Kukhtin–Ramirez-Reaction-Inspired Deprotection of Sulfamidates for the Synthesis of Amino Sugars
Abstract
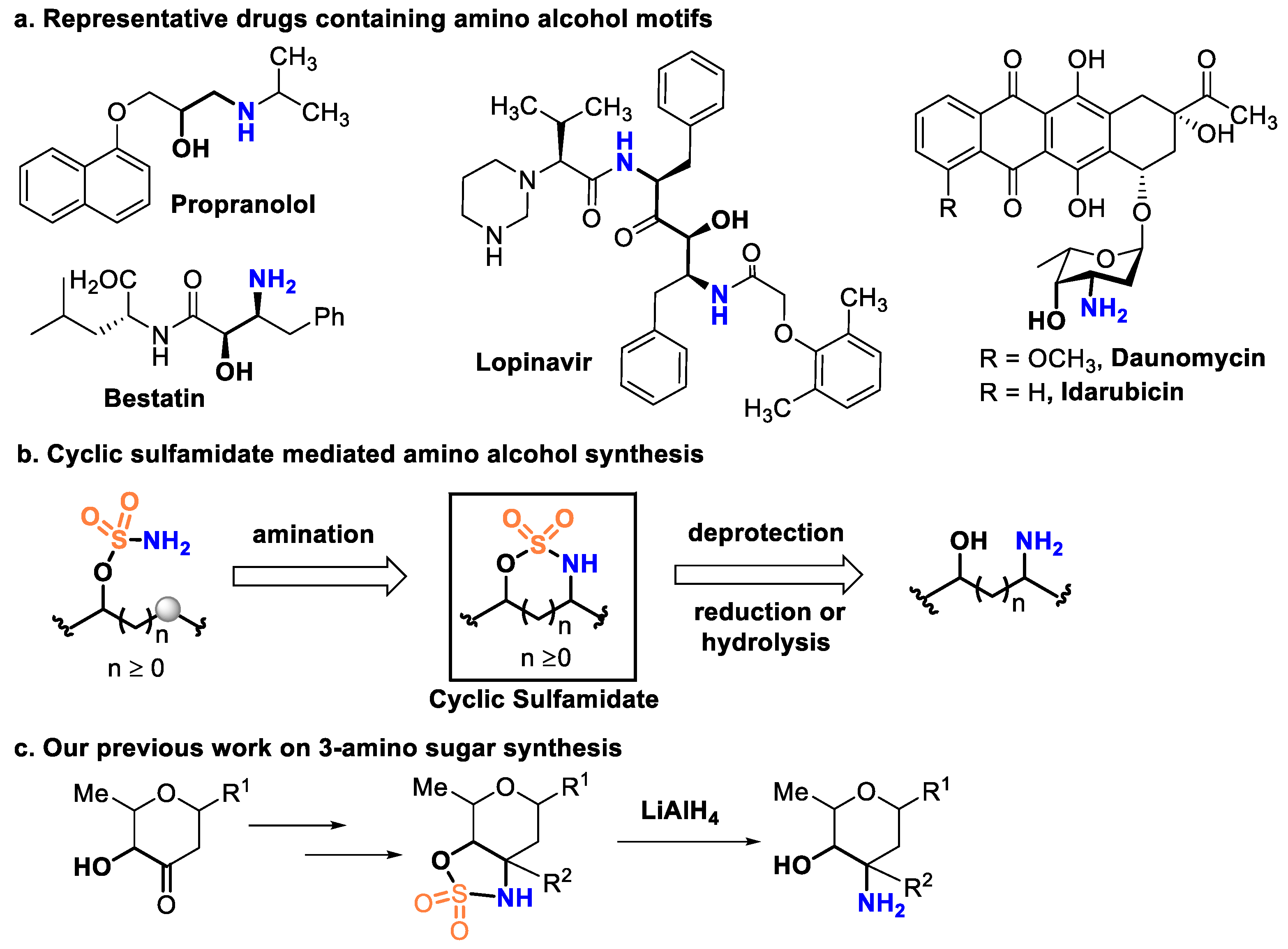
1. Introduction
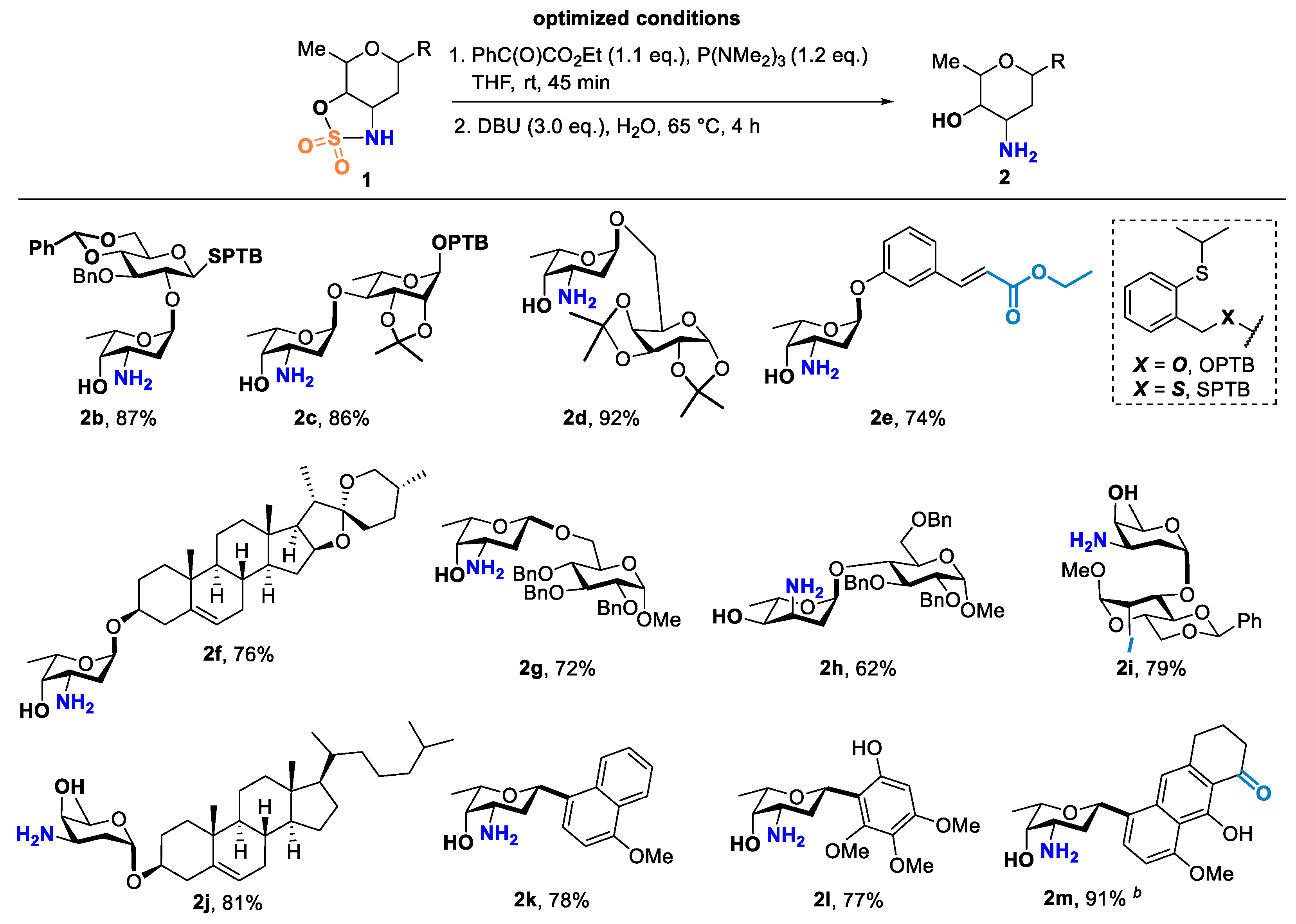
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Procedures for Compound 3a and 4a
3.2.1. Procedures for Compound 3a
3.2.2. Procedures for Compound 4a
3.3. General Procedure for Deprotection and Characterization of the Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heravi, M.M.; Lashaki, T.B.; Fattahi, B.; Zadsirjan, V. Application of asymmetric Sharpless aminohydroxylation in total synthesis of natural products and some synthetic complex bio-active molecules. RSC Adv. 2018, 8, 6634–6659. [Google Scholar] [CrossRef] [PubMed]
- Dimarco, A.; Gaetani, M.; Orezzi, P.; Scarpinato, B.M.; Silvestrini, R.; Soldati, M.; Dasdia, T.; Valentini, L. ‘Daunomycin’, a new antibiotic of the rhodomycin group. Nature 1964, 201, 706–707. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, S.C.; Stanchina, D.M. Acylnitrene route to vicinal amino alcohols. Application to the synthesis of (-)-Bestatin and analogues. J. Org. Chem. 1999, 64, 2852–2859. [Google Scholar] [CrossRef] [PubMed]
- Ager, D.J.; Prakash, I.; Schaad, D.R. 1,2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 1996, 96, 835–876. [Google Scholar] [CrossRef] [PubMed]
- Fache, F.; Schulz, E.; Tommasino, M.L.; Lemaire, M. Nitrogen-containing ligands for asymmetric homogeneous and heterogeneous catalysis. Chem. Rev. 2000, 100, 2159–2231. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Rose, B.T.; Olen, C.L.; Roth, A.; Kwong, A.C.; Wang, Y.; Denmark, S.E. A unified strategy for the asymmetric synthesis of highly substituted 1,2-amino alcohols leading to highly substituted bisoxazoline ligands. J. Org. Chem. 2021, 86, 3490–3534. [Google Scholar] [CrossRef] [PubMed]
- Malkov, A.V.; Kabeshov, M.A.; Bella, M.; Kysilka, O.; Malyshev, D.A.; Pluháčková, K.; Kočovský, P. Vicinal amino alcohols as organocatalysts in asymmetric cross-aldol reaction of ketones: Application in the synthesis of convolutamydine A. Org. Lett. 2007, 9, 5473–5476. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Farajpour, B. Applications of oxazolidinones as chiral auxiliaries in the asymmetric alkylation reaction applied to total synthesis. RSC Adv. 2016, 6, 30498–30551. [Google Scholar] [CrossRef]
