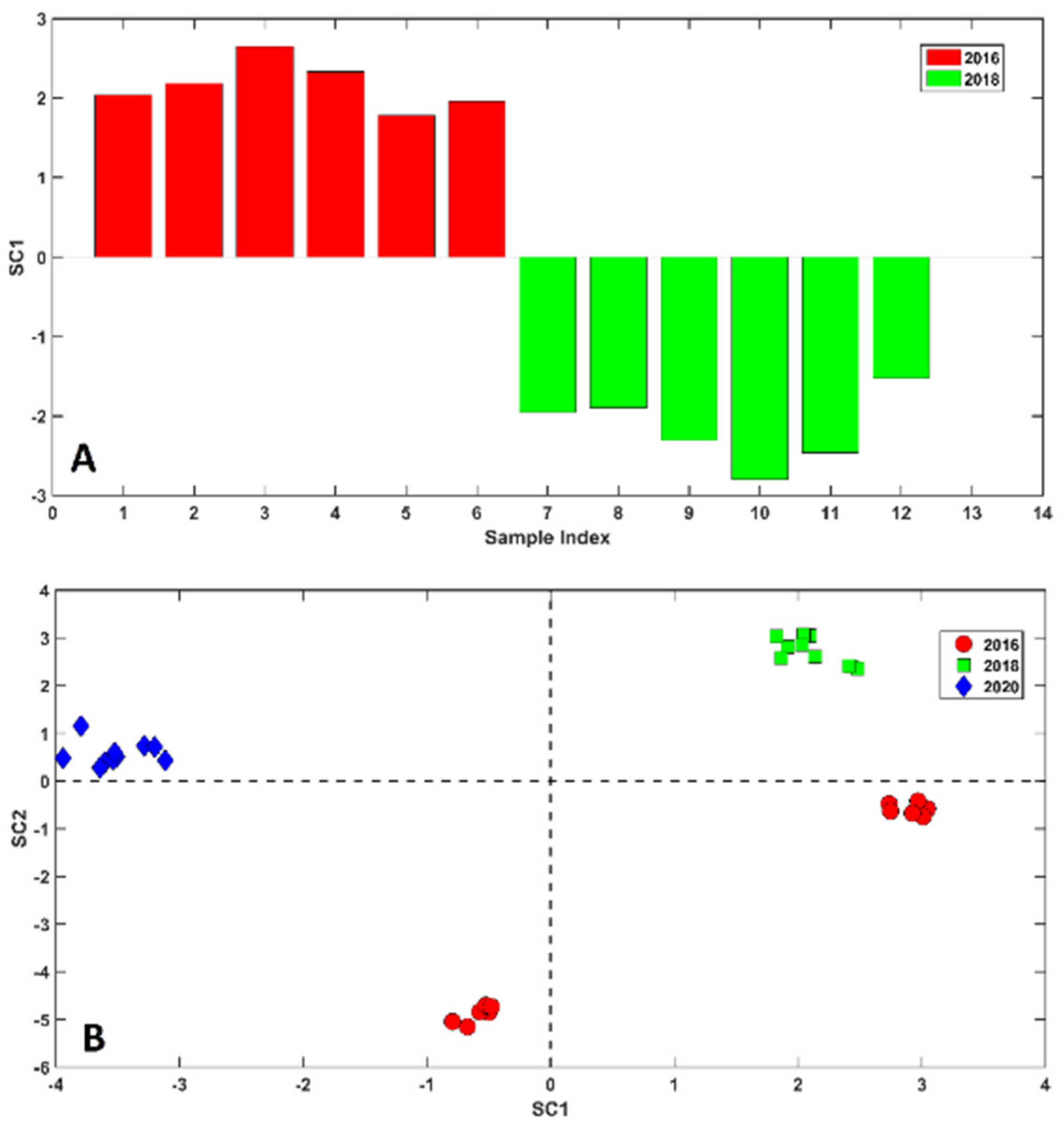
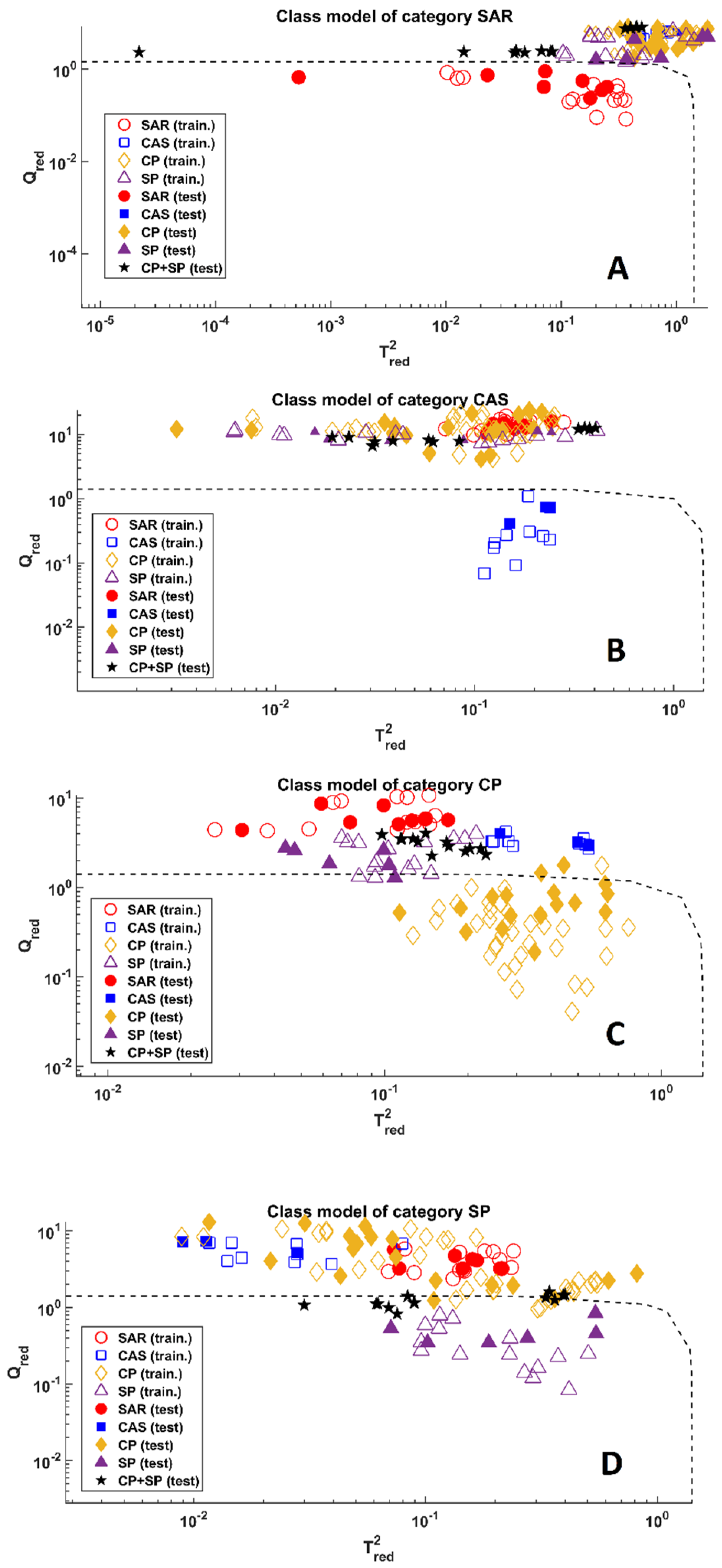
2.2. Chemometric Analysis
3. Materials and Methods
3.1. Samples
3.2. LC-MS/MS Analysis
3.3. ANOVA–Simultaneous Component Analysis (ASCA)
3.4. Soft Independent Modelling by Class Analogy (SIMCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- D’Archivio, A.A.; Giannitto, A.; Maggi, M.A.; Ruggieri, F. Geographical classification of Italian saffron (Crocus sativus L.) based on chemical constituents determined by high-performance liquid-chromatography and by using linear discriminant analysis. Food Chem. 2016, 212, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Bathaie, S.Z.; Farajzade, A.; Hoshyar, R. A review of the chemistry and uses of crocins and crocetin, the carotenoid natural dyes in saffron, with particular emphasis on applications as colorants including their use as biological stains. Biotech. Histochem. 2014, 89, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Castellar, M.R.; Simancas, M.J.; Iborra, J.L. A quantitative high-performance liquid chromatographic method to analyse commercial saffron (Crocus sativus L.) products. J. Chromatogr. A 1999, 830, 477–483. [Google Scholar] [CrossRef]
- Moras, B.; Loffredo, L.; Rey, S. Quality assessment of saffron (Crocus sativus L.) extracts via UHPLC-DAD-MS analysis and detection of adulteration using gardenia fruit extract (Gardenia jasminoides Ellis). Food Chem. 2018, 257, 325–332. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Donato, F.D.; Foschi, M.; Maggi, M.A.; Ruggieri, F. Uhplc analysis of saffron (Crocus sativus L.): Optimization of separation using chemometrics and detection of minor crocetin esters. Molecules 2018, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Gikas, E.; Koulakiotis, N.S.; Tsarbopoulos, A. Phytochemical differentiation of saffron (Crocus sativus L.) by high resolution mass spectrometry metabolomic studies. Molecules 2021, 26, 2180. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.J.; Hoefsloot, H.C.J.; van der Greef, J.; Timmerman, M.E.; Westerhuis, J.A.; Smilde, A.K. ASCA: Analysis of multivariate data obtained from an experimental design. J. Chemom. 2005, 19, 469–481. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M. SIMCA: A Method for Analysing Chemical Data in Terms of Similarity and Analogy. In Chemometrics, Theory and Application; Kowalski, B.R., Ed.; American Chemical Society: Washington, DC, USA, 1977; pp. 243–282. [Google Scholar]
- D’Archivio, A.A.; Di Vacri, M.L.; Ferrante, M.; Maggi, M.A.; Nisi, S.; Ruggieri, F. Geographical discrimination of saffron (Crocus sativus L.) using ICP-MS elemental data and class modeling of PDO Zafferano dell’Aquila produced in Abruzzo (Italy). Food Anal. Methods 2019, 12, 2572–2581. [Google Scholar] [CrossRef]
- Shawky, E.; Abu El-Khair, R.A.; Selim, D.A. NIR spectroscopy-multivariate analysis for rapid authentication, detection and quantification of common plant adulterants in saffron (Crocus sativus L.) stigmas. LWT 2020, 122, 109032. [Google Scholar] [CrossRef]
- Hashemi-Nasab, F.S.; Parastar, H. Vis-NIR hyperspectral imaging coupled with independent component analysis for saffron authentication. Food Chem. 2022, 393, 133450. [Google Scholar] [CrossRef] [PubMed]
- Gunning, Y.; Davies, K.S.; Kemsley, E.K. Authentication of saffron using 60 MHz 1H NMR spectroscopy. Food Chem. 2023, 404, 134649. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, F.; D’Archivio, A.A.; Maggi, M.A.; Rossi, L. Detection of plant-derived adulterants in saffron (Crocus sativus L.) by HS-SPME/GC-MS profiling of volatiles and chemometrics. Food Anal. Methods 2021, 14, 784–796. [Google Scholar] [CrossRef]
