A Short Review of Research Progress on the Synthesis Approaches of Aza-Dibenzocyclooctyne Derivatives
Abstract
1. Introduction
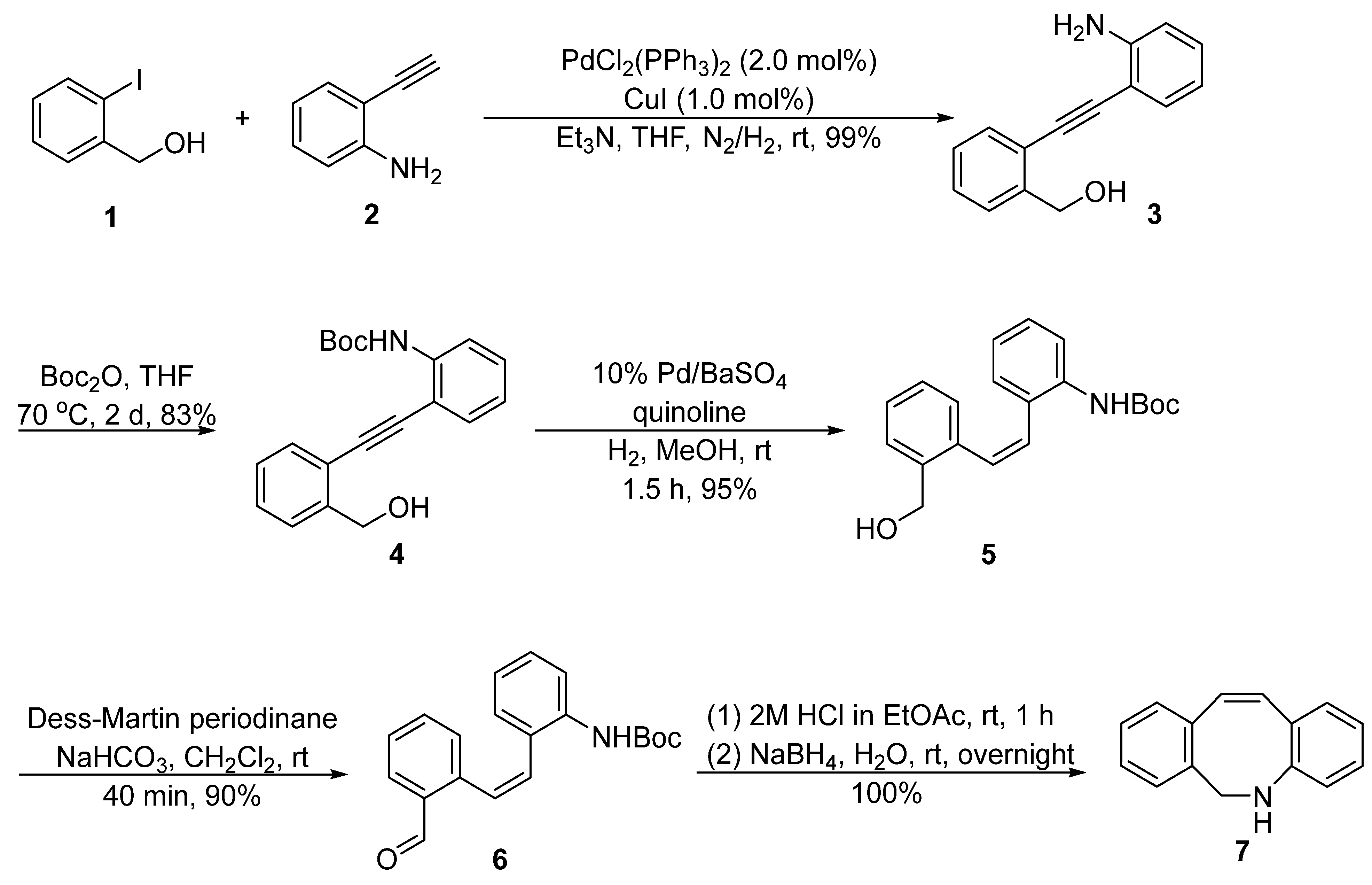
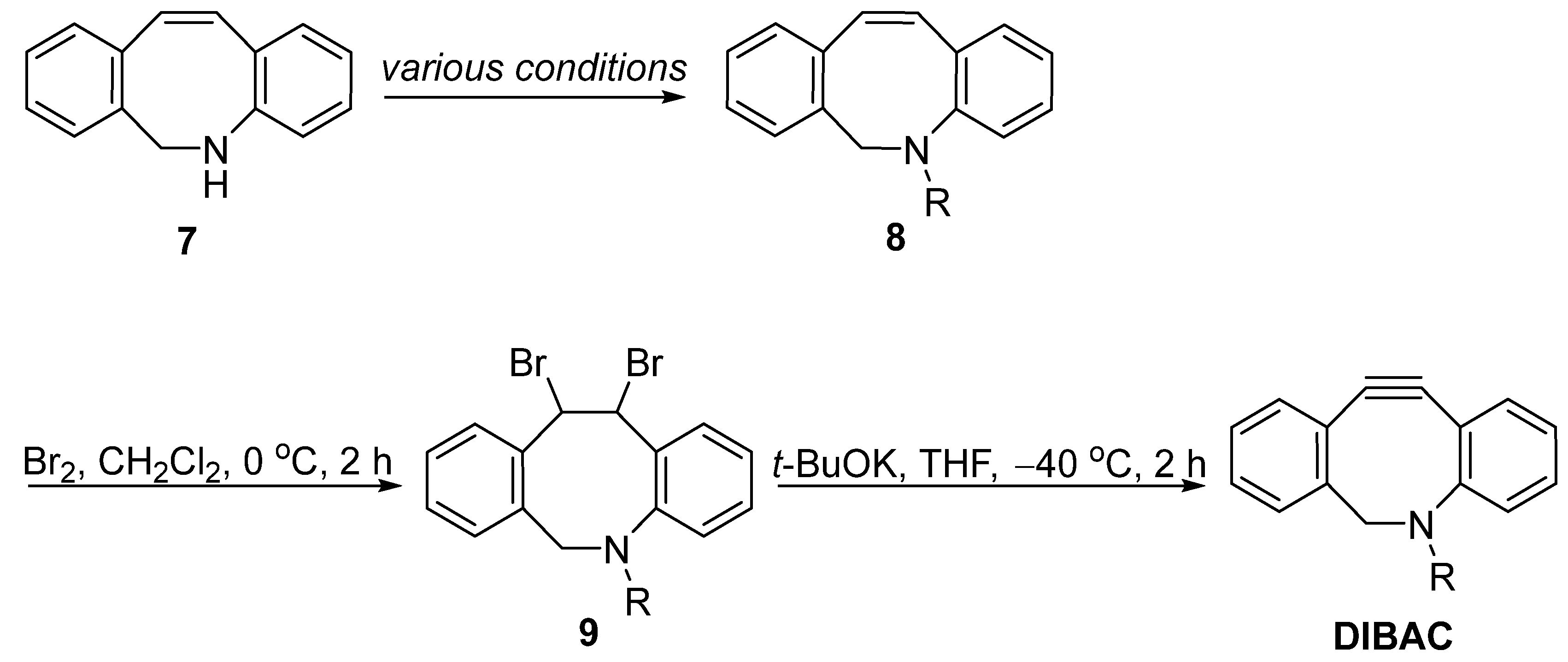
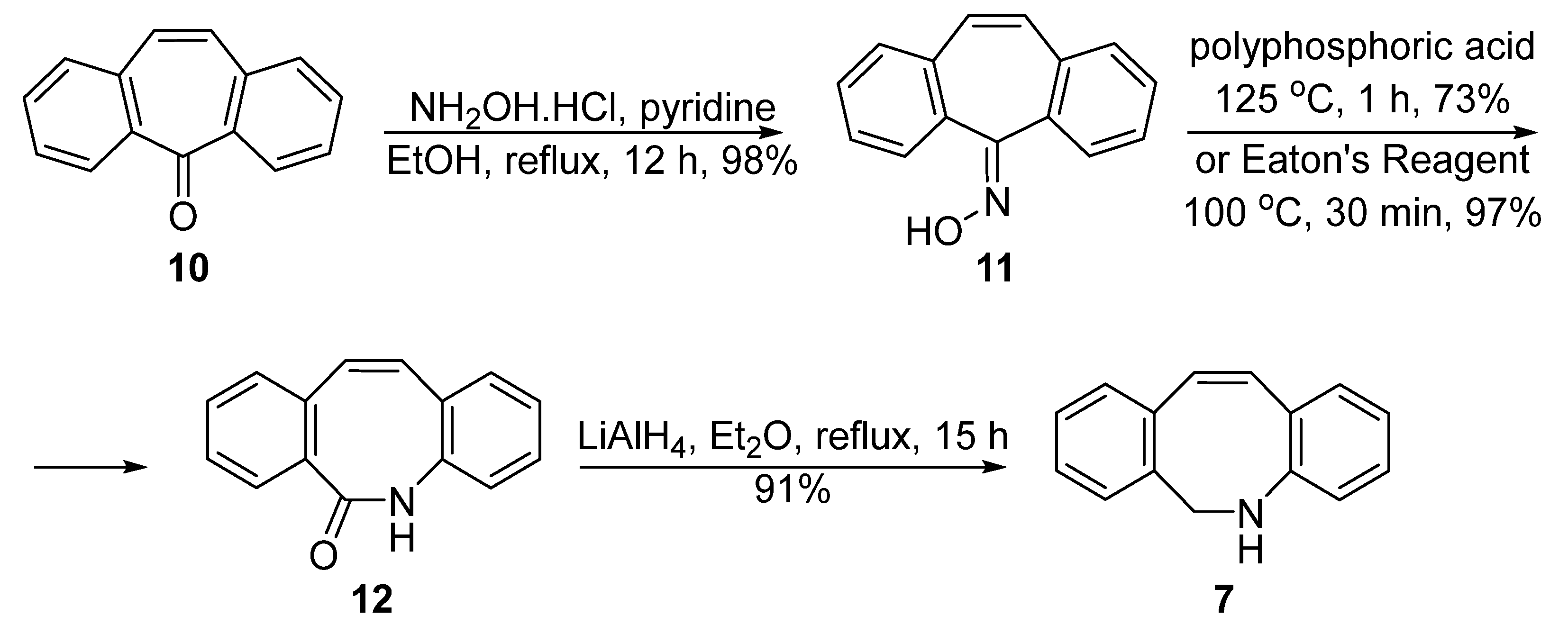
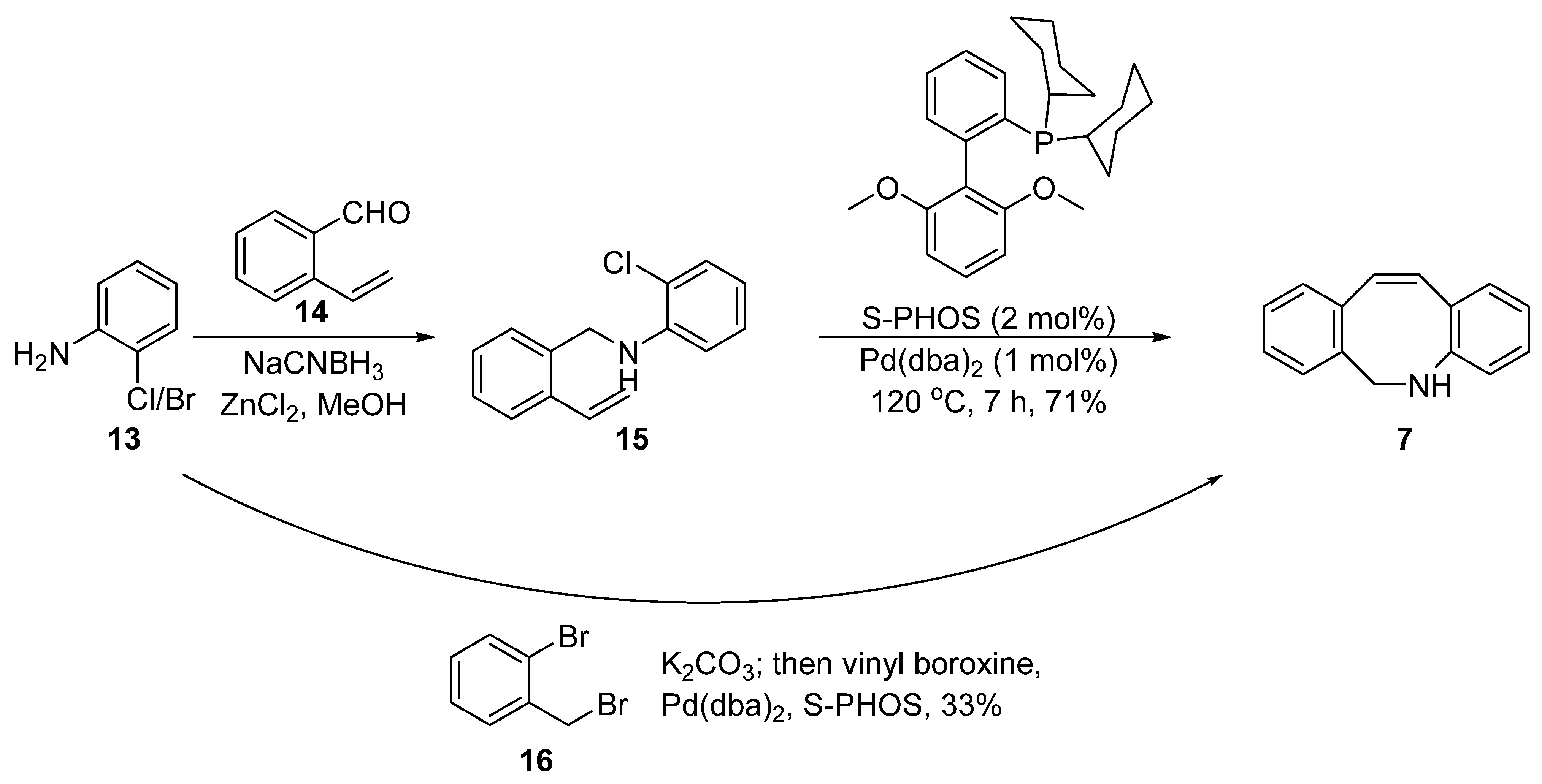
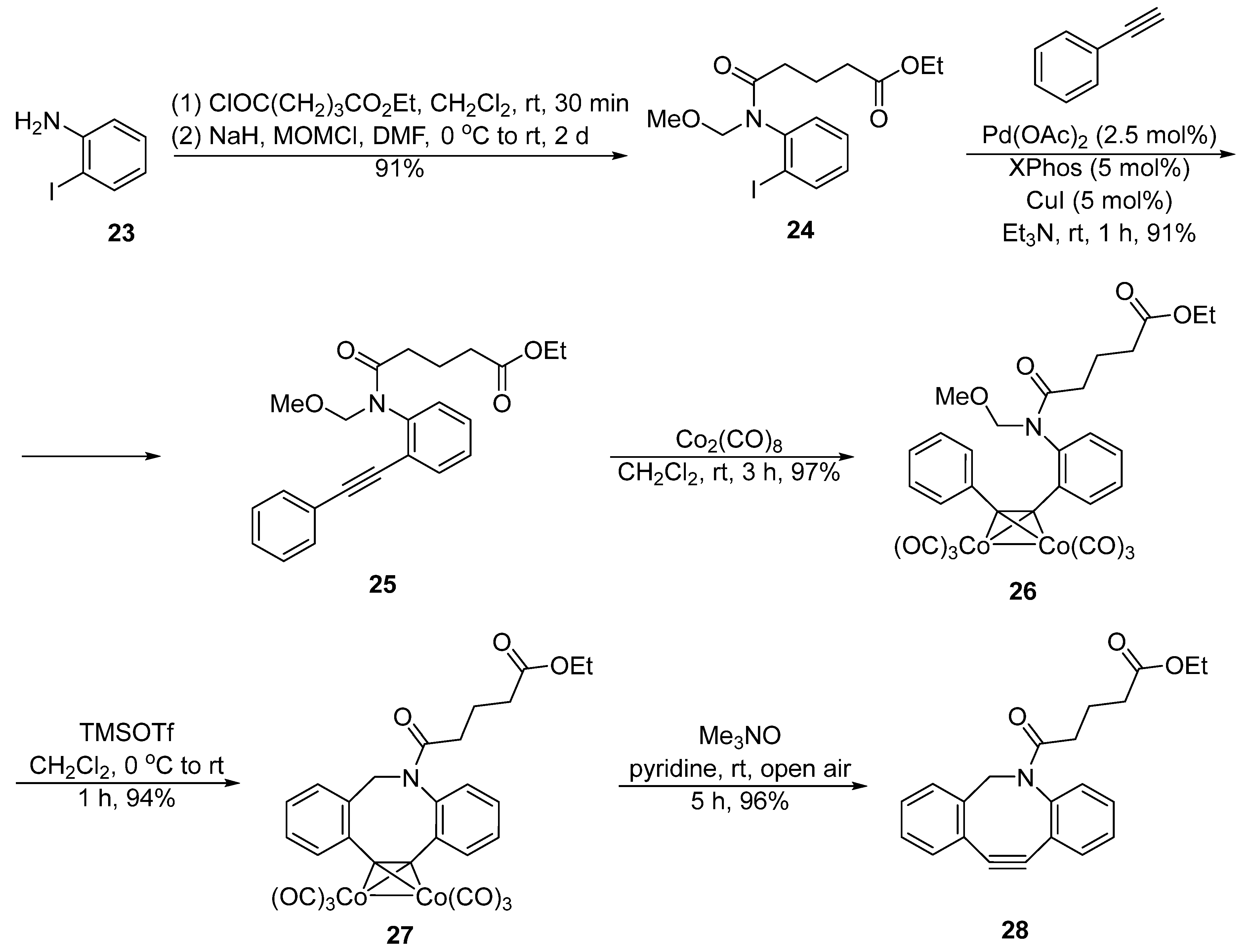
2. Synthesis Approaches for DIBAC
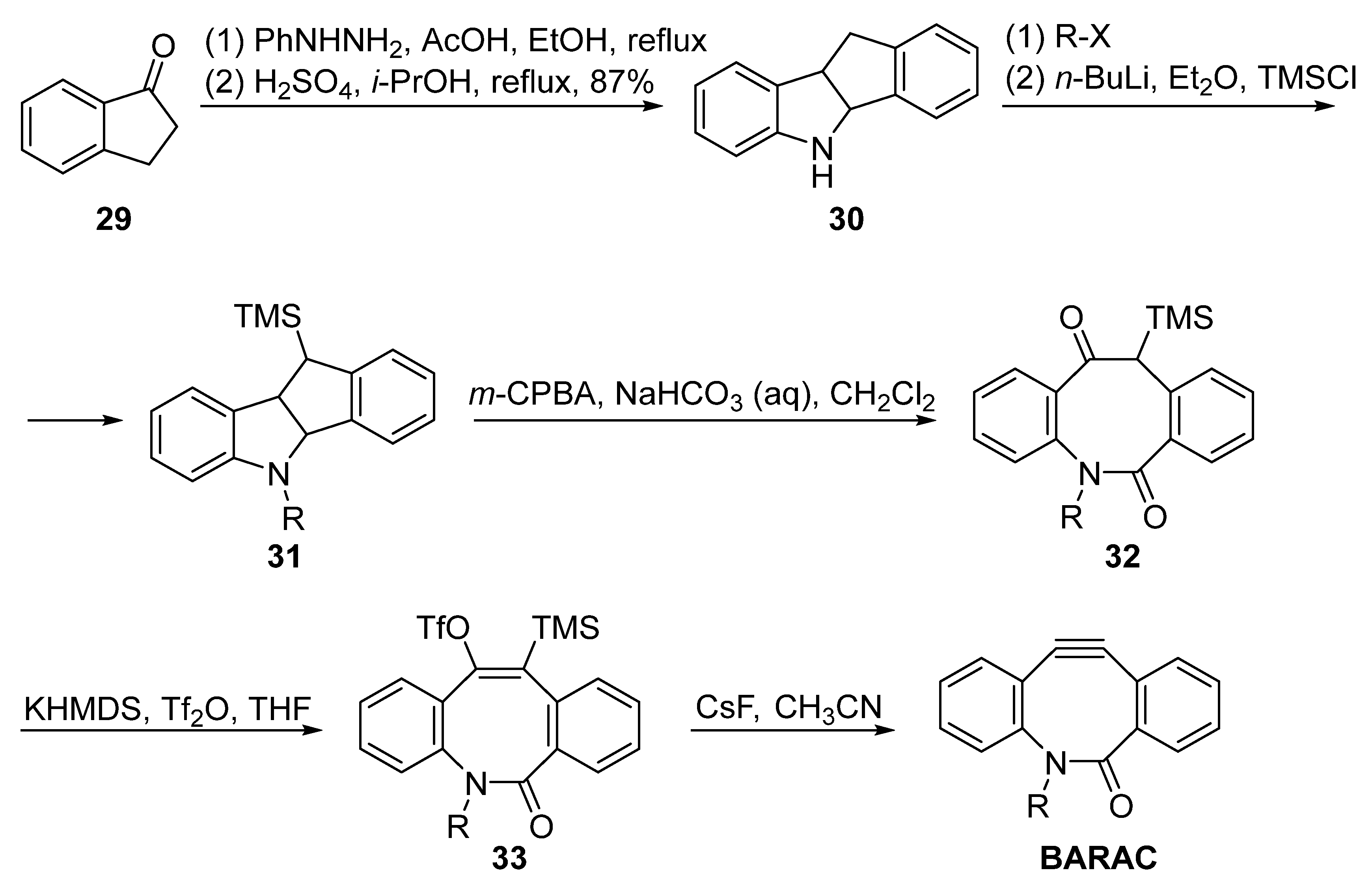
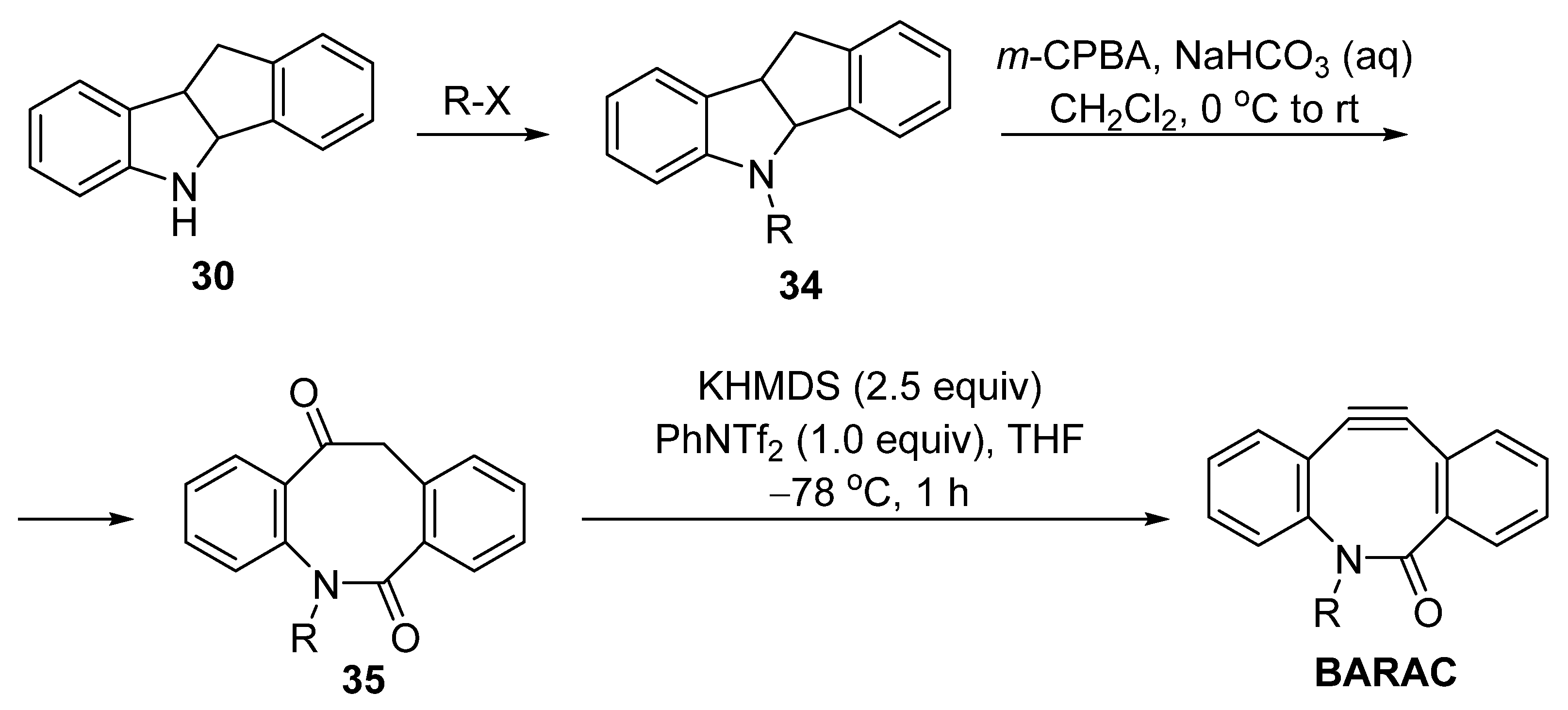
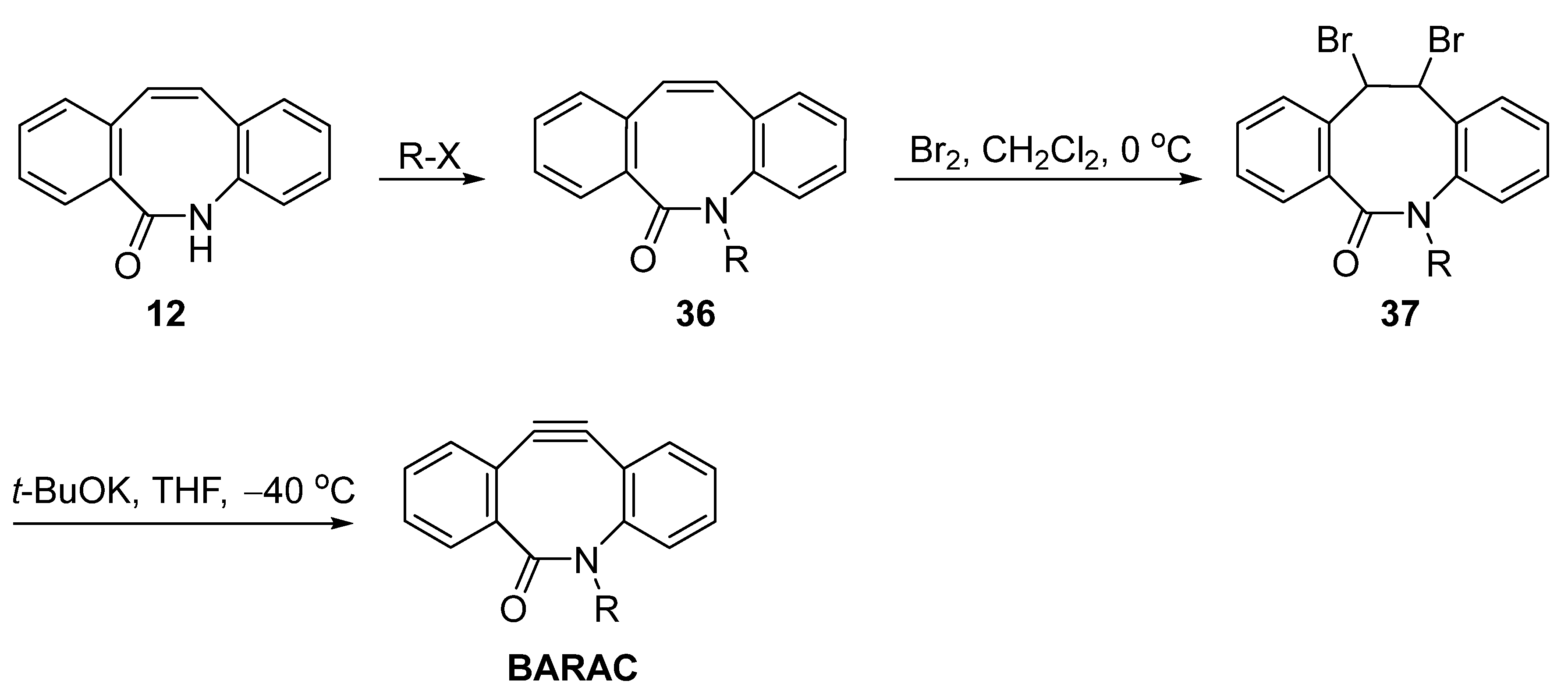
3. Synthesis Approaches for BARAC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. Engl. 2009, 48, 6974–6998. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi, C.R. A Decade of Bioorthogonal Chemistry. Acc. Chem. Res. 2011, 44, 651–653. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, J.; Chen, P.R. Bioorthogonal chemistry in living animals. Natl. Sci. Rev. 2017, 4, 300–302. [Google Scholar] [CrossRef]
- Prescher, J.A.; Bertozzi, C.R. Chemistry in living systems. Nat. Chem. Biol. 2005, 1, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.; Chen, P.R. Unnatural Amino Acid Mediated Protein Bioorthogonal Labeling. Huaxue Xuebao 2012, 70, 1439. [Google Scholar] [CrossRef]