- Lait, S.M.; Rankic, D.A.; Keay, B.A. 1,3-Aminoalcohols and their derivatives in asymmetric organic synthesis. Chem. Rev. 2007, 107, 767–796. [Google Scholar] [CrossRef]
- Donohoe, T.J.; Callens, C.K.; Flores, A.; Lacy, A.R.; Rathi, A.H. Recent developments in methodology for the direct oxyamination of olefins. Chemistry 2011, 17, 58–76. [Google Scholar] [CrossRef]
- Palomo, C.; Oiarbide, M.; Laso, A. Recent advances in the catalytic asymmetric nitroaldol (Henry) reaction. Eur. J. Org. Chem. 2007, 2007, 2561–2574. [Google Scholar] [CrossRef]
- Gupta, P.; Mahajan, N. Biocatalytic approaches towards the stereoselective synthesis of vicinal amino alcohols. New J. Chem. 2018, 42, 12296–12327. [Google Scholar] [CrossRef]
- Karjalainen, O.K.; Koskinen, A.M. Diastereoselective synthesis of vicinal amino alcohols. Org. Biomol. Chem. 2012, 10, 4311–4326. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, S.C. The synthesis of vicinal amino alcohols. Tetrahedron 2000, 56, 2561–2576. [Google Scholar] [CrossRef]
- Burchak, O.N.; Py, S. Reductive cross-coupling reactions (RCCR) between C=N and C=O for β-amino alcohol synthesis. Tetrahedron 2009, 65, 7333–7356. [Google Scholar] [CrossRef]
- Appel, R.; Berger, G. Hydrazinsulfonsäure-amide, I. Über das Hydrazodisulfamid. Chem. Ber. 1958, 91, 1339–1341. [Google Scholar] [CrossRef]
- Armitage, I.; Berne, A.M.; Elliott, E.L.; Fu, M.; Hicks, F.; McCubbin, Q.; Zhu, L. N-(tert-butoxycarbonyl)-N-[(triethylenediammonium)sulfonyl] azanide: A convenient sulfamoylation reagent for alcohols. Org. Lett. 2012, 14, 2626–2629. [Google Scholar] [CrossRef]
- Okada, M.; Iwashita, S.; Koizumi, N. Efficient general method for sulfamoylation of a hydroxyl group. Tetrahedron Lett. 2000, 41, 7047–7051. [Google Scholar] [CrossRef]
- Rapp, P.B.; Murai, K.; Ichiishi, N.; Leahy, D.K.; Miller, S.J. Catalytic sulfamoylation of alcohols with activated aryl sulfamates. Org. Lett. 2020, 22, 168–174. [Google Scholar] [CrossRef]
- Sguazzin, M.A.; Johnson, J.W.; Magolan, J. Hexafluoroisopropyl sulfamate: A useful reagent for the synthesis of sulfamates and sulfamides. Org. Lett. 2021, 23, 3373–3378. [Google Scholar] [CrossRef]
- Wang, H.M.; Xiong, C.D.; Chen, X.Q.; Hu, C.; Wang, D.Y. Preparation of sulfamates and sulfamides using a selective sulfamoylation agent. Org. Lett. 2021, 23, 2595–2599. [Google Scholar] [CrossRef] [PubMed]
