Bioactive Chemical Constituents and Pharmacological Activities of Ponciri Fructus
Abstract
1. Introduction
2. Cultivation of P. trifoliata
3. Traditional Uses of Ponciri Fructus
4. Bioactive Chemical Constituents
4.1. Phytochemical Constituents of Ponciri Fructus
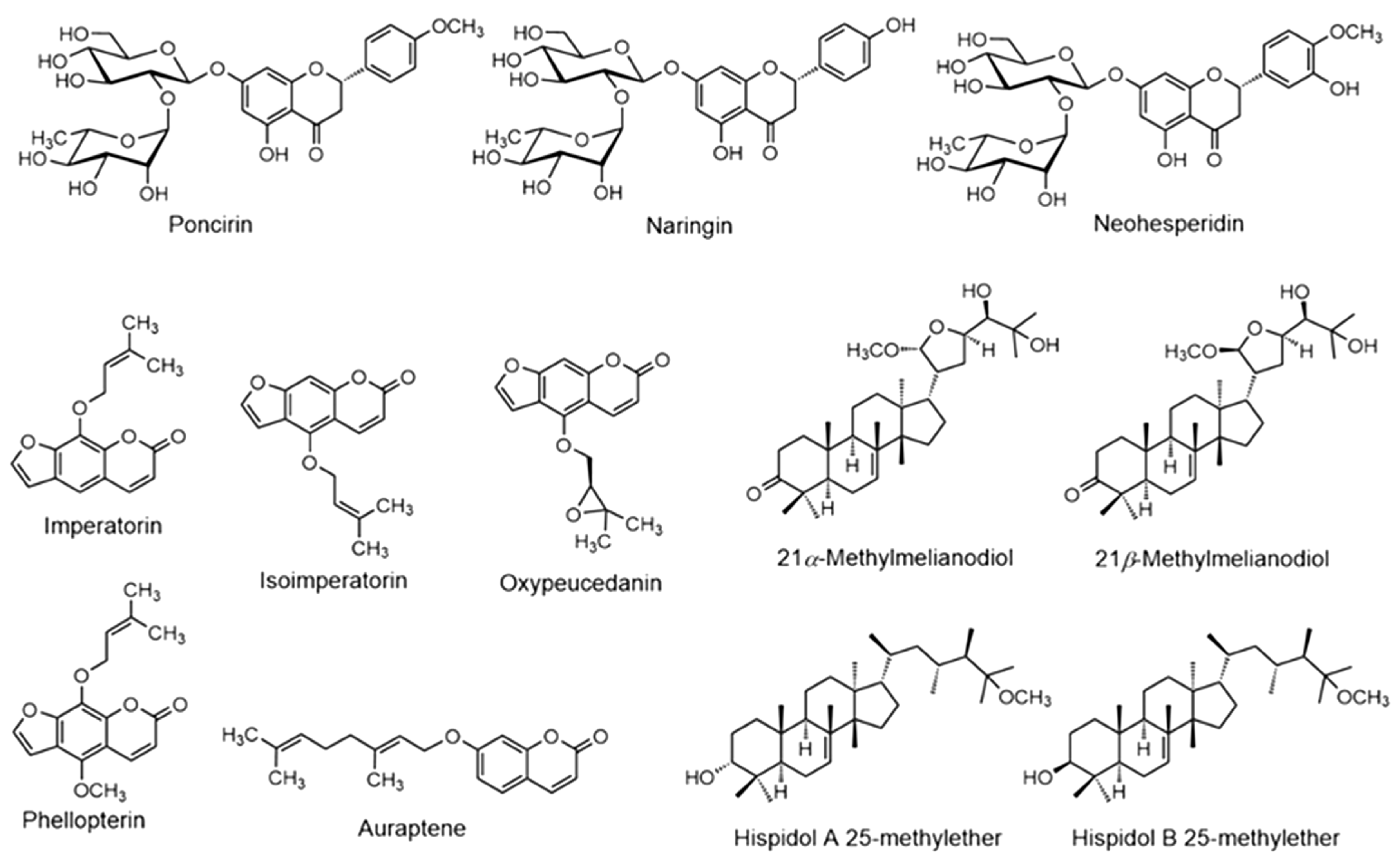
4.1.1. Flavonoids
4.1.2. Terpenoids
4.1.3. Coumarins
4.1.4. Miscellaneous Compounds
4.2. Volatile Constituents from the Peel of P. trifoliata Fresh Fruits
4.3. Chemical Constituents from the Fresh Fruit Juice and Seed Extract of P. trifoliata
5. Pharmacological Activities
5.1. Anti-Obesity Effects
5.2. Activity in Prostatic Hyperplasia
5.3. Anti-Inflammatory Activity/Anti-Allergic Effect/Immunoprotective Effects
5.4. Inhibition of Melanogenesis
5.5. Hypolipidemic Acactivity
5.6. Anti-Cancer Activity
5.7. Cosmeceutical Uses
5.8. Anti-Helicobactor pylori Effect (Gastroprotective)
5.9. Prokinetic Effect
5.10. Hepatoprotective Activity
5.11. Tick Repellent Effect
5.12. Effect on Acute Pancreatis
5.13. Neuroprotective Effects
5.14. Antiviral Activity
6. Polyherbal Formulations Containing Ponciri Fructus
6.1. Sam-Chul-Kun-Bi-Tang
6.2. Jeechool-Whan
6.3. Mahwangyounpae-Tang
6.4. Cheonggan (CGX)
6.5. Ojeok-San
7. Safety Issues
8. Conclusion and Future Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kunwar, R.M.; Bussmann, R.W. Ethnobotany in the Nepal Himalaya. J. Ethnobiol. Ethnomed. 2008, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Lee, U.W.; Kim, D.W. Chungtaejeon, A Korean fermented tea, prevents the risk of atherosclerosis in rats fed a high-fat atherogenic diet. J. Integr. Med. 2016, 14, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Paudel, K.R.; Chin, L.H.; Hon, C.M.; Madheswaran, T.; Gupta, G.; Panneerselvam, J.; Lakshmi, T.; Singh, S.K.; Gulati, M.; et al. Anti-inflammatory and anticancer activities of naringenin-loaded liquid crystalline nanoparticles in vitro. J. Food Biochem. 2021, 45, e13572. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Karki, R.; Kim, D.W. Cepharanthine inhibits in vitro VSMC proliferation and migration and vascular inflammatory responses mediated by RAW264.7. Toxicol. Vitr. 2016, 34, 16–25. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Gong, D.S.; Oak, M.H. Vascular protection by ethanol extract of Morus alba root bark: Endothelium dependent relaxation of rat aorta and decrease of smooth muscle cell migration and proliferation. Evid. Based Complement. Altern. Med. 2018, 2018, 7905763. [Google Scholar] [CrossRef]
- Devkota, H.P.; Paudel, K.R.; Khanal, S.; Baral, A.; Panth, N.; Adhikari-Devkota, A.; Jha, N.K.; Das, N.; Singh, S.K.; Chellappan, D.K.; et al. Stinging nettle (Urtica dioica L.): Nutritional composition, bioactive compounds, and food functional properties. Molecules 2022, 27, 5219. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Carresi, C.; Musolino, V.; Oppedisano, F.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Macri, R.; et al. Nutraceuticals and cancer: Potential for natural polyphenols. Nutrients 2021, 13, 3834. [Google Scholar] [CrossRef]
- Devkota, H.P.; Adhikari-Devkota, A.; Paudel, K.R.; Panth, N.; Chellappan, D.K.; Hansbro, P.M.; Dua, K. Tea (Catechins Including (−)-Epigallocatechin-3-Gallate) and Cancer. In Nutraceuticals and Cancer Signaling. Food Bioactive Ingredients; Jafari, S.M., Nabavi, S.M., Silva, A.S., Eds.; Springer: Cham, Switzerland, 2021; pp. 451–466. [Google Scholar]
- Lee, H.H.; Paudel, K.R.; Jeong, J.; Wi, A.J.; Park, W.S.; Kim, D.W.; Oak, M.H. Antiatherogenic effect of Camellia japonica fruit extract in high fat diet-fed rats. Evid. Based Complement. Altern. Med. 2016, 2016, 9679867. [Google Scholar] [CrossRef]
- Rehman, A.; Mehmood, M.H.; Haneef, M.; Gilani, A.H.; Ilyas, M.; Siddiqui, B.S.; Ahmed, M. Potential of black pepper as a functional food for treatment of airways disorders. J. Funct. Foods 2015, 19, 126–140. [Google Scholar] [CrossRef]
- De Vico, G.; Guida, V.; Carella, F. Urtica dioica (Stinging Nettle): A neglected plant with emerging growth promoter/immunostimulant properties for farmed fish. Front. Physiol. 2018, 9, 285. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as Functional Food: A Review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, E.K.; Shim, W.S. Phytotherapeutic effects of the fruits of Poncirus trifoliata (L.) Raf. on cancer, inflammation, and digestive dysfunction. Phytother. Res. 2018, 32, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Cho, C.W.; Vinh, L.B.; Kim, J.H.; Kim, Y.H.; Kang, J.S. Phytochemical analysis of trifoliate orange during fermentation by HPLC–DAD–ESI–MS/MS coupled with multivariate statistical analysis. Acta Chromatogr. 2021, 33, 371–377. [Google Scholar] [CrossRef]
- Pang, X.M.; Wen, X.P.; Hu, C.G.; Deng, X.X. Genetic diversity of Poncirus accessions as revealed by amplified fragment length polymorphism (AFLP). J. Hortic. Sci. Biotechnol. 2006, 81, 269–275. [Google Scholar] [CrossRef]
- Yu, D.J.; Jun, J.H.; Kim, T.J.; Suh, D.K.; Youn, D.H.; Kim, T.W. The relaxing effect of Poncirus fructus and its flavonoid content on porcine coronary artery. Lab. Anim. Res. 2015, 31, 33–39. [Google Scholar] [CrossRef]
- Park, S.H.; Kwak, I.S. Effect of Photoprotective activities of Poncirus trifoliata immature Fruit extract and Naringin compound. J. Korea Converg. Soc. 2019, 10, 267–279. [Google Scholar]
- Wang, M.; Dai, W.; Du, J.; Ming, R.; Dahro, B.; Liu, J.H. ERF 109 of trifoliate orange (Poncirus trifoliata (L.) Raf.) contributes to cold tolerance by directly regulating expression of Prx1 involved in antioxidative process. Plant. Biotechnol. J. 2019, 17, 1316–1332. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, S.J.; Bae, H.; Kim, C.R.; Cho, H.Y.; Kim, Y.J.; Lim, S.T.; Kim, C.J.; Kim, H.K.; Peterson, S.; et al. Effects of methoxsalen from Poncirus trifoliata on acetylcholinesterase and trimethyltin-induced learning and memory impairment. Biosci. Biotechnol. Biochem. 2011, 75, 1984–1989. [Google Scholar] [CrossRef][Green Version]
- World Flora Online (WFO). Poncirus trifoliata L. 2022. Available online: https://wfoplantlist.org/plant-list/taxon/wfo-0000608305-2022-06?matched_id=wfo-0001132893 (accessed on 25 October 2022).
- Hong, S.H.; Kim, H.M. Anti-Allergic Effect of Ponciri fructus. In Proceedings of the Korean Society of Food Science and Nutrition Conference; The Korean Society of Food Science and Nutrition: Jeju, Korea, 2004; pp. 110–115. [Google Scholar]