- Saxon, E.; Bertozzi, C.R. Cell surface engineering by a modified Staudinger reaction. Science 2000, 287, 2007–2010. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A Strain-Promoted [3 + 2] Azide−Alkyne Cycloaddition for Covalent Modification of Biomolecules in Living Systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Debets, M.F.; van Berkel, S.S.; Schoffelen, S.; Rutjes, F.P.J.T.; van Hest, J.C.M.; van Delft, F.L. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3 + 2) cycloaddition. Chem. Commun. 2010, 46, 97–99. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Versteegen, R.M.; Rossin, R.; ten Hoeve, W.; Janssen, H.M.; Robillard, M.S. Click to release: Instantaneous doxorubicin elimination upon tetrazine ligation. Angew. Chem. Int. Ed. Engl. 2013, 52, 14112–14116. [Google Scholar] [CrossRef]
- Li, J.; Jia, S.; Chen, P.R. Diels-Alder reaction-triggered bioorthogonal protein decaging in living cells. Nat. Chem. Biol. 2014, 10, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, D.A.; Sherratt, A.R.; Chigrinova, M.; Cheung, L.L.W.; Pezacki, J.P. Strain-promoted cycloadditions involving nitrones and alkynes—Rapid tunable reactions for bioorthogonal labeling. Curr. Opin. Chem. Biol. 2014, 21, 81–88. [Google Scholar] [CrossRef]
- Ramil, C.P.; Lin, Q. Photoclick chemistry: A fluorogenic light-triggered in vivo ligation reaction. Curr. Opin. Chem. Biol. 2014, 21, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.-W.; Kamber, D.N.; Prescher, J.A. Building better bioorthogonal reactions. Curr. Opin. Chem. Biol. 2014, 21, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels–Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef]
- Nguyen, S.S.; Prescher, J.A. Developing bioorthogonal probes to span a spectrum of reactivities. Nat. Rev. Chem. 2020, 4, 476–489. [Google Scholar] [CrossRef]
- Murtagh, J.; Frimannsson, D.O.; O’Shea, D.F. Azide conjugatable and pH responsive near-infrared fluorescent imaging probes. Org. Lett. 2009, 11, 5386–5389. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, Y.; Shi, H.; Hu, Y.; Feng, L.; Luo, Z.; Zhou, M.; He, J.; Zhou, Z.; Zhang, Y.; et al. Redox-mediated disassembly to build activatable trimodal probe for molecular imaging of biothiols. ACS Nano 2016, 10, 10075–10085. [Google Scholar] [CrossRef] [PubMed]
- Huisgen, R. 1, 3-dipolar cycloadditions. Past and future. Angew. Chem. Int. Ed. Engl. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Breugst, M.; Reissig, H.-U. The Huisgen Reaction: Milestones of the 1, 3-Dipolar Cycloaddition. Angew. Chem. Int. Ed. Engl. 2020, 59, 12293–12307. [Google Scholar] [CrossRef]
- Padwa, A.; Pearson, W.H. (Eds.) Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products; Wiley-Interscience: New York, NY, USA, 2003. [Google Scholar]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper (I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Wittig, G.; Krebs, A. Zur Existenz niedergliedriger cycloalkine, I. Chem. Ber. 1961, 94, 3260–3275. [Google Scholar] [CrossRef]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kang, D.W.; Leucke, H.F.; Bond, M.R.; Ghosh, S.; Love, D.C.; Ahn, J.-S.; Kang, D.-O.; Hanover, J.A. Optimizing the selectivity of DIFO-based reagents for intracellular bioorthogonal application. Carbohydr. Res. 2013, 377, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Mbua, N.E.; Guo, J.; Wolfert, M.A.; Steet, R.; Boons, G.-J. Strain-promoted alkyne–azide cycloadditions (SPAAC) reveal new features of glycoconjugate biosynthesis. ChemBioChem 2011, 12, 1912–1921. [Google Scholar] [CrossRef]
- Friscourt, F.; Ledin, P.A.; Mbua, N.E.; Flanagan-Steet, H.R.; Wolfert, M.A.; Steet, R.; Boons, G.-J. Polar dibenzocyclooctynes for selective labeling of extracellular glycoconjugates of living cells. J. Am. Chem. Soc. 2012, 134, 5381–5389. [Google Scholar] [CrossRef]
- Ning, X.; Guo, J.; Wolfert, M.A.; Boons, G.-J. Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast huisgen cycloadditions. Angew. Chem. Int. Ed. Engl. 2008, 47, 2253–2255. [Google Scholar] [CrossRef]