- Collet, F.; Dodd, R.H.; Dauban, P. Catalytic C-H amination: Recent progress and future directions. Chem. Commun. 2009, 5061–5074. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.F.; Rujirawanich, J.; Gallagher, T. N-heterocycle construction via cyclic sulfamidates. Applications in synthesis. Org. Biomol. Chem. 2010, 8, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.H.; Hyland, C.J.T.; Pyne, S.G. Five-membered cyclic sulfamidate imines: Versatile scaffolds for organic synthesis. Org. Biomol. Chem. 2020, 18, 7467–7484. [Google Scholar] [CrossRef]
- Meléndez, R.E.; Lubell, W.D. Synthesis and reactivity of cyclic sulfamidites and sulfamidates. Tetrahedron 2003, 59, 2581–2616. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Huang, X.; Snyder, S.A.; Bheema Rao, P.; Bella, M.; Reddy, M.V. A novel regio- and stereoselective synthesis of sulfamidates from 1,2-diols using Burgess and related reagents: A facile entry into β-amino alcohols. Angew. Chem. Int. Ed. 2002, 41, 834–838. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Longbottom, D.A.; Nalbandian, A.Z.; Huang, X. New uses for the Burgess reagent in chemical synthesis: Methods for the facile and stereoselective formation of sulfamidates, glycosylamines, and sulfamides. Chemistry 2004, 10, 5581–5606. [Google Scholar] [CrossRef]
- Lee, H.K.; Kang, S.; Choi, E.B. Stereoselective synthesis of norephedrine and norpseudoephedrine by using asymmetric transfer hydrogenation accompanied by dynamic kinetic resolution. J. Org. Chem. 2012, 77, 5454–5460. [Google Scholar] [CrossRef]
- Kang, S.; Han, J.; Lee, E.S.; Choi, E.B.; Lee, H.K. Enantioselective synthesis of cyclic sulfamidates by using chiral Rhodium-catalyzed asymmetric transfer hydrogenation. Org. Lett. 2010, 12, 4184–4187. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yu, C.B.; Wang, D.W.; Wang, X.B.; Zhou, Y.G. Enantioselective synthesis of cyclic sulfamidates via Pd-catalyzed hydrogenation. Org. Lett. 2008, 10, 2071–2074. [Google Scholar] [CrossRef]
- Alderson, J.M.; Phelps, A.M.; Scamp, R.J.; Dolan, N.S.; Schomaker, J.M. Ligand-controlled, tunable silver-catalyzed C-H amination. J. Am. Chem. Soc. 2014, 136, 16720–16723. [Google Scholar] [CrossRef] [PubMed]
- Espino, C.G.; Wehn, P.M.; Chow, J.; Du Bois, J. Synthesis of 1,3-difunctionalized amine derivatives through selective C−H bond oxidation. J. Am. Chem. Soc. 2001, 123, 6935–6936. [Google Scholar] [CrossRef]
