Radiochemical Synthesis of 4-[18F]FluorobenzylAzide and Its Conjugation with EGFR-Specific Aptamers
Abstract
1. Introduction
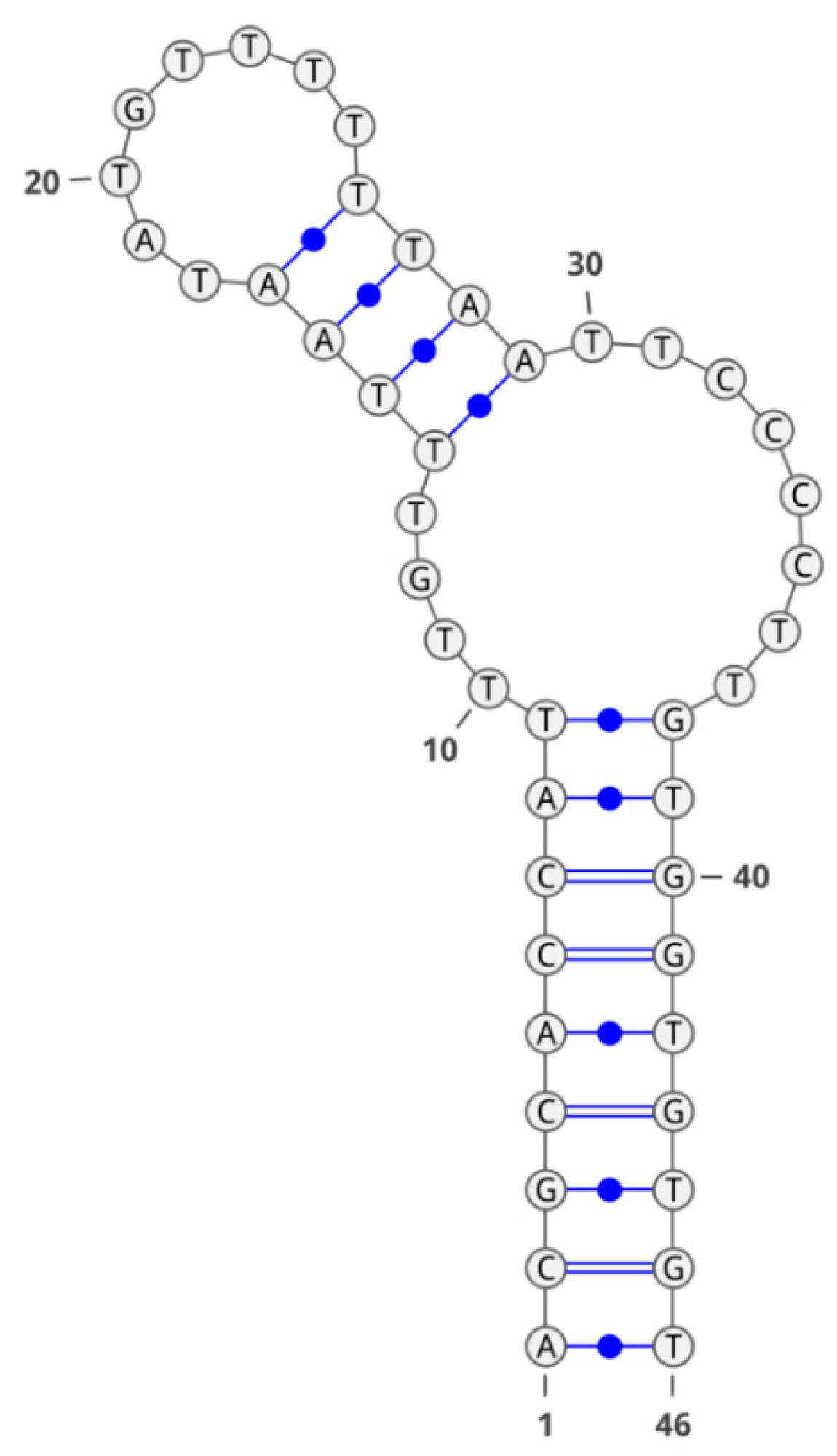
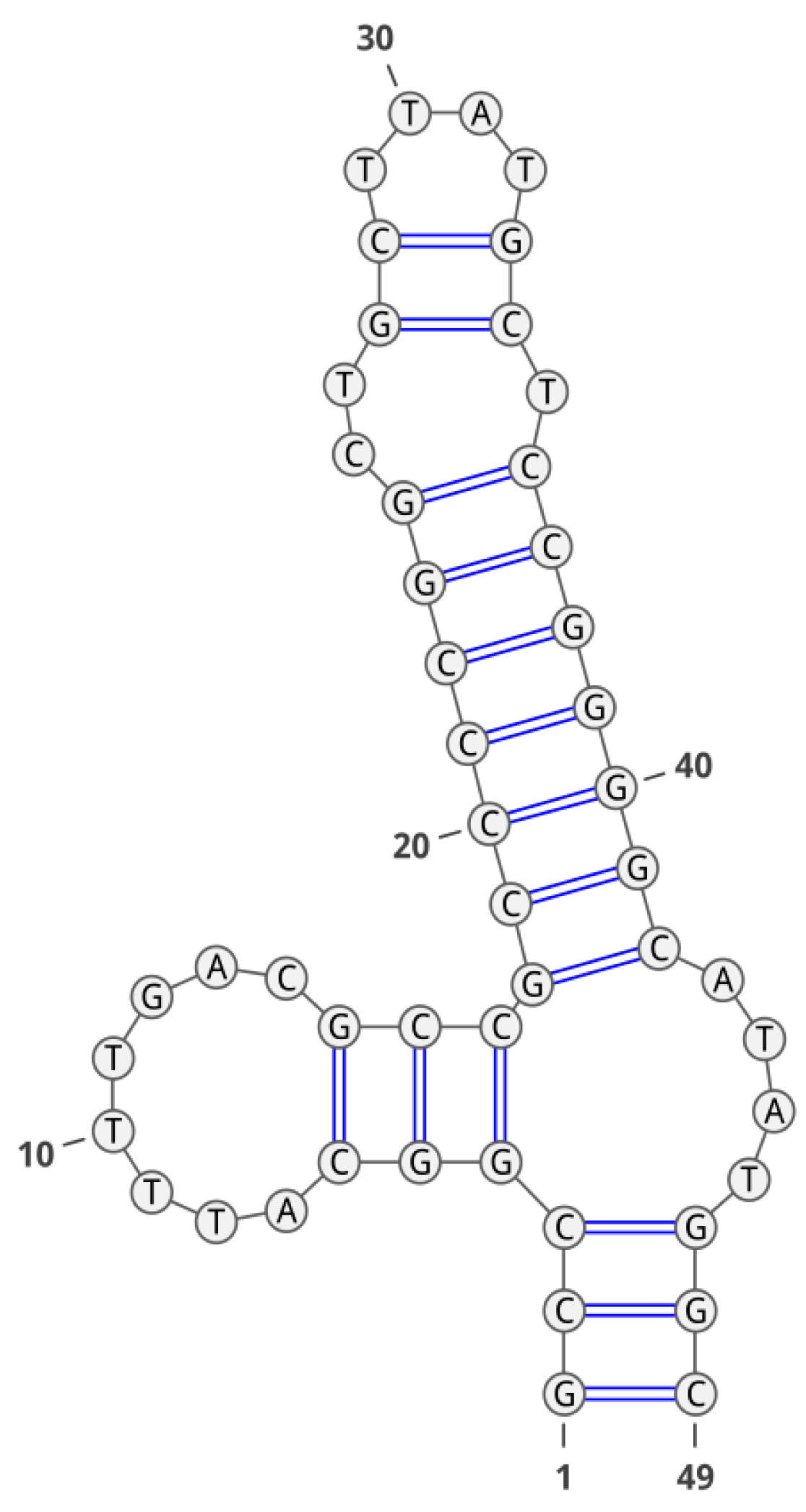
2. Results
3. Discussion
4. Materials and Methods
4.1. General
4.2. Aptamers
4.3. Quality Control
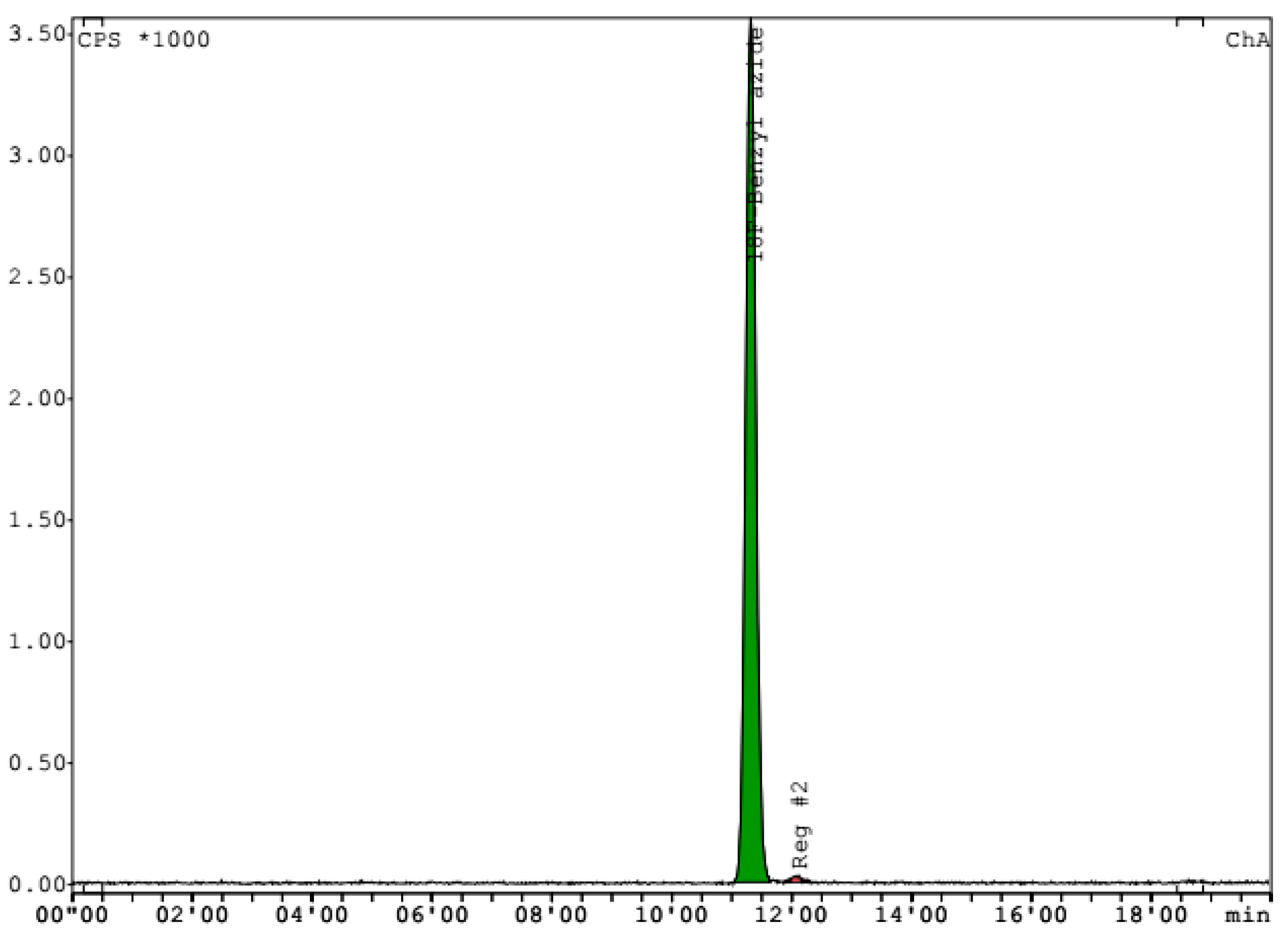
4.3.1. Quality Control 4-[18F]FluorobenzylAzide
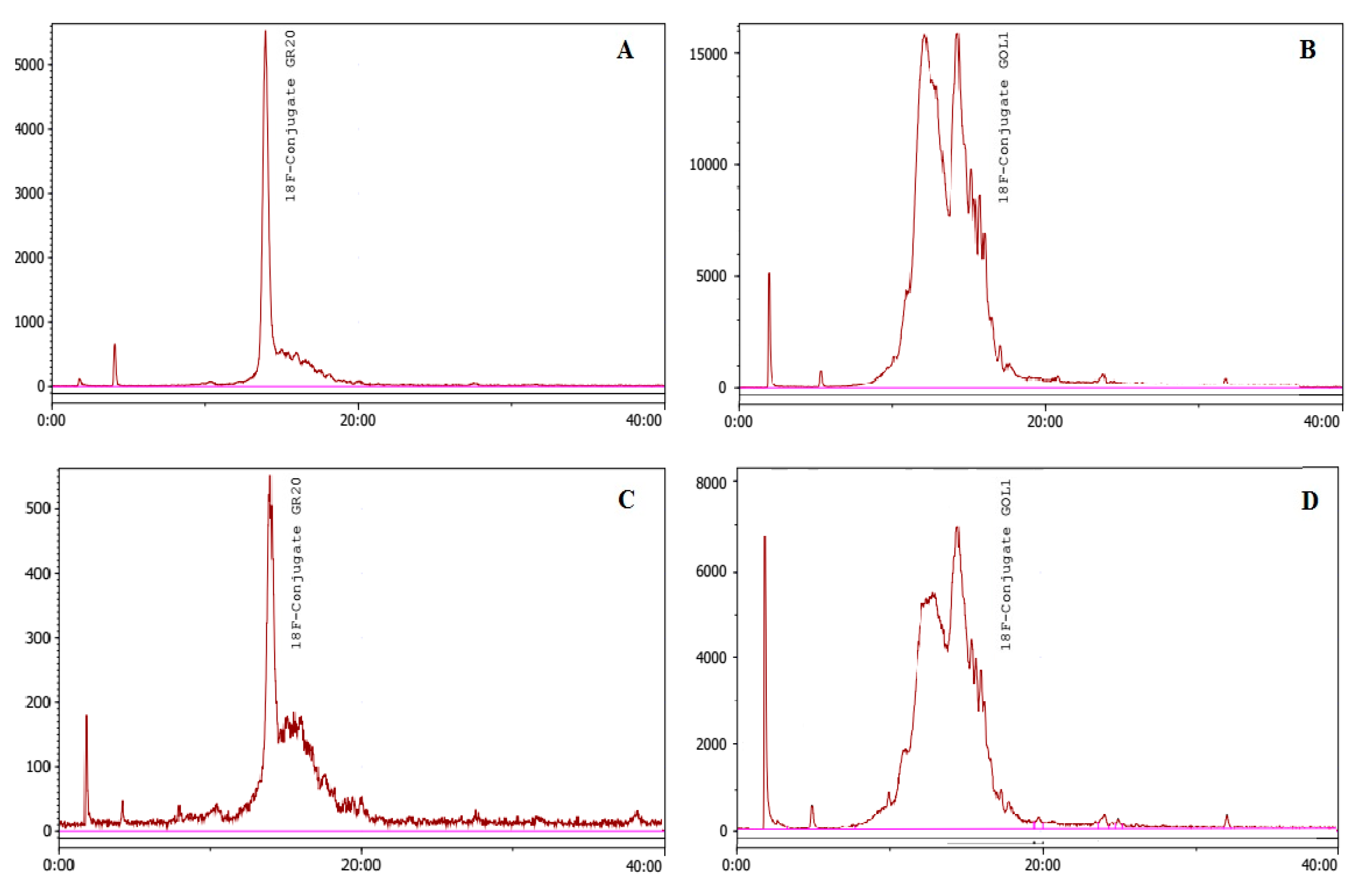
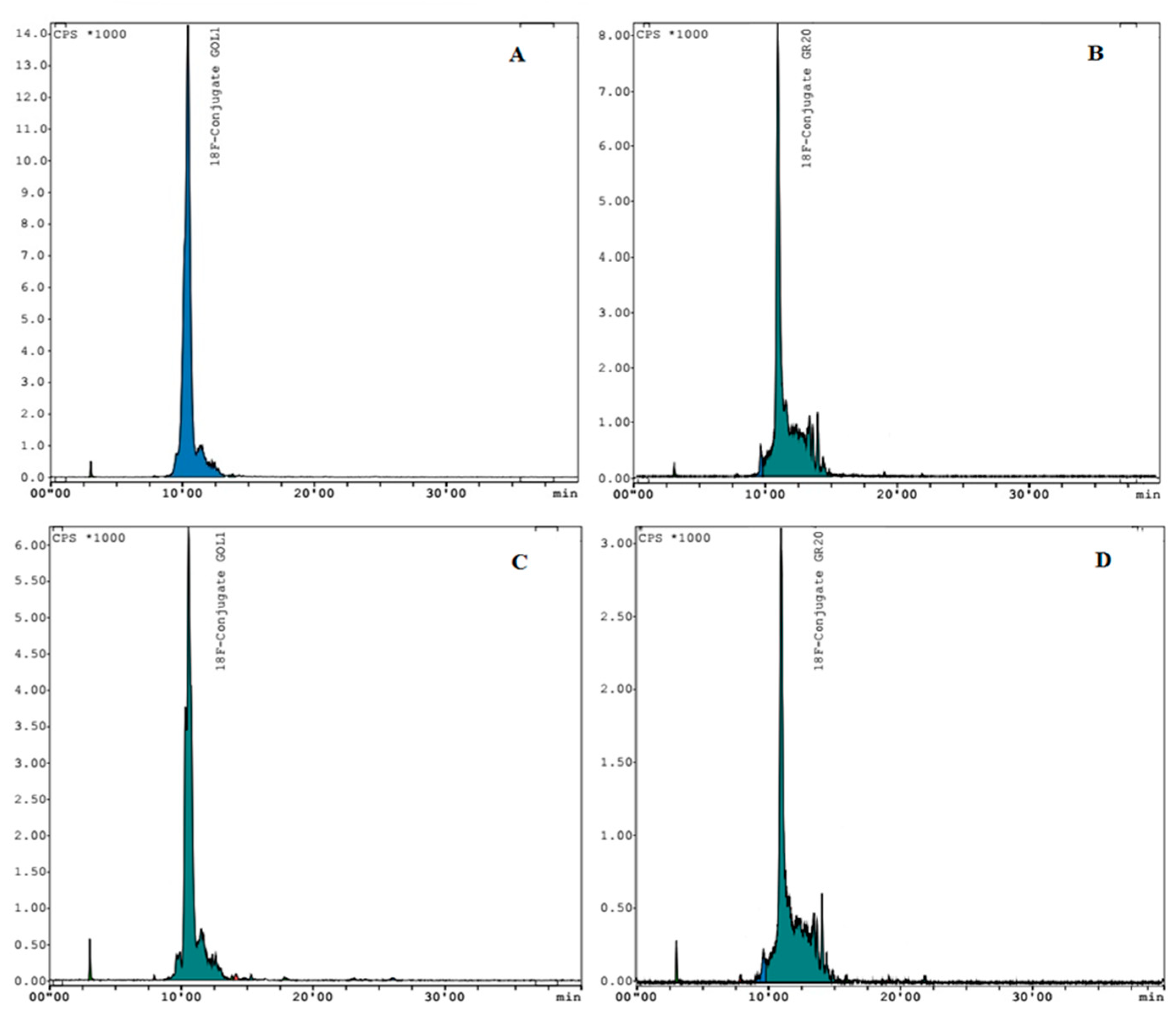
4.3.2. Quality Control of Conjugates and Aptamers
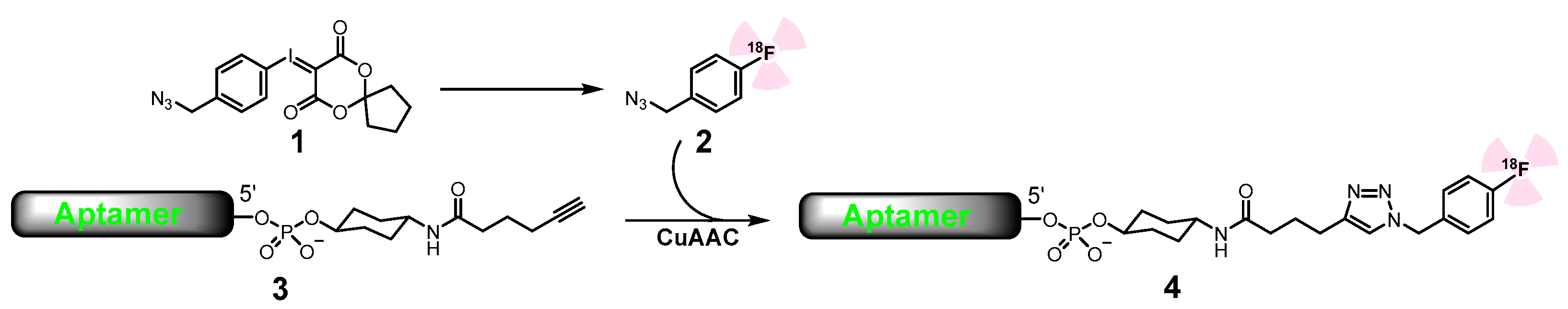
4.4. Radiochemical Synthesis
4.5. Conjugation of 4-[18F]Fluorobenzyl Azide with Aptamers
4.5.1. Conjugation of Aptamers without Cu-TBTA
4.5.2. Conjugation of Aptamers with Cu-TBTA
4.5.3. Coprecipitation of Conjugates with Acetone in the Presence of Lithium Perchlorate (LiClO4)
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Bauchet, L.; Davis, F.G.; Deltour, I.; Fisher, J.L.; Langer, C.E.; Pekmezci, M.; Schwartzbaum, J.A.; Turner, M.C.; Walsh, K.M.; et al. The epidemiology of glioma in adults: A “state of science” review. Neuro-Oncology 2014, 16, 896–913. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; Brennan, M.C.; Li, M.; Church, E.W.; Brandmeir, N.J.; Rakszawski, K.L.; Patel, A.S.; Rizk, E.B.; Suki, D.; Sawaya, R.; et al. Association of the extent of resection with survival in glioblastoma: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro-Oncology 2017, 19 (Suppl. S5), v1–v88. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, A.M.; Zavyalova, E.G.; Pavlova, G.V.; Pronin, I.N. Theranostics for glioblastoma with monoclonal antibodies to the epidermal growth factor receptor. Zh. Vopr. Neirokhir. Im. N. N. Burdenko 2020, 84, 113–118. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Hassanzadeh, L.; Chen, S.; Veedu, R.N. Radiolabeling of nucleic acid aptamers for highly sensitive disease-specific molecular imaging. Pharmaceuticals 2018, 11, 106. [Google Scholar] [CrossRef]
- Khalid, U.; Vi, C.; Henri, J.; Macdonald, J.; Eu, P.; Mandarano, G.; Shigdar, S. Radiolabelledaptamers for theranostic treatment of cancer. Pharmaceuticals 2019, 12, 2. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Stephenson, N.A.; Vasdev, N.; Liang, S.H. Spirocyclic hypervalent iodine (III)-mediated radiofluorination of non-activated and hindered aromatics. Nat. Commun. 2014, 5, 4365. [Google Scholar] [CrossRef]
- Hedberg, E.; LångstrÖm, B. Synthesis of 4-([18F]-fluoromethyl)phenylisothiocyanate and its use in labelling oligonucleotides. Acta Chem. Scand. 1997, 51, 1236–1240. [Google Scholar] [CrossRef]
- Jacobson, O.; Weiss, I.D.; Wang, L.; Wang, Z.; Yang, X.; Dewhurst, A.; Ma, Y.; Zhu, G.; Niu, G.; Kiesewetter, D.O.; et al. 18F-labeled single-stranded DNA aptamer for PET imaging of protein tyrosine kinase-7 expression. J. Nucl. Med. 2015, 56, 1780–1785. [Google Scholar] [CrossRef]
- Jacobson, O.; Weiss, I.D.; Kiesewetter, D.O.; Farber, J.M.; Chen, X. PET of tumor CXCR4 expression with 4-18F-T140. J. Nucl. Med. 2010, 51, 1796–1804. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jacobson, O.; Yan, X.; Niu, G.; Weiss, I.D.; Ma, Y.; Szajek, L.P.; Shen, B.; Kiesewetter, D.O.; Chen, X. PET imaging of tenascin-C with a radiolabeled single-stranded DNA aptamer. J. Nucl. Med. 2015, 56, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jacobson, O.; Avdic, D.; Rotstein, B.H.; Weiss, I.D.; Collier, L.; Chen, X.; Vasdev, N.; Liang, S.H. Ortho-stabilized 18F-azido click agents and their application in PET imaging with single-stranded DNA aptamers. Angew. Chem. Int. Ed. 2015, 54, 12777–12781. [Google Scholar] [CrossRef]
- Rotstein, B.H.; Wang, L.; Liu, R.Y.; Patteson, J.; Kwan, E.E.; Vasdev, N.; Liang, S.H. Mechanistic Studies and Radiofluorination of Structurally Diverse Pharmaceuticals with Spirocyclic Iodonium(III) Ylides. Chem. Sci. 2016, 7, 4407–4417. [Google Scholar] [CrossRef] [PubMed]
- Zavyalova, E.; Turashev, A.; Novoseltseva, A.; Legatova, V.; Antipova, O.; Savchenko, E.; Balk, S.; Golovin, A.; Pavlova, G.; Kopylov, A. Pyrene-modified