- Tong, R.; Peng, M.; Tong, C.; Guo, K.; Shi, S. Online extraction–high performance liquid chromatography–diode array detector–quadrupole time-of-flight tandem mass spectrometry for rapid flavonoid profiling of Fructus aurantii immaturus. J. Chromatogr. B 2018, 1077, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Poncirus trifoliata. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2012; pp. 893–899. [Google Scholar]
- Changxun, G.; Zhiyong, P.; Shu’ang, P. Effect of biochar on the growth of Poncirus trifoliata (L.) Raf. seedlings in Gannan acidic red soil. Soil. Scie. Plant. Nutr. 2016, 62, 194–200. [Google Scholar] [CrossRef]
- Chatzissavvidis, C.; Antonopoulou, C.; Therios, I.; Dimassi, K. Responses of trifoliate orange (Poncirus trifoliata (L.) Raf.) to continuously and gradually increasing NaCl concentration. Acta. Bot. Croat. 2014, 73, 275–280. [Google Scholar] [CrossRef]
- Martínez-Alcántara, B.; Rodriguez-Gamir, J.; Martínez-Cuenca, M.R.; Iglesias, D.J.; Primo-Millo, E.; Forner-Giner, M.A. Relationship between hydraulic conductance and citrus dwarfing by the Flying Dragon rootstock (Poncirus trifoliata L. Raft var. monstruosa). Trees 2013, 27, 629–638. [Google Scholar] [CrossRef]
- Kroeff Schmitz, J.A.; Dutra de Souza, P.V.; Koller, O.C. Vegetative growth of Poncirus trifoliata L. Raf. inoculated with mycorrhizal fungi in three growing media. Commun. Soil. Sci. Plant Anal. 2001, 32, 3031–3043. [Google Scholar] [CrossRef]
- Wu, Q.S.; Xia, R.X.; Zou, Y.N.; Wang, G.Y. Osmotic solute responses of mycorrhizal citrus (Poncirus trifoliata) seedlings to drought stress. Acta Physiol. Plant. 2007, 29, 543–549. [Google Scholar] [CrossRef]
- Xu, G.H.; Kim, J.A.; Kim, S.Y.; Ryu, J.C.; Kim, Y.S.; Jung, S.H.; Kim, M.K.; Lee, S.H. Terpenoids and coumarins isolated from the fruits of Poncirus trifoliata. Chem. Pharm. Bull. 2008, 56, 839–842. [Google Scholar] [CrossRef]
- Jeon, W.Y.; Kim, O.S.; Seo, C.S.; Jin, S.E.; Kim, J.; Shin, H.K.; Kim, Y.U.; Lee, M.Y. Inhibitory effects of Ponciri Fructus on testosterone-induced benign prostatic hyperplasia in rats. BMC Complement. Altern. Med. 2017, 17, 384. [Google Scholar] [CrossRef]
- Kim, D.H.; Bae, E.A.; Han, M.J. Anti-Helicobacter pylori activity of the metabolites of poncirin from Poncirus trifoliata by human intestinal bacteria. Biol. Pharm. Bull. 1999, 22, 422–424. [Google Scholar] [CrossRef]
- Cho, H.E.; Ahn, S.Y.; Kim, S.C.; Woo, M.H.; Hong, J.T.; Moon, D.C. Determination of flavonoid glycosides, polymethoxyflavones, and coumarins in herbal drugs of citrus and poncirus fruits by high performance liquid chromatography–electrospray ionization/tandem mass spectrometry. Anal. Lett. 2014, 47, 1299–1323. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Jang, J.P.; Park, S.H.; Kim, A.; Jang, J.H.; Yoon, H.R.; Yoon, S.R.; Park, J.H.; Cho, H.J.; Lee, H.G. Ponciri Fructus Immaturus ethanol extract attenuates septic shock through inhibition of the STAT1 signaling pathway. Front. Nutr. 2022, 9, 988309. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, Y.R.; Han, E.H.; Kim, J.Y.; Oh, S.J.; Kim, S.K.; Woo, E.R.; Jeong, H.G.; Kang, K.W. Potent protective effect of isoimperatorin against aflatoxin B 1-inducible cytotoxicity in H4IIE cells: Bifunctional effects on glutathione S-transferase and CYP1A. Carcinogenesis 2006, 27, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.R.; Seo, C.S.; Kim, O.S.; Shin, H.K.; Jeong, S.J. Anti-adipogenic and antioxidant effects of the traditional Korean herbal formula Samchulgeonbi-tang: An in vitro study. Int. J. Clin. Exp. Med. 2015, 8, 8698–8708. [Google Scholar] [PubMed]
- Park, M.Y.; Choi, H.Y.; Kim, J.D.; Lee, H.S.; Ku, S.K. 28 Days repeated oral dose toxicity test of aqueous extracts of mahwangyounpae-tang, a polyherbal formula. Food Chem. Toxicol. 2010, 48, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Park, H.J.; Kwon, M.; Son, C.G. Scientific evaluation of the chronic toxicity of the herbal medicine CGX in beagle dogs. Food. Chem. Toxicol. 2010, 48, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.W.; Kim, H.G.; Park, H.J.; Sung, N.W.; Son, C.G. Safety of the traditional Korean herbal medicine CGX: A 6-month repeated-dose study in rats. J. Ethnopharmacol. 2010, 128, 221–229. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, K.Y.; Kim, S.H.; Jeon, M.J.; Cho, J.H.; Oh, M.H.; Baek, J.H.; Kim, H.J.; Sung, S.H. Simultaneous determination of paeoniflorin and glycyrrhizin in sayuk-san by HPLC/DAD. J. Pharm. Investig. 2009, 39, 23–27. [Google Scholar] [CrossRef]
- Oh, H.A.; Ryu, J.G.; Cha, W.S.; Kim, H.M.; Jeong, H.J. Therapeutic effects of traditional Korean medicine, Jeechool-Whan in allergic rhinitis model. Cellmed 2012, 2, 1–9. [Google Scholar] [CrossRef]
- Weon, J.B.; Park, H.; Yang, H.J.; Ma, J.Y.; Ma, C.J. Simultaneous quantification of marker components in Ojeok-san by HPLC–DAD. J Nat. Med. 2011, 65, 375–380. [Google Scholar] [CrossRef]
- Han, S.H.; Jeong, B.J.; Woo, S.H.; Kim, B.C.; Kim, Y.H.; Seo, H.S.; Hwang, G.D.; Cho, C.J.; Nam, H.I.; Kim, J.W. A case report of diabetic hyperlipidemia in a patient with cerebral infarction treated with Ojeok-san. J. Int. Korean Med. 2005, 26, 275–280. [Google Scholar]
- Moon, Y.H.; Park, Y.J. Studies on the anti-inflammatory and analgesic activities of Ohjuksan. Korean J. Pharmacogn. 1994, 25, 258–263. [Google Scholar]
- Kim, M.; Seol, M.H.; Lee, B.C. The Effects of Poncirus fructus on insulin resistance and the macrophage-mediated inflammatory response in high fat diet-induced obese mice. Int. J. Mol. Sci. 2019, 20, 2858. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, S.H.; Kim, Y.S.; Jeong, C.S. Protective effects of neohesperidin and poncirin isolated from the fruits of Poncirus trifoliata on potential gastric disease. Phytother. Res. 2009, 23, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jang, D.; Phung, H.M.; Choi, T.J.; Kim, C.E.; Lee, S.; Kang, K.S.; Choi, S.H. The potential of pharmacological activities of the multi-compound treatment for GERD: Literature review and a network pharmacology-based analysis. Appl. Biol. Chem. 2021, 64, 48. [Google Scholar] [CrossRef]
- Han, H.Y.; Park, B.S.; Lee, G.S.; Jeong, S.H.; Kim, H.; Ryu, M.H. Autophagic cell death by Poncirus trifoliata Rafin., a traditional oriental medicine, in human oral cancer HSC-4 cells. Evid. Based Complement. Altern. Med. 2015, 2015, 394263. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.M.; Kim, M.S.; Koo, H.N.; Song, B.K.; Yoo, Y.H.; Kim, H.M. Poncirus trifoliata fruit induces apoptosis in human promyelocytic leukemia cells. Clin. Chim. Acta. 2004, 340, 179–185. [Google Scholar] [CrossRef]