- Verma, R.S.; Middha, D. Analysis of saffron (Crocus sativus L. Stigma) components by LC–MS–MS. Chromatographia 2010, 71, 117–123. [Google Scholar] [CrossRef]
- Rocchi, R.; Mascini, M.; Sergi, M.; Compagnone, D.; Mastrocola, D.; Pittia, P. Crocins pattern in saffron detected by UHPLC-MS/MS as marker of quality, process and traceability. Food Chem. 2018, 264, 241–249. [Google Scholar] [CrossRef]
- Snee, R.D. Validation of regression models: Methods and examples. Technometrics 1977, 19, 415–428. [Google Scholar] [CrossRef]
- Searle, S.R. Linear Models, 1st ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1971. [Google Scholar]
- Ten Berge, J.M.F.; Kiers, H.A.L.; Van der Stel, V. Simultaneous component analysis. Stat. Appl. 1992, 4, 377–392. [Google Scholar]
- Jolliffe, I. Principal Component Analysis. In Encyclopedia of Statistics in Behavioral Science; American Cancer Society: Atlanta, GA, USA, 2005; ISBN 9780470013199. [Google Scholar]
- Hotelling, H. The generalization of student’s ratio. Ann. Math. Stat. 1931, 2, 360–378. [Google Scholar] [CrossRef]
- Jackson, J.E.; Mudholkar, G.S. Control procedures for residuals associated with principal component analysis. Technometrics 1979, 21, 341–349. [Google Scholar] [CrossRef]
| Analytes | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (eV) |
|---|---|---|---|
| Crocetin | 327 | 283 * | 15 |
| 239 | 20 | ||
| 165 ** | 25 | ||
| Crocetin digentobiose ester | 975 | 652 | 16 |
| 651 * | 20 | ||
| 327.1 ** | 20 | ||
| 59.5 | 60 | ||
| Crocetin gentiobiosylglucosyl ester | 813 | 652 * | 15 |
| 327.1 ** | 15 | ||