- Paradine, S.M.; Griffin, J.R.; Zhao, J.; Petronico, A.L.; Miller, S.M.; Christina White, M. A manganese catalyst for highly reactive yet chemoselective intramolecular C(sp3)-H amination. Nat Chem. 2015, 7, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Hazelard, D.; Nocquet, P.A.; Compain, P. Catalytic C–H amination at its limits: Challenges and solutions. Org. Chem. Front. 2017, 4, 2500–2521. [Google Scholar] [CrossRef]
- Ruppel, J.V.; Kamble, R.M.; Zhang, X.P. Cobalt-catalyzed intramolecular C−H amination with arylsulfonyl azides. Org. Lett. 2007, 9, 4889–4892. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Lu, Y.; Hayashi, T. High performance of a palladium phosphinooxazoline catalyst in the asymmetric arylation of cyclic N-sulfonyl ketimines. Angew. Chem. Int.Ed. 2014, 53, 9936–9939. [Google Scholar] [CrossRef]
- Higginbotham, M.C.; Bebbington, M.W. Gold(I)-catalysed synthesis of cyclic sulfamidates by intramolecular allene hydroamination. Chem. Commun. 2012, 48, 7565–7567. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, Y.H.; Feng, C.G.; Lin, G.Q. Enantioselective Rhodium-catalyzed arylation of cyclic N-sulfamidate alkylketimines: A new access to chiral β-alkyl-β-aryl amino alcohols. Org. Lett. 2014, 16, 3400–3403. [Google Scholar] [CrossRef]
- Weymouth-Wilson, A.C. The role of carbohydrates in biologically active natural products. Nat. Prod. Rep. 1997, 14, 99–110. [Google Scholar] [CrossRef]
- William Lown, J. Anthracycline and anthraquinone anticancer agents: Current status and recent developments. Pharmacol. Ther. 1993, 60, 185–214. [Google Scholar] [CrossRef]
- Zeng, J.; Sun, G.; Yao, W.; Zhu, Y.; Wang, R.; Cai, L.; Liu, K.; Zhang, Q.; Liu, X.W.; Wan, Q. 3-Aminodeoxypyranoses in glycosylation: Diversity-oriented synthesis and assembly in oligosaccharides. Angew. Chem. Int.Ed. 2017, 56, 5227–5231. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Wang, R.; Yao, W.; Zhang, S.; Sun, G.; Liao, Z.; Meng, L.; Wan, Q. Diversified synthesis and α-selective glycosylation of 3-amino-2,3,6-trideoxy sugars. Org. Chem. Front. 2018, 5, 3391–3395. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, S.; Xu, B.; Peng, P.; Wan, Q.; Zeng, J. Selective reduction leading to 3,5-cis-3-aminosugars: Synthesis and stereoselective glycosylation. J. Org. Chem. 2022. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Himuro, M.; Hayashi, Y.; Hirama, M. Remote C–H bond functionalization of androstane C-ring: C12-amination. Tetrahedron Lett. 2013, 54, 1307–1308. [Google Scholar] [CrossRef]