- Hong, J.Y.; Min, H.Y.; Xu, G.H.; Lee, J.G.; Lee, S.H.; Kim, Y.S.; Kang, S.S.; Lee, S.K. Growth inhibition and G1 cell cycle arrest mediated by 25-methoxyhispidol A, a novel triterpenoid, isolated from the fruit of Poncirus trifoliata in human hepatocellular carcinoma cells. Planta Med. 2008, 74, 151–155. [Google Scholar] [CrossRef]
- Yan, H.; Ma, Z.; Peng, S.A.; Deng, X. Anti-inflammatory effect of auraptene extracted from trifoliate orange (Poncirus trifoliate) on LPS-stimulated RAW 264.7 cells. Inflammation 2013, 36, 1525–1532. [Google Scholar] [CrossRef]
- Rahman, A.; Alam Siddiqui, S.; Jakhar, R.; Chul Kang, S. Growth inhibition of various human cancer cell lines by imperatorin and limonin from Poncirus trifoliata Rafin. seeds. Anti Cancer Agents Med. Chem. 2015, 15, 236–241. [Google Scholar] [CrossRef]
- Kim, B.J.; San Lee, G.; Kim, H.W. Involvement of transient receptor potential melastatin type 7 channels on Poncirus fructus-induced depolarizations of pacemaking activity in interstitial cells of cajal from murine small intestine. Integr. Med. Res. 2013, 2, 62–69. [Google Scholar] [CrossRef][Green Version]
- Shim, W.S.; Back, H.; Seo, E.K.; Lee, H.T.; Shim, C.K. Long-term administration of an aqueous extract of dried, immature fruit of Poncirus trifoliata (L.) Raf. Suppresses body weight gain in rats. J. Ethnopharmacol. 2009, 126, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.T.; Seo, E.K.; Chung, S.J.; Shim, C.K. Prokinetic activity of an aqueous extract from dried immature fruit of Poncirus trifoliata (L.) Raf. J. Ethnopharmacol. 2005, 102, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, C.; Bucheli, P.; Wei, D. Citrus flavonoids in fruit and traditional Chinese medicinal food ingredients in China. Plant Foods Hum. Nutr. 2006, 61, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.T.; Kim, E.J.; Son, K.H.; Son, J.K.; Min, B.S.; Woo, M.H. Quality evaluation and pattern recognition analyses of marker compounds from five medicinal drugs of Rutaceae family by HPLC/PDA. Arch. Pharm. Res. 2015, 38, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Han, A.R.; Kim, J.B.; Lee, J.; Nam, J.W.; Lee, I.S.; Shim, C.K.; Lee, K.T.; Seo, E.K. A new flavanone glycoside from the dried immature fruits of Poncirus trifoliata. Chem. Pharm. Bull. 2007, 55, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Jeong, J.J.; Xu, G.H.; Lee, S.H.; Kang, S.S.; Kim, Y.S.; Chang, K.C.; Kim, H.J. Hesperidin, hesperidin methyl chalone and phellopterin from Poncirus trifoliata (Rutaceae) differentially regulate the expression of adhesion molecules in tumor necrosis factor-α-stimulated human umbilical vein endothelial cells. Int. Immunopharmacol. 2008, 8, 670–678. [Google Scholar] [CrossRef]
- Martin-Smith, M.; Khatoon, T. Biological activity of the terpenoids and their derivatives. Prog. Drug Res./Fortschr. Der Arzneim./Progrès Des Rech. Pharm. 1963, 279–346. [Google Scholar]
- Park, H.R. Pancastatin A and B have selective cytotoxicity on glucose-deprived PANC-1human pancreatic cancer cells. J. Microbiol. Biotechnol. 2020, 30, 733–738. [Google Scholar] [CrossRef]
- Chung, H.J.; Park, E.J.; Pyee, Y.; Xu, G.H.; Lee, S.H.; Kim, Y.S.; Lee, S.K. 25-Methoxyhispidol A, a novel triterpenoid of Poncirus trifoliata, inhibits cell growth via the modulation of EGFR/c-Src signaling pathway in MDA-MB-231 human breast cancer cells. Food. Chem. Toxicol. 2011, 49, 2942–2946. [Google Scholar] [CrossRef]
- Genovese, S.; Taddeo, V.A.; Epifano, F.; Fiorito, S. Prenylated coumarins of the genus citrus: An overview of the 2006–2016 literature data. Curr. Med. Chem. 2018, 25, 1186–1193. [Google Scholar] [CrossRef]
- Rho, T.C.; Choi, H.C.; Kim, B.Y.; Kim, Y.H.; Ahn, J.S.; Kim, Y.K.; Lee, H.S. Inhibitory Effect of Coumarins on Nitric Oxide Production in LPS-Activated Murine Macrophages. Korean J. Pharmacogn. 1999, 30, 413–416. [Google Scholar]
- Lamichhane, G.; Pandeya, P.R.; Lamichhane, R.; Rhee, S.J.; Devkota, H.P.; Jung, H.J. Anti-obesity potential of Ponciri Fructus: Effects of extracts, fractions and compounds on adipogenesis in 3T3-L1 preadipocytes. Molecules 2022, 27, 676. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W. Isolation and Structure Determination of Bioactive Constituents from Poncirus trifoliata Raf. Master’s Thesis, Seoul National University, Seoul, South Korea, 2018. [Google Scholar]
- Pokharel, Y.R.; Jeong, J.E.; Oh, S.J.; Kim, S.K.; Woo, E.R.; Kang, K.W. Screening of potential chemopreventive compounds from Poncirus trifoliata Raf. Int. J. Pharm. Sci. 2006, 61, 796–798. [Google Scholar]
- Tundis, R.; Bonesi, M.; Sicari, V.; Pellicanò, T.M.; Tenuta, M.C.; Leporini, M.; Menichini, F.; Loizzo, M.R. Poncirus trifoliata (L.) Raf.: Chemical composition, antioxidant properties and hypoglycaemic activity via the inhibition of α-amylase and α-glucosidase enzymes. J. Funct. Foods. 2016, 25, 477–485. [Google Scholar] [CrossRef]
- Pan, X.; Liang, M. Analysis of essential Oil from fructus Ponciri trifoliatae immaturi by GC-MS. Tradit. Chin. Drug Res. Clin. Pharmacol. 2000, 6. [Google Scholar]
- Son, A.R.; Choi, J.Y.; Kim, J.A.; Cho, S.H.; Xu, G.H.; Park, S.H.; Chung, S.R.; Chung, T.C.; Jahng, Y.D.; Son, J.K.; et al. Isolation of melanogenesis inhibitors from Ponciri fructus. Korean J. Pharmacogn. 2005, 36, 1–8. [Google Scholar]
- Lee, H.T.; Seo, E.K.; Chung, S.J.; Shim, C.K. Effect of an aqueous extract of dried immature fruit of Poncirus trifoliata (L.) Raf. on intestinal transit in rodents with experimental gastrointestinal motility dysfunctions. J. Ethnopharmacol. 2005, 102, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Mandadi, K.K.; Poulose, S.M.; Jadegoud, Y.; Gowda, G.N.; Patil, B.S. Inhibition of colon cancer cell growth and antioxidant activity of bioactive compounds from Poncirus trifoliata (L.) Raf. Bioorg. Med. Chem. 2007, 15, 4923–4932. [Google Scholar] [CrossRef]
- Ban, S.S.; Yoon, H.D.; Shin, O.C.; Shin, Y.J.; Park, C.S.; Park, J.H.; Seo, B.I. The effects of Artemisiae Capillaris, Ponciri Fructus and Cartaegi Fructus in obese rats induced by high fat diet. Korea J. Herbol. 2006, 21, 55–67. [Google Scholar]
- Jia, S.; Gao, Z.; Yan, S.; Chen, Y.; Sun, C.; Li, X.; Chen, K. Anti-obesity and hypoglycemic effects of Poncirus trifoliata L. extracts in high-fat diet C57BL/6 mice. Molecules 2016, 21, 453. [Google Scholar] [CrossRef]