- Debets, M.F.; Prins, J.S.; Merkx, D.; van Berkel, S.S.; van Delft, F.L.; van Hest, J.C.M.; Rutjes, F.P.J.T. Synthesis of DIBAC analogues with excellent SPAAC rate constants. Org. Biomol. Chem. 2014, 12, 5031–5037. [Google Scholar] [CrossRef]
- Jewett, J.C.; Sletten, E.M.; Bertozzi, C.R. Rapid Cu-free click chemistry with readily synthesized biarylazacyclooctynones. J. Am. Chem. Soc. 2010, 132, 3688–3690. [Google Scholar] [CrossRef]
- Kuzmin, A.; Poloukhtine, A.; Wolfert, M.A.; Popik, V.V. Surface functionalization using catalyst-free azide–alkyne cycloaddition. Bioconjug. Chem. 2010, 21, 2076–2085. [Google Scholar] [CrossRef]
- Adronov, A.; Chadwick, R.; Van Gyzen, S.; Liogier, S. Scalable synthesis of strained cyclooctyne derivatives. Synthesis 2014, 46, 669–677. [Google Scholar] [CrossRef]
- Eaton, P.E.; Carlson, G.R.; Lee, J.T. Phosphorus pentoxide-methanesulfonic acid. Convenient alternative to polyphosphoric acid. J. Org. Chem. 1973, 38, 4071–4073. [Google Scholar] [CrossRef]
- Jäger, M.; Görls, H.; Günther, W.; Schubert, U.S. Pd-Catalyzed Ring Assembly by Vinylation and Intramolecular Heck Coupling: A Versatile Strategy Towards Functionalized Azadibenzocyclooctynes. Chem.-Eur. J. 2013, 19, 2150–2157. [Google Scholar] [PubMed]