- Liu, M.-Q.; Jiang, T.; Chen, W.-W.; Xu, M.-H. Highly enantioselective Rh/chiral sulfur-olefin-catalyzed arylation of alkyl-substituted non-benzofused cyclic N-sulfonyl ketimines. Org. Chem. Front. 2017, 4, 2159–2162. [Google Scholar] [CrossRef]
- Kukhtin, V.A. Some new types of Arbuzov’s regrouping. Dokl. Akad. Nauk SSSR 1958, 121, 466–469. [Google Scholar]
- Ramirez, F. Condensations of carbonyl compounds with phosphite esters. Pure Appl. Chem. 1964, 9, 337–370. [Google Scholar] [CrossRef]
- Ramirez, F. Oxyphosphoranes. Acc. Chem. Res. 1968, 1, 168–174. [Google Scholar] [CrossRef]
- Miller, E.J.; Zhao, W.; Herr, J.D.; Radosevich, A.T. A nonmetal approach to α-heterofunctionalized carbonyl derivatives by formal reductive X-H insertion. Angew. Chem. Int. Ed. 2012, 51, 10605–10609. [Google Scholar] [CrossRef]
- Harpp, D.N.; Mathiaparanam, P. 1,3,2-Dioxaphospholene-sulfenyl chloride condensation. Scope and mechanism. J. Org. Chem. 1972, 37, 1367–1374. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, P.K.; Radosevich, A.T. A phosphetane catalyzes deoxygenative condensation of α-keto esters and carboxylic acids via PIII/PV=O redox cycling. J. Am. Chem. Soc. 2015, 137, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Fink, D.M.; Labutta, C.A.; Radosevich, A.T. A Csp3-Csp3 bond forming reductive condensation of α-keto esters and enolizable carbon pronucleophiles. Org. Lett. 2013, 15, 3090–3093. [Google Scholar] [CrossRef] [PubMed]
- Haugen, K.C.; Rodriguez, K.X.; Chavannavar, A.P.; Oliver, A.G.; Ashfeld, B.L. Phosphine-mediated addition of 1,2-dicarbonyls to diazenes: An umpolung approach toward N-acyl hydrazone synthesis. Tetrahedron Lett. 2015, 56, 3527–3530. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, H.; Xu, G.Q.; Wang, Z.Y.; Xu, P.F. PPh3 mediated reductive annulation reaction between isatins and electron deficient dienes to construct spirooxindole compounds. J. Org. Chem. 2017, 82, 5782–5789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yang, C.; Liu, Y.; Li, R.; He, Z. Diastereoselective synthesis of functionalized spirocyclopropyl oxindoles via P(NMe2)3-mediated reductive cyclopropanation. J. Org. Chem. 2014, 79, 10709–10715. [Google Scholar] [CrossRef]
- Rodriguez, K.X.; Vail, J.D.; Ashfeld, B.L. Phosphorus(III)-mediated stereoconvergent formal [4+1]-cycloannulation of 1,2-dicarbonyls and o-quinone methides: A multicomponent assembly of 2,3-dihydrobenzofurans. Org. Lett. 2016, 18, 4514–4517. [Google Scholar] [CrossRef]