- Lee, S.M.; Kang, Y.H.; Kim, K.K.; Kim, T.W.; Choe, M. A study of the lipoprotein lipase inhibitory mechanism of Poncirus trifoliata water extracts. J. Nutr. Health 2015, 48, 9–18. [Google Scholar] [CrossRef]
- Lee, J.W.; Jung, H.S.; Sohn, Y.; Kang, Y.J. Anti-inflammatory Effects of Ponciri Fructus Extracts on Raw 264.7 Cells. In Proceedings of the Plant Resources Society of Korea Conference; The Plant Resources Society of Korea: Chungbuk, Korea, 2018; p. 91. [Google Scholar]
- Song, C.S.; Park, E.J.; Jeong, G.M. Effects of Jiyutang with Fructus Immaturus Ponciri on the immune response in the Mouse. J. Pediatr. Korean Med. 1992, 6, 15–32. [Google Scholar]
- Jung, S.A.; Choi, Y.Y.; Yang, W.M. Anti-atopic dermatitis effects of poncirus trifoliata rafinesque via regulation of immune response and nerve growth factor. J. Korean Med. 2016, 37, 10–20. [Google Scholar] [CrossRef]
- Aeom, Y.D.; Kim, D.H.; Jeong, J.G.; Shin, M.K.; Song, H.J. The comparative study of Fructus Immaturus Ponciri and Fructus Ponciri effect on allergic reaction. J. Korean Orient. Med. 2001, 22, 10–21. [Google Scholar]
- Lee, Y.M.; Kim, C.Y.; Kim, Y.C.; Kim, H.M. Effects of Poncirus trifoliata on type I hypersensitivity reaction. Am. J. Chin. Med. 1997, 25, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Shal, B.; Khan, A.; Naveed, M.; Khan, N.U.; AlSharari, S.D.; Kim, Y.S.; Khan, S. Effect of 25-methoxy hispidol A isolated from Poncirus trifoliate against bacteria-induced anxiety and depression by targeting neuroinflammation, oxidative stress and apoptosis in mice. Biomed. Pharmacother. 2019, 111, 209–223. [Google Scholar] [CrossRef]
- Shin, E.M.; Zhou, H.Y.; Xu, G.H.; Lee, S.H.; Merfort, I.; Kim, Y.S. Anti-inflammatory activity of hispidol A 25-methyl ether, a triterpenoid isolated from Ponciri Immaturus Fructus. Eur. J. Pharmacol. 2010, 627, 318–324. [Google Scholar] [CrossRef]
- Ko, J.S.; Lee, J.C.; Jang, Y.M.; Mun, Y.J.; Woo, W.H.; Lim, K.S.; Lee, Y.C. Inhibitory effect on melanogenesis of Ponciri fructus ethanol extract. J. Physiol. Pathol. Korean Med. 2008, 22, 829–834. [Google Scholar]
- Lee, G.W.; Park, S.M.; Yoo, Y.C.; Cho, Y.H. Effect of ponciri fructus extracts fermented with ganoderma lucidum on the collagen synthesis and expression of matrix metalloproteinase-1. KSBB J. 2013, 28, 106–114. [Google Scholar] [CrossRef][Green Version]
- Lee, E. Antihyperlipidemic and antioxidant effects of Poncirus trifoliata. Korean J. Plant Resour. 2006, 19, 273–276. [Google Scholar]
- Ham, I.H.; Lee, U.C.; Lee, B.H.; Choi, H.Y. Lipid lowering activity of Ponciri Fructus and Aurantii Fructus Immaturus on hyperlipemia rats induced by Triton WR-1339. Korea J. Herbol. 2007, 22, 109–116. [Google Scholar]
- Cha, M.R.; Yoon, M.Y.; Son, E.S.; Park, H.R. Selective cytotoxicity of Ponciri Fructus against glucose-deprived PANC-1 human pancreatic cancer cells via blocking activation of GRP78. Biosci. Biotechnol. Biochem. 2009, 73, 2167–2171. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Kim, B.W.; Ha, Y.M.; Park, J.K.; Nam, S.Y.; Choi, K.C.; Choi, Y.M. Experimental Studies on Antitumor Activity of Herb Drugs (I)-Effectiveness on Rat Natural Killer Cell Activity. Korean J. Pharmacogn. 1987, 18, 118–126. [Google Scholar]
- Sim, J.R.; Jea, Y.; Kim, B.W. Study on Anticancer Effects of Putative Ponciri Fruit, Houttuyniae Herb, Manitis Squama and Polyporus in Rats. Kyung Hee Univ. Orient. Med. Diss. 1998, 11, 99–112. [Google Scholar]
- Kim, S.Y.; Yi, H.K.; Yun, B.S.; Lee, D.Y.; Hwang, P.H.; Park, H.R.; Kim, M.S. The extract of the immature fruit of Poncirus trifoliata induces apoptosis in colorectal cancer cells via mitochondrial autophagy. Food Sci. Hum. Wellness 2020, 9, 237–244. [Google Scholar] [CrossRef]
- Choi, Y.M.; Gu, J.B.; Kim, M.H.; Lee, J.S. Antioxidant and antiproliferative activities of methanolic extracts from thirty Korean medicinal plants. Food Sci. Biotechnol. 2008, 17, 1235–1239. [Google Scholar]
- Han, H.Y.; Ryu, M.H.; Son, Y.; Lee, G.; Jeong, S.H.; Kim, H. Poncirus trifoliata Rafin. induces the apoptosis of triple-negative breast cancer cells via activation of the c-Jun NH (2)-terminal kinase and extracellular signal-regulated kinase pathways. Pharmacogn. Mag. 2015, 11 (Suppl. S2), S237. [Google Scholar]
- Kim, Y.M. An Anti-MRSA (Methicillin Resistant Staphylococcus aureus) Activity and Antioxidant Activity of Poncirus trifoliata Extract. Ph.D. Thesis, Pukyung National University, Busan, Republic of Korea, 2008. [Google Scholar]
- Park, I.H.; Moon, G.; Kim, H.R.; Kim, J.H.; Hong, S.H. A Case of Liver Cirrhosis Patients Senile Pruritus Treated by External Preparation Containing Ponciri fructus and Radix lithospermi. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2016, 29, 231–239. [Google Scholar] [CrossRef][Green Version]
- Choi, K.H.; Jeong, S.I.; Hwang, B.S.; Lee, J.H.; Ryoo, H.K.; Lee, S.; Choi, B.K.; Jung, K.Y. Hexane extract of Poncirus trifoliata (L.) Raf. stimulates the motility of rat distal colon. J. Ethnopharmacol. 2010, 127, 718–724. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.K.; Joo, M.C. Effects and safety of aqueous extract of Poncirus fructus in spinal cord injury with neurogenic bowel. Evid. Based Complement. Altern. Med. 2016, 2016, 7154616. [Google Scholar] [CrossRef]
- Lee, J.N. Studies on the Tick Killing and Repellent Effects of Two Korean Indigenous Crude Drugs, Radix Jingyu and Fructus Ponciri. Korean J. Vet. Res. 1962, 2, 15–26. [Google Scholar]
- Park, K.C.; Bae, G.S.; Choi, S.B.; Jo, I.J.; Gwak, T.S.; Lee, G.S.; Park, S.J.; Song, H.J. Protective effect of Poncirus trifoliata and Citrus aurantium extract on acute pancreatitis in mice model. Korea J. Herbol. 2012, 27, 9–14. [Google Scholar] [CrossRef]
- Heo, Y.; Cho, Y.; Cho, H.; Park, K.H.; Choi, H.; Yoon, J.K.; Moon, C.; Kim, Y.B. Antiviral activity of Poncirus trifoliata seed extract against oseltamivir-resistant influenza virus. J. Microbiol. 2018, 56, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Venkatarame Gowda Saralamma, V.; Nagappan, A.; Hong, G.E.; Lee, H.J.; Yumnam, S.; Raha, S.; Heo, J.D.; Lee, S.J.; Lee, W.S.; Kim, E.H.; et al. Poncirin induces apoptosis in AGS human gastric cancer cells through extrinsic apoptotic pathway by up-regulation of fas ligand. Int. J. Mol. Sci. 2015, 16, 22676–22691. [Google Scholar] [CrossRef]