| Crocetin β D gentobiosyl ester | 651 | 327.1 * | 20 |
| 283 | 20 | ||
| 239 ** | 20 | ||
| Crocetin β D glucosyl ester | 489 | 327.1 * | 15 |
| 324 | 15 | ||
| 323 ** | 15 | ||
| 283 | 20 | ||
| Crocetin gentiobiosyl neapolitanosyl ester | 1137 | 1137 ** | 5 |
| 813 * | 20 | ||
| Dimethyl_crocetin | 355 | 327.1 | 15 |
| Picocrocin | 329 | 303 * | 15 |
| 285 | 20 | ||
| 283 | 20 | ||
| 167 ** | 15 |
| Class Model | PCs | Sensitivity (%CV) | Specificity (%CV) | Efficiency (%CV) | Sensitivity (%Test) | Specificity (%Test) |
|---|---|---|---|---|---|---|
| Sardinia (SAR) | 1 | 100.0 | 99.2 | 99.6 | 100.0 | 100.0 |
| Cascia (CAS) | 1 | 88.9 | 100.0 | 94.3 | 100.0 | 100.0 |
| Città della Pieve (CP) | 5 | 91.2 | 95.5 | 93.3 | 88.9 | 96.7 |
| Spoleto (SP) | 2 | 93.3 | 94.2 | 93.8 | 100.0 | 71.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spinelli, M.; Biancolillo, A.; Battaglia, G.; Foschi, M.; Amoresano, A.; Maggi, M.A. Saffron Characterization by a Multidisciplinary Approach. Molecules 2023, 28, 42. https://doi.org/10.3390/molecules28010042
Spinelli M, Biancolillo A, Battaglia G, Foschi M, Amoresano A, Maggi MA. Saffron Characterization by a Multidisciplinary Approach. Molecules. 2023; 28(1):42. https://doi.org/10.3390/molecules28010042
Chicago/Turabian StyleSpinelli, Michele, Alessandra Biancolillo, Gennaro Battaglia, Martina Foschi, Angela Amoresano, and Maria Anna Maggi. 2023. "Saffron Characterization by a Multidisciplinary Approach" Molecules 28, no. 1: 42. https://doi.org/10.3390/molecules28010042
APA StyleSpinelli, M., Biancolillo, A., Battaglia, G., Foschi, M., Amoresano, A., & Maggi, M. A. (2023). Saffron Characterization by a Multidisciplinary Approach. Molecules, 28(1), 42. https://doi.org/10.3390/molecules28010042