- Corre, E.; Foucaud, A. Synthesis of 2-acetyl-3-aryl-1,1-dicyano-2-methyl-cyclopropanes. J. Chem. Soc. D 1971, 570a. [Google Scholar] [CrossRef]
- Choi, G.; Kim, H.E.; Hwang, S.; Jang, H.; Chung, W.J. Phosphorus(III)-mediated, tandem deoxygenative geminal chlorofluorination of 1,2-diketones. Org. Lett. 2020, 22, 4190–4195. [Google Scholar] [CrossRef]
- Wang, S.R.; Radosevich, A.T. P(NMe2)3-mediated umpolung alkylation and nonylidic olefination of α-keto esters. Org. Lett. 2015, 17, 3810–3813. [Google Scholar] [CrossRef]
- Calcatelli, A.; Denton, R.M.; Ball, L.T. Modular synthesis of α,α-diaryl α-amino esters via Bi(V)-mediated arylation/SN2-displacement of Kukhtin–Ramirez intermediates. Org. Lett. 2022, 24, 8002–8007. [Google Scholar] [CrossRef]
- Fier, P.S.; Kim, S.; Maloney, K.M. Reductive cleavage of secondary sulfonamides: Converting terminal functional groups into versatile synthetic handles. J. Am. Chem. Soc. 2019, 141, 18416–18420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-R.; Liao, Y.-Y.; Deng, J.-C.; Tang, Z.-L.; Xu, Y.-L.; Xu, L.; Tang, R.-Y. DABCO-promoted decarboxylative acylation: Synthesis of α-keto and α,β-unsaturated amides or esters. Asian J. Org. Chem. 2017, 6, 305–312. [Google Scholar] [CrossRef]
- Yu, T.T.; Nizalapur, S.; Ho, K.K.K.; Yee, E.; Berry, T.; Cranfield, C.G.; Willcox, M.; Black, D.S.; Kumar, N. Design, Synthesis and biological evaluation of N-sulfonylphenyl glyoxamide-based antimicrobial peptide mimics as novel antimicrobial agents. ChemistrySelect 2017, 2, 3452–3461. [Google Scholar] [CrossRef]
- Ryu, H.; Seo, J.; Ko, H.M. Synthesis of spiro[oxindole-3,2’-pyrrolidine] derivatives from benzynes and azomethine ylides through 1,3-dipolar cycloaddition reactions. J. Org. Chem. 2018, 83, 14102–14109. [Google Scholar] [CrossRef] [PubMed]
- Shu, P.; Xiao, X.; Zhao, Y.; Xu, Y.; Yao, W.; Tao, J.; Wang, H.; Yao, G.; Lu, Z.; Zeng, J.; et al. Interrupted Pummerer reaction in latent-active glycosylation: Glycosyl donors with a recyclable and regenerative leaving group. Angew. Chem. Int. Ed. 2015, 54, 14432–14436. [Google Scholar] [CrossRef]