- Zhu, X.; Luo, F.; Zheng, Y.; Zhang, J.; Huang, J.; Sun, C.; Li, X.; Chen, K. Characterization, purification of poncirin from edible citrus ougan (Citrus reticulate cv. suavissima) and its growth inhibitory effect on human gastric cancer cells SGC-7901. Int. J. Mol. Sci. 2013, 14, 8684–8697. [Google Scholar] [CrossRef]
- Ali, M.Y.; Zaib, S.; Rahman, M.M.; Jannat, S.; Iqbal, J.; Park, S.K.; Chang, M.S. Poncirin, an orally active flavonoid exerts antidiabetic complications and improves glucose uptake activating PI3K/Akt signaling pathway in insulin resistant C2C12 cells with anti-glycation capacities. Bioorg. Chem. 2020, 102, 104061. [Google Scholar]
- Ullah, H.; Khan, A.; Baig, M.W.; Ullah, N.; Ahmed, N.; Tipu, M.K.; Ali, H.; Khan, S. Poncirin attenuates CCL4-induced liver injury through inhibition of oxidative stress and inflammatory cytokines in mice. BMC Complement. Med. Ther. 2020, 20, 115. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, A.; Bibi, T.; Ahmad, S.; Shehzad, O.; Ali, H.; Seo, E.K.; Khan, S. Comprehensive in vivo and in silico approaches to explore the hepatoprotective activity of poncirin against paracetamol toxicity. Naunyn Schmiedeberg’s Arch. Pharmacol. 2022, 395, 195–215. [Google Scholar] [CrossRef]
- Afridi, R.; Khan, A.U.; Khalid, S.; Shal, B.; Rasheed, H.; Ullah, M.Z.; Shehzad, O.; Kim, Y.S.; Khan, S. Anti-hyperalgesic properties of a flavanone derivative Poncirin in acute and chronic inflammatory pain models in mice. BMC Pharmacol. Toxicol. 2019, 20, 57. [Google Scholar] [CrossRef]
- Yang, L.X.; Chen, F.Y.; Yu, H.L.; Liu, P.Y.; Bao, X.Y.; Xia, S.N.; Gu, Y.; Xu, Y.; Cao, X. Poncirin suppresses lipopolysaccharide (LPS)-induced microglial inflammation and ameliorates brain ischemic injury in experimental stroke in mice. Ann. Transl. Med. 2020, 8, 1344. [Google Scholar] [CrossRef]
- Kang, G.D.; Kim, D.H. Poncirin and its metabolite ponciretin attenuate colitis in mice by inhibiting LPS binding on TLR4 of macrophages and correcting Th17/Treg imbalance. J. Ethnopharmacol. 2016, 189, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhu, J.; Wang, Y.; Zhang, N.; Gober, H.J.; Qiu, X.; Li, D.; Wang, L. Chinese single herbs and active ingredients for postmenopausal osteoporosis: From preclinical evidence to action mechanism. Biosci. Trends 2017, 11, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, L.; Wang, B. Poncirin ameliorates oxygen glucose deprivation/reperfusion injury in cortical neurons via inhibiting NOX4-mediated NLRP3 inflammasome activation. Int. Immunopharmacol. 2022, 102, 107210. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.H.; Jin, H.C.; Kang, K.S.; Chang, T.S.; Hwang, G.S. Poncirin inhibits osteoclast differentiation and bone loss through down-regulation of NFATc1 in vitro and in vivo. Biomol. Ther. 2020, 28, 337. [Google Scholar] [CrossRef]
- Yao, Q.; Lin, M.T.; Zhu, Y.D.; Xu, H.L.; Zhao, Y.Z. Recent trends in potential therapeutic applications of the dietary flavonoid didymin. Molecules 2018, 23, 2547. [Google Scholar] [CrossRef]
- Ali, M.Y.; Zaib, S.; Rahman, M.M.; Jannat, S.; Iqbal, J.; Park, S.K.; Chang, M.S. Didymin, a dietary citrus flavonoid exhibits anti-diabetic complications and promotes glucose uptake through the activation of PI3K/Akt signaling pathway in insulin-resistant HepG2 cells. Chem. Biol. Interact. 2019, 305, 180–194. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef]
- Kiran, S.D.; Rohini, P.; Bhagyasree, P. Flavonoid: A review on Naringenin. J. Pharmacogn. Phytochem. 2017, 6, 2778–2783. [Google Scholar]
- Da Silva, R.R.; De Oliveira, T.T.; Nagem, T.J.; Pinto, A.S.; Albino, L.F.; De Almeida, M.R.; De Moraes, G.H.; Pinto, J.G. Hypocholesterolemic effect of naringin and rutin flavonoids. Arch. Latinoam. Nutr. 2001, 51, 258–264. [Google Scholar]
- Sehrawat, N.; Upadhyay, S.K.; Sharma, A.K.; Kumar, S.; Yadav, M. Emerging renoprotective role of citrus flavonoid naringin: Current pharmaceutical status and future perspectives. Curr. Pharmacol. Rep. 2021, 7, 96–101. [Google Scholar] [CrossRef]
- Pereira, R.M.; Andrades, N.E.; Paulino, N.; Sawaya, A.C.; Eberlin, M.N.; Marcucci, M.C.; Favero, G.M.; Novak, E.M.; Bydlowski, S.P. Synthesis and characterization of a metal complex containing naringin and Cu, and its antioxidant, antimicrobial, antiinflammatory and tumor cell cytotoxicity. Molecules 2007, 12, 1352–1366. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef] [PubMed]
- Dhanya, R.; Jayamurthy, P. In vitro evaluation of antidiabetic potential of hesperidin and its aglycone hesperetin under oxidative stress in skeletal muscle cell line. Cell Biochem. Funct. 2020, 38, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Arman, M.S.; Tayab, M.A.; Islam, M.N.; Xiao, J. Recent advances in the biosynthesis, bioavailability, toxicology, pharmacology, and controlled release of citrus neohesperidin. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Shishir, M.R.; Karim, N.; Gowd, V.; Xie, J.; Zheng, X.; Chen, W. Pectin-chitosan conjugated nanoliposome as a promising delivery system for neohesperidin: Characterization, release behavior, cellular uptake, and antioxidant property. Food Hydrocoll. 2019, 95, 432–444. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, C.; Yan, Y.; Chen, Q.; Luo, F.; Zhu, X.; Li, X.; Chen, K. Purification of naringin and neohesperidin from Huyou (Citrus changshanensis) fruit and their effects on glucose consumption in human HepG2 cells. Food Chem. 2012, 135, 1471–1478. [Google Scholar] [CrossRef]
- Han Jie, L.; Jantan, I.; Yusoff, S.D.; Jalil, J.; Husain, K. Sinensetin: An insight on its pharmacological activities, mechanisms of action and toxicity. Front. Pharmacol. 2021, 11, 553404. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Shen, J.; Huang, L.; Men, S.; Wang, T. Sinensetin attenuates oxygen–glucose deprivation/reperfusion-induced neurotoxicity by MAPK pathway in human cerebral microvascular endothelial cells. J. Appl. Toxicol. 2022, 42, 683–693. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, S.M.; Ha, S.E.; Vetrivel, P.; Saralamma, V.V.; Kim, E.H.; Kim, G.S. Sinensetin regulates age-related sarcopenia in cultured primary thigh and calf muscle cells. BMC Complement. Altern. Med. 2019, 19, 287. [Google Scholar] [CrossRef]
- Xiong, Y.J.; Deng, Z.B.; Liu, J.N.; Qiu, J.J.; Guo, L.; Feng, P.P.; Sui, J.R.; Chen, D.P.; Guo, H.S. Enhancement of epithelial cell autophagy induced by sinensetin alleviates epithelial barrier dysfunction in colitis. Pharmacol. Res. 2019, 148, 104461. [Google Scholar] [CrossRef]