- Slugovc, C.; Perner, B.; Stelzer, F.; Mereiter, K. “Second Generation” Ruthenium Carbene Complexes with a cis-Dichloro Arrangement. Organometallics 2004, 23, 3622–3626. [Google Scholar] [CrossRef]
- Denmark, S.E.; Butler, C.R. Vinylation of aryl bromides using an inexpensive vinylpolysiloxane. Org. Lett. 2006, 8, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Starke, F.; Walther, M.; Pietzsch, H.-J. A Novel Dibenzoazacyclooctyne Precursor in Regioselective Copper-Free Click Chemistry. Arkivoc 2010, 2010, 350–359. [Google Scholar] [CrossRef]
- Sakata, Y.; Nabekura, R.; Hazama, Y.; Hany, M.; Nishiyama, T.; Kii, I.; Hosoya, T. Synthesis of Functionalized Dibenzoazacyclooctynes by a Decomplexation Method for Dibenzo-Fused Cyclooctyne–Cobalt Complexes. Org. Lett. 2023, 25, 1051–1055. [Google Scholar] [CrossRef]
- Hioki, Y.; Itoh, M.; Mori, A.; Okano, K. One-Pot Deprotonative Synthesis of Biarylazacyclooctynones. Synlett 2020, 31, 189–193. [Google Scholar] [CrossRef]