- Xiao, X.; Zhao, Y.; Shu, P.; Zhao, X.; Liu, Y.; Sun, J.; Zhang, Q.; Zeng, J.; Wan, Q. Remote activation of disarmed thioglycosides in latent-active glycosylation via interrupted Pummerer reaction. J. Am. Chem. Soc. 2016, 138, 13402–13407. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, J.; Meng, L.; Wan, Q. Application of interrupted Pummerer reaction mediated (IPRm) glycosylation in natural product synthesis. Chem. Rec. 2020, 20, 743–751. [Google Scholar] [CrossRef]
- Xiao, X.; Zeng, J.; Fang, J.; Sun, J.; Li, T.; Song, Z.; Cai, L.; Wan, Q. One-pot relay glycosylation. J. Am. Chem. Soc. 2020, 142, 5498–5503. [Google Scholar] [CrossRef]
- Cai, L.; Chen, Q.; Liang, Z.; Fu, D.; Meng, L.; Zeng, J.; Wan, Q. Recyclable fluorous-tag assisted two-directional oligosaccharide synthesis enabled by interrupted Pummerer reaction mediated glycosylation. Chem. Sci. 2022, 13, 8759–8765. [Google Scholar] [CrossRef]
- Paradine, S.M.; White, M.C. Iron-catalyzed intramolecular allylic C-H amination. J. Am. Chem.Soc. 2012, 134, 2036–2039. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L. TBHP/I2-promoted oxidative coupling of acetophenones with amines at room temperature under metal-free and solvent-free conditions for the synthesis of α-ketoamides. Green Chem. 2012, 14, 2141–2145. [Google Scholar] [CrossRef]


 | ||
|---|---|---|
| Entry | Variation from Standard Conditions | Yield a |
| 1 | Step 1 only | 3a, 95% |
| 2 | Without H2O | 4a, 79% |
| 3 | Standard conditions | 2a, 88% |
| 4 | DBU instead of BTMG | 2a, 86% |
| 5 | KOH instead of BTMG | 2a, 80% |
| 6 | K2CO3 instead of BTMG | 2a, 16% |
| 7 | Et3N instead of BTMG | 2a, trace |
| 8 | Ambersep® 900 (OH) instead of BTMG | 2a, 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Xu, B.; Fu, D.; Wan, Q.; Zeng, J. Kukhtin–Ramirez-Reaction-Inspired Deprotection of Sulfamidates for the Synthesis of Amino Sugars. Molecules 2023, 28, 182. https://doi.org/10.3390/molecules28010182
Li T, Xu B, Fu D, Wan Q, Zeng J. Kukhtin–Ramirez-Reaction-Inspired Deprotection of Sulfamidates for the Synthesis of Amino Sugars. Molecules. 2023; 28(1):182. https://doi.org/10.3390/molecules28010182
Chicago/Turabian StyleLi, Ting, Bingbing Xu, Dengxian Fu, Qian Wan, and Jing Zeng. 2023. "Kukhtin–Ramirez-Reaction-Inspired Deprotection of Sulfamidates for the Synthesis of Amino Sugars" Molecules 28, no. 1: 182. https://doi.org/10.3390/molecules28010182
APA StyleLi, T., Xu, B., Fu, D., Wan, Q., & Zeng, J. (2023). Kukhtin–Ramirez-Reaction-Inspired Deprotection of Sulfamidates for the Synthesis of Amino Sugars. Molecules, 28(1), 182. https://doi.org/10.3390/molecules28010182