- Singh, A.P.; Kandpal, J.B.; Sharma, R.K.; Chitme, H. Nobiletin a biologically active phytoconstituent: Systematic review. J. Biol. Act. Prod. Nat. 2021, 11, 204–211. [Google Scholar] [CrossRef]
- Li, S.; Wang, H.; Guo, L.; Zhao, H.; Ho, C.T. Chemistry and bioactivity of nobiletin and its metabolites. J. Funct. Foods 2014, 6, 2–10. [Google Scholar] [CrossRef]
- Mileykovskaya, E.; Yoo, S.H.; Dowhan, W.; Chen, Z. Nobiletin: Targeting the circadian network to promote bioenergetics and healthy aging. Biochemistry 2020, 85, 1554–1559. [Google Scholar] [CrossRef]
- Salehi, B.; Cruz-Martins, N.; Butnariu, M.; Sarac, I.; Bagiu, I.C.; Ezzat, S.M.; Wang, J.; Koay, A.; Sheridan, H.; Adetunji, C.O.; et al. Hesperetin’s health potential: Moving from preclinical to clinical evidence and bioavailability issues, to upcoming strategies to overcome current limitations. Crit. Rev. Food Sci. Nutr. 2022, 62, 4449–4464. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wang, Y.; Xu, S.; Ren, J.; Tang, L.; Gong, J.; Lin, Y.; Fang, H.; Su, D. Hesperetin, a promising treatment option for diabetes and related complications: A literature review. J. Agric. Food Chem. 2022, 70, 8582–8592. [Google Scholar] [CrossRef] [PubMed]
- Famurewa, A.C.; Renu, K.; Eladl, M.A.; Chakraborty, R.; Myakala, H.; El-Sherbiny, M.; Elsherbini, D.M.; Vellingiri, B.; Madhyastha, H.; Wanjari, U.R.; et al. Hesperidin and hesperetin against heavy metal toxicity: Insight on the molecular mechanism of mitigation. Biomed. Pharmacother. 2022, 149, 112914. [Google Scholar] [CrossRef] [PubMed]
- Scoditti, E. Neuroinflammation and neurodegeneration: The promising protective role of the citrus flavanone hesperetin. Nutrients 2020, 12, 2336. [Google Scholar] [CrossRef]
- Mitra, S.; Lami, M.S.; Uddin, T.M.; Das, R.; Islam, F.; Anjum, J.; Hossain, M.J.; Emran, T.B. Prospective multifunctional roles and pharmacological potential of dietary flavonoid narirutin. Biomed. Pharmacother. 2022, 150, 112932. [Google Scholar] [CrossRef]
- Yang, H.; Shan, Z.; Guo, W.; Wang, Y.; Cai, S.; Li, F.; Huang, Q.; Liu, J.A.; Cheung, C.W.; Cai, S. Reversal of Peripheral Neuropathic Pain by the Small-Molecule Natural Product Narirutin via Block of Nav1. 7 Voltage-Gated Sodium Channel. Int. J. Mol. Sci. 2022, 23, 14842. [Google Scholar] [CrossRef]
- Qurtam, A.A.; Mechchate, H.; Es-Safi, I.; Al-Zharani, M.; Nasr, F.A.; Noman, O.M.; Aleissa, M.; Imtara, H.; Aleissa, A.M.; Bouhrim, M.; et al. Citrus flavanone narirutin, in vitro and in silico mechanistic antidiabetic potential. Pharmaceutics 2021, 13, 1818. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Shin, E.M.; Guo, L.Y.; Zou, L.B.; Xu, G.H.; Lee, S.H.; Ze, K.R.; Kim, E.K.; Kang, S.S.; Kim, Y.S. Anti-inflammatory activity of 21 (α, β)-methylmelianodiols, novel compounds from Poncirus trifoliata Rafinesque. Eur. J. Pharmacol. 2007, 572, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Shal, B.; Khan, A.; Naveed, M.; Ali, H.; Seo, E.K.; Choi, H.; Khan, S. Neuroprotective effect of 25-Methoxyhispidol A against CCl4-induced behavioral alterations by targeting VEGF/BDNF and caspase-3 in mice. Life Sci. 2020, 253, 117684. [Google Scholar] [CrossRef] [PubMed]
- Aldonza, M.B.; Hong, J.Y.; Bae, S.Y.; Song, J.; Kim, W.K.; Oh, J.; Shin, Y.; Lee, S.H.; Lee, S.K. Suppression of MAPK signaling and reversal of mTOR-dependent MDR1-associated multidrug resistance by 21α-methylmelianodiol in lung cancer cells. PLoS ONE 2015, 10, e0127841. [Google Scholar]
- Garg, B.J.; Garg, N.K.; Beg, S.; Singh, B.; Katare, O.P. Nanosized ethosomes-based hydrogel formulations of methoxsalen for enhanced topical delivery against vitiligo: Formulation optimization, in vitro evaluation and preclinical assessment. J. Drug Target. 2016, 24, 233–246. [Google Scholar] [CrossRef]
- Deng, M.; Xie, L.; Zhong, L.; Liao, Y.; Liu, L.; Li, X. Imperatorin: A review of its pharmacology, toxicity and pharmacokinetics. Eur. J. Pharmacol. 2020, 879, 173124. [Google Scholar] [CrossRef]
- Nasser, M.I.; Zhu, S.; Hu, H.; Huang, H.; Guo, M.; Zhu, P. Effects of imperatorin in the cardiovascular system and cancer. Biomed. Pharmacother. 2019, 120, 109401. [Google Scholar] [CrossRef]
- Wang, N.; Wang, J.; Zhang, Y.; Zeng, Y.; Hu, S.; Bai, H.; Hou, Y.; Wang, C.; He, H.; He, L. Imperatorin ameliorates mast cell-mediated allergic airway inflammation by inhibiting MRGPRX2 and CamKII/ERK signaling pathway. Biochem. Pharmacol. 2021, 184, 114401. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Chen, C.Y.; Lin, I.W.; Leu, Y.L.; Yang, S.C.; Syu, Y.T.; Chen, P.J.; Hwang, T.L. Imperatorin alleviates psoriasiform dermatitis by blocking neutrophil respiratory burst, adhesion, and chemotaxis through selective phosphodiesterase 4 inhibition. Antioxid. Redox Signal. 2021, 35, 885–903. [Google Scholar] [CrossRef]
- Kim, T.; Hyun, C.G. Imperatorin Positively Regulates Melanogenesis through Signaling Pathways Involving PKA/CREB, ERK, AKT, and GSK3β/β-Catenin. Molecules 2022, 27, 6512. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Nakos-Bimpos, M.; Polissidis, A.; Dalla, C.; Kokras, N.; Skalicka-Woźniak, K.; Budzyńska, B. Imperatorin Influences Depressive-like Behaviors: A Preclinical Study on Behavioral and Neurochemical Sex Differences. Molecules 2022, 27, 1179. [Google Scholar] [CrossRef]
- Kim, N.Y.; Jung, Y.Y.; Yang, M.H.; Um, J.Y.; Sethi, G.; Ahn, K.S. Isoimperatorin down-regulates epithelial mesenchymal transition through modulating NF-κB signaling and CXCR4 expression in colorectal and hepatocellular carcinoma cells. Cell. Signal. 2022, 99, 110433. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Xin, C.; Chen, W. Isoimperatorin induces apoptosis of the SGC-7901 human gastric cancer cell line via the mitochondria-mediated pathway. Oncol. Lett. 2017, 13, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Han, T.; Zhan, S.; Jiang, Y.; Liu, X.; Li, G. Antiviral Activity of Isoimperatorin against Influenza a Virus in vitro and its Inhibition of Neuraminidase. Front. Pharmacol. 2021, 12, 657826. [Google Scholar] [CrossRef]
- Wijerathne, C.U.; Seo, C.S.; Song, J.W.; Park, H.S.; Moon, O.S.; Won, Y.S.; Kwon, H.J.; Son, H.Y. Isoimperatorin attenuates airway inflammation and mucus hypersecretion in an ovalbumin-induced murine model of asthma. Int. Immunopharmacol. 2017, 49, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Wu, J.; Fan, J.; Yuan, P.; Shi, Q.; Jin, K.; Cheng, W.; Zhao, X.; Zhang, Y.; Li, W.; et al. In vitro activity of isoimperatorin, alone and in combination, against M ycobacterium tuberculosis. Lett. Appl. Microbiol. 2014, 58, 344–349. [Google Scholar] [CrossRef]