| DBCO Compound | Solvent | k (10−3 M−1 s−1) |
|---|---|---|
| OCT | CD3CN | 2.4 |
| DIFO DIBO | CD3CN CH3OH | 76 57 |
| DIBAC BARAC | CD3OD CD3CN | 310 960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Liu, L.; Cheng, L. A Short Review of Research Progress on the Synthesis Approaches of Aza-Dibenzocyclooctyne Derivatives. Molecules 2023, 28, 3715. https://doi.org/10.3390/molecules28093715
He Y, Liu L, Cheng L. A Short Review of Research Progress on the Synthesis Approaches of Aza-Dibenzocyclooctyne Derivatives. Molecules. 2023; 28(9):3715. https://doi.org/10.3390/molecules28093715
Chicago/Turabian StyleHe, Yinming, Li Liu, and Liang Cheng. 2023. "A Short Review of Research Progress on the Synthesis Approaches of Aza-Dibenzocyclooctyne Derivatives" Molecules 28, no. 9: 3715. https://doi.org/10.3390/molecules28093715
APA StyleHe, Y., Liu, L., & Cheng, L. (2023). A Short Review of Research Progress on the Synthesis Approaches of Aza-Dibenzocyclooctyne Derivatives. Molecules, 28(9), 3715. https://doi.org/10.3390/molecules28093715