DNA aptamers with high affinity to wild-type EGFR and EGFRvIII. Nucleic Acid Ther. 2020, 30, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liang, H.; Tan, Y.; Yuan, C.; Li, S.; Li, X.; Li, G.; Shi, Y.; Zhang, X. Cell-SELEX aptamer for highly specific molecular imaging of glioblastoma in vivo. PLoS ONE 2014, 9, e90752. [Google Scholar] [CrossRef]
- Fantoni, N.Z.; El-Sagheer, A.H.; Brown, T. A Hitchhiker’s Guide to Click-Chemistry with Nucleic Acids. Chem. Rev. 2021, 121, 7122–7154. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Chan, T.R.; Hilgraf, R.; Sharpless, K.B.; Fokin, V.V. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org. Lett. 2004, 6, 2853–2855. [Google Scholar] [CrossRef]
- Cheng, S.; Jacobson, O.; Zhu, G.; Chen, Z.; Liang, S.H.; Tian, R.; Yang, Z.; Niu, G.; Zhu, X.; Chen, X. PET imaging of EGFR expression using an 18F-labeled RNA aptamer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 948–956. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Liang, Z.; Kou, Z.; Chen, Y.; Shi, G.; Li, X.; Liang, Y.; Wang, F.; Shi, Y. Effects of aptamer to U87-EGFRvIII cells on the proliferation, radiosensitivity and radiotherapy of glioblastoma cells. Mole. Ther. Nucleic Acids 2018, 10, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Kopylov, A.M.; Fab, L.V.; Antipova, O.; Savchenko, E.A.; Revishchin, A.V.; Parshina, V.V.; Pavlova, S.V.; Kireev, I.I.; Golovin, A.V.; Usachev, D.Y.; et al. RNA aptamers for theranostics of glioblastoma of human brain. Biochemistry 2021, 86, 1012–1024. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Il’in, V.A.; Pyzhik, E.V.; Balakhonov, A.B.; Kiryushin, M.A.; Shcherbatova, E.V.; Kuznetsov, A.A.; Kostin, P.A.; Golovin, A.V.; Korshun, V.A.; Brylev, V.A.; et al. Radiochemical Synthesis of 4-[18F]FluorobenzylAzide and Its Conjugation with EGFR-Specific Aptamers. Molecules 2023, 28, 294. https://doi.org/10.3390/molecules28010294