- Jiang, T.; Shi, X.; Yan, Z.; Wang, X.; Gun, S. Isoimperatorin enhances 3T3 L1 preadipocyte differentiation by regulating PPARγ and C/EBPα through the Akt signaling pathway. Exp. Ther. Med. 2019, 18, 2160–2166. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, Y.; Xu, Y.; Zhang, M.; Guo, S.; Zhang, G. Isoimperatorin exerts anti-inflammatory activity by targeting the LPS-TLR4/MD-2-NF-κB pathway. Eur. J. Inflamm. 2021, 19, 1–10. [Google Scholar] [CrossRef]
- Ouyang, J.; Jiang, H.; Fang, H.; Cui, W.; Cai, D. Isoimperatorin ameliorates osteoarthritis by downregulating the mammalian target of rapamycin C1 signaling pathway. Mol. Med. Rep. 2017, 16, 9636–9644. [Google Scholar] [CrossRef]
- Zou, J.; Duan, Y.; Wang, Y.; Liu, A.; Chen, Y.; Guo, D.; Guo, W.; Li, S.; Su, Z.; Wu, Y.; et al. Phellopterin cream exerts an anti-inflammatory effect that facilitates diabetes-associated cutaneous wound healing via SIRT1. Phytomedicine 2022, 107, 154447. [Google Scholar] [CrossRef]
- Bao, R.; Wang, S.; Yang, X.; Chen, G.; Meng, X. Phellopterin-induced caspase-dependent apoptosis through PI3K/AKT pathway inhibition in SMMC-7721 human hepatoma cells. Lat. Am. J. Pharm. 2018, 37, 2498–2501. [Google Scholar]
- Fiorito, S.; Preziuso, F.; Sharifi-Rad, M.; Marchetti, L.; Epifano, F.; Genovese, S. Auraptene and umbelliprenin: A review on their latest literature acquisitions. Phytochem. Rev. 2022, 21, 317–326. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Kawaguchi, S.; Miyazaki, Y.; Katanasaka, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Hamabe-Horiike, T.; Kawase, Y.; Komiyama, M.; et al. Auraptene, a citrus peel-derived natural product, prevents myocardial infarction-induced heart failure by activating PPARα in rats. Phytomedicine 2022, 107, 154457. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.H.; Jayakumar, T.; Lu, W.J.; Sheu, J.R.; Hsia, C.W.; Saravana Bhavan, P.; Manubolu, M.; Huang, W.C.; Chang, Y. Auraptene, a Monoterpene Coumarin, Inhibits LTA-Induced Inflammatory Mediators via Modulating NF-κB/MAPKs Signaling Pathways. Evid. Based Complement. Altern. Med. 2021, 2021, 5319584. [Google Scholar] [CrossRef] [PubMed]
- Charmforoshan, E.; Karimi, E.; Oskoueian, E.; Iranshahi, M. Antibacterial, Antioxidant and Melanogenesis Inhibitory Activity of Auraptene, a Coumarin from Ferula szowitsiana Root. Nutr. Cancer 2022, 74, 1829–1836. [Google Scholar] [CrossRef]
- Mazimba, O. Umbelliferone: Sources, chemistry and bioactivities review. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 223–232. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, X.; Xiong, Z.; Tang, H.; Guo, H.; Ye, G.; Chen, D.; Yang, S.; Yin, Z.; Fu, H.; et al. The Antivirulence Activity of Umbelliferone and Its Protective Effect against A. hydrophila-Infected Grass Carp. Int. J. Mol. Sci. 2022, 23, 11119. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.F.; de Figueiredo, G.F.; Pedro, L.P.; Amorin, Y.M.; Andrade, J.T.; Passos, T.F.; Rodrigues, F.F.; Souza, I.L.; Gonçalves, T.P.; dos Santos Lima, L.A.; et al. Umbelliferone (7-hydroxycoumarin): A non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed. Pharmacother. 2020, 129, 110432. [Google Scholar] [CrossRef]
- Khan, A.; Shehzad, O.; Seo, E.K.; Onder, A.; Khan, S. Anti-allergic activities of Umbelliferone against histamine-and Picryl chloride-induced ear edema by targeting Nrf2/iNOS signaling in mice. BMC Complement. Med. Ther. 2021, 21, 215. [Google Scholar]
- Hindam, M.O.; Sayed, R.H.; Skalicka-Woźniak, K.; Budzyńska, B.; El Sayed, N.S. Xanthotoxin and umbelliferone attenuate cognitive dysfunction in a streptozotocin-induced rat model of sporadic Alzheimer’s disease: The role of JAK2/STAT3 and Nrf2/HO-1 signalling pathway modulation. Phytother. Res. 2020, 34, 2351–2365. [Google Scholar] [CrossRef]
- Mottaghipisheh, J. Oxypeucedanin: Chemotaxonomy, isolation, and bioactivities. Plants 2021, 10, 1577. [Google Scholar] [CrossRef]
- Tavakoli, S.; Delnavazi, M.R.; Hadjiaghaee, R.; Jafari-Nodooshan, S.; Khalighi-Sigaroodi, F.; Akhbari, M.; Hadjiakhoondi, A.; Yassa, N. Bioactive coumarins from the roots and fruits of Ferulago trifida Boiss., an endemic species to Iran. Nat. Prod. Res. 2018, 32, 2724–2728. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Pyne, S.G.; Keller, P.A.; Taweechotipatr, M.; Kamchonwongpaisan, S. Phenylpropanoids and furanocoumarins as antibacterial and antimalarial constituents of the Bhutanese medicinal plant Pleurospermum amabile. Nat. Prod. Commun. 2014, 9, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Okada, R.; Abe, H.; Okuyama, T.; Nishidono, Y.; Ishii, T.; Sato, T.; Shirako, S.; Tanaka, K.; Ikeya, Y.; Nishizawa, M. Comparison of the anti-inflammatory activities of furanocoumarins from the roots of Angelica dahurica. Bioact. Compd. Health Dis. 2021, 4, 287–300. [Google Scholar] [CrossRef]
- Lee, J.A.; Ha, H.K.; Jung, D.Y.; Lee, H.Y.; Lee, N.H.; Lee, J.K.; Huang, D.S.; Shin, H.K. Anti-inflammatory Effects of Sam-chul-kun-bi-tang. J. Korean Med. 2010, 31, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.N.; Kwon, Y.K.; Kim, B.J. Effects of Samchulkunbi-tang in Cultured Interstitial Cells of Cajal of Murine Small Intestine. J. Physiol. Pathol. Korean Med. 2013, 27, 112–117. [Google Scholar]
- Kim, J.Y.; Kim, J.D.; Kam, C.W. Effects of mahwangyoonpye-tang on asthma induced by ovalbumin in mouse. J. Physiol. Pathol. Korean Med. 2003, 17, 1453–1462. [Google Scholar]
- Liu, H.H.; Park, M.Y.; Choi, H.Y.; Gu, D.M.; Kim, J.D.; Song, K.K. Synergic effect of Mahwangyounpae-tang and ciprofloxacin on 5 strains of aerobic Gram-negative bacteria. J. Physiol. Pathol. Korean Med. 2005, 19, 684–689. [Google Scholar]
- Um, Y.R.; Lee, J.H.; Moon, H.J.; Park, H.Y.; Ma, J.Y. Acute toxicity study on Ojeok-san (Wuji-san) in mice. J. Korean Obstet. Gynecol. 2009, 22, 135–142. [Google Scholar]
- Jung, Y.P.; Yim, N.H.; Kim, A.; Hwang, Y.H.; Park, H.; Ma, J.Y. Single Oral Dose Toxicity Test of Fermented Samchulgeonbi-tang Extract in ICR mice. Korea J. Herbol. 2013, 28, 61–65. [Google Scholar] [CrossRef]


| Compounds | Relative Amount (%) |
|---|---|
| Limonene | 41.73 |
| Myrcene | 15.68 |
| (E)-β-Ocimene | 5.05 |
| α-Phellandrene | 4.11 |
| β-Pinene | 3.95 |
| trans-Caryophyllene | 3.59 |
| Ethyl hexanoate | 2.22 |
| Ethyl octanoate | 2.12 |
| p-Cimene | 1.5 |
| α-Pinene | 1.19 |
| Sabinene | 1.17 |
| β-Farnesene | 1.16 |
| (E,Z)-Farnesol | 1.02 |
| Hexyl butanoate | 0.98 |
| δ-Cadinene | 0.73 |
| α-Humulene | 0.72 |
| Ethyl decanoate | 0.72 |
| γ-Terpinene | 0.70 |
| Linalool | 0.69 |
| Nerol | 0.65 |
| β-Elemene | 0.59 |
| Geranyl acetate | 0.56 |
| Terpinolene | 0.54 |
| Geraniol | 0.52 |
| Germacrene B | 0.51 |
| p-Mentha-1,3,8-triene | 0.46 |
| Ethyl laurate | 0.46 |
| Spathulenol | 0.44 |
| (E)-Nerolidol | 0.42 |
| Decanal | 0.42 |
| Pentacosane | 0.42 |
| Ethyl stearate | 0.35 |
| Eicosane | 0.35 |
| Neryl acetate | 0.34 |
| Heptacosane | 0.32 |
| Ethyl myristate | 0.31 |
| α-Terpineol | 0.30 |
| α-Cubebene | 0.28 |
| Myristic acid | 0.26 |
| Ethyl linoleate | 0.23 |
| Dodecanal | 0.22 |
| γ-Cadinene | 0.20 |
| Octadecane | 0.14 |
| Nonadecane | 0.12 |
| Nonacosane | <0.1 |
| Nonanal | <0.1 |
| Terpinen-4-ol | <0.1 |
| α-Terpinene | <0.1 |
| Identified Compounds | Fresh Fruit Juice (µg/mL) | Seed Extract (µg/g of Dry Extract) |
|---|---|---|
| Flavanone aglycones | ||
| Hesperetin | 55.13 | Not detected |
| Naringenin | 28.65 | Not detected |
| Flavanone-O-glycosides | ||
| Didymin | 78.83 | 156.42 |
| Hesperidin | 129.33 | Not detected |
| Naringin | 115.79 | Not detected |
| Narirutin | 75.73 | 37.62 |
| Neohesperedin | 32.75 | 80.12 |
| Poncirin | 49.37 | Not detected |
| Flavone aglycones | ||
| Rhamnetin | 0.37 | Not detected |
| Quercetin | 0.76 | Not detected |
| Flavone-O-glycoside | ||
| Rutin | 1.85 | Not detected |
| Phenolic acids | ||
| Caffeic acid | 18.46 | 32.85 |
| Chlorogenic acid | 112.54 | Not Detected |
| Bioactive Compound | Pharmacological Activities |
|---|---|
| Poncirin | Anticancer [101,102], antidiabetic [103], hepatoprotective [104,105], analgesics [106], anti-inflammatory [106,107], attenuation of colitis [108], anti-bacterial, anti-adipogenic [109], neuroprotective [110], suppression of osteoclast differentiation [111] |
| Neoponcirin (didymin) | Anticancer, neuroprotective, anxiolytic, antinociceptive hepatoprotective, and cardioprotective, anti-inflammatory activities [112], antidiabetic [113] |
| Naringin | Anticancer, antioxidant, anti-inflammatory, neuroprotective, increased bone regeneration, genoprotective, amelioration of metabolic syndrome [114], hepatoprotective, cardioprotective, gastroprotective, immuno-promotive [115], hypocholesterolemic [116], renoprotective [117], antibacterial [118] |
| Hespiridin | Antioxident, anti-inflammatory, anticancer, antiviral, cardioprotective, neuroprotective, antibacterial, radioprotective, wound healing [119], antidiabetic [120] |
| Neohesperidin | Neuroprotective, anti-inflammatory, antidiabetic, antimicrobial, anticancer, gastroprotective, cardioprotective, hepatoprotective, anti-obesity [121], antioxidant [122], increased glucose uptake [123] |
| Sinensetin | Antiangiogenetic, anticancer, anti-dementia, anti-inflammatory, antimicrobial, anti-obesity, antidiabetic, antioxidant, anti-trypanosomal, diuretic, hypolipidemic, vasorelaxant [124], neuroprotective [125], alleviation of age-related muscle loss [126], gastroprotective [127] |
| Nobiletin | Anticancer, anti-obesity, antioxidant, memory enhancing, chondroprotective, anti-tuberculosis, hepatoprotective, cardioprotective, anti-hypertensive, immunoenhancing, gastroprotective, antiviral [128], amelioration of metabolic disorders [129], anti-aging [130] |
| Hesperetin | Anticancer, cardioprotective, regulation of carbohydrate and lipids metabolism, osteoprotective, anti-asthmatic, radioprotective, regulation of melanogenesis, anti-inflammatory [131], antidiabetic [132], mitigation of heavy metals toxicity [133], neuroprotective [134] |
| Narirutin | Anticancer, neuroprotective, anti-inflammatory, antidepressant, hepatoprotective, antioxidant, anti-adipogenic, immunomodulatory, antiallergic [135], analgesics [136], antidiabetic [137] |
| 21β-Methylmelianodiol | Anti-inflammatory [138] |
| Hispidol β 25-methyl ether | Anti-inflammatory [83], neuroprotective [82] |
| Hispidol A 25-methyl ether | Neuroprotective [139] |
| 21α-Methylmelianodiol | Anti-inflammatory [138], anticancer [140] |
| Pancastatin A and B | Anticancer [62] |
| 25-Methoxyhispidol A and B | Neuroprotective [139], anticancer [51], anti-inflammatory [83] |
| Methoxsalen | Vitiligo management [141] |
| Imperatorin | Neuroprotective, anticancer, anti-inflammatory, antihypertensive, antibacterial, antiviral, anticoagulant [142], antioxidant [143], antiallergic [144], anti-psoriatic [145], regulation of melanogenesis [146], antidepressant [147] |
| Isoimperatorin | Anticancer [148,149], antiviral [150], anti-asthmatic [151], antibacterial [152], anti-adipogenic [153], anti-inflammatory [154], alleviation of osteoarthritis [155] |
| Phellopterin | Wound healing, anti-inflammatory [156], anticancer [157], anti-adipogenic [66] |
| Auraptene | Neuroprotective, antioxidant, anticancer, inhibition of platelet aggregation, hepatoprotective, antibacterial [158], cardioprotection [159], anti-inflammatory [160], melanogenesis inhibition [161] |
| Umbelliferone | Antibacterial, antifungal, antioxidant, antidiabetic, anticancer, molluscicidal [162], antiviral [163], antidiarrheal [164], antiallergic [165], anti- Alzheimer [166] |
| Oxypeucedanin | Antiallergic, antiarrhythmic, anticonvulsant, antifeedant, antigenotoxic, ant inflammatory, antimalarial, antimicrobial, antioxidant, antiproliferative, antiviral, calcium antagonistic, cytotoxic, insecticidal, phytotoxic [167], anti-adipogenic [66] |
| Oxypeucedanin methanolate | Antioxidant, antibacterial [168], antimalarial [169], anti-inflammatory [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamichhane, G.; Pandey, J.; Devkota, H.P. Bioactive Chemical Constituents and Pharmacological Activities of Ponciri Fructus. Molecules 2023, 28, 255. https://doi.org/10.3390/molecules28010255
Lamichhane G, Pandey J, Devkota HP. Bioactive Chemical Constituents and Pharmacological Activities of Ponciri Fructus. Molecules. 2023; 28(1):255. https://doi.org/10.3390/molecules28010255
Chicago/Turabian StyleLamichhane, Gopal, Jitendra Pandey, and Hari Prasad Devkota. 2023. "Bioactive Chemical Constituents and Pharmacological Activities of Ponciri Fructus" Molecules 28, no. 1: 255. https://doi.org/10.3390/molecules28010255
APA StyleLamichhane, G., Pandey, J., & Devkota, H. P. (2023). Bioactive Chemical Constituents and Pharmacological Activities of Ponciri Fructus. Molecules, 28(1), 255. https://doi.org/10.3390/molecules28010255