Il’in VA, Pyzhik EV, Balakhonov AB, Kiryushin MA, Shcherbatova EV, Kuznetsov AA, Kostin PA, Golovin AV, Korshun VA, Brylev VA, et al. Radiochemical Synthesis of 4-[18F]FluorobenzylAzide and Its Conjugation with EGFR-Specific Aptamers. Molecules. 2023; 28(1):294. https://doi.org/10.3390/molecules28010294
Chicago/Turabian StyleIl’in, Viktor A., Elena V. Pyzhik, Anton B. Balakhonov, Maksim A. Kiryushin, Evgeniya V. Shcherbatova, Andrey A. Kuznetsov, Pavel A. Kostin, Andrey V. Golovin, Vladimir A. Korshun, Vladimir A. Brylev, and et al. 2023. "Radiochemical Synthesis of 4-[18F]FluorobenzylAzide and Its Conjugation with EGFR-Specific Aptamers" Molecules 28, no. 1: 294. https://doi.org/10.3390/molecules28010294
APA StyleIl’in, V. A., Pyzhik, E. V., Balakhonov, A. B., Kiryushin, M. A., Shcherbatova, E. V., Kuznetsov, A. A., Kostin, P. A., Golovin, A. V., Korshun, V. A., Brylev, V. A., Sapozhnikova, K. A., Kopylov, A. M., Pavlova, G. V., & Pronin, I. N. (2023). Radiochemical Synthesis of 4-[18F]FluorobenzylAzide and Its Conjugation with EGFR-Specific Aptamers. Molecules, 28(1), 294. https://doi.org/10.3390/molecules28010294







