Abstract
Low-dimensional graphene-based nanomaterials are interesting due to their cutting-edge electronic and magnetic properties. Their large surface area, strong mechanical resistance, and electronic properties have enabled potential pharmaceutical and opto-electronic applications. Graphene nanoribbons (GNRs) are graphene strips of nanometer size possessing zigzag and armchair edge geometries with tunable widths. Despite the recent developments in the characterization, design and synthesis of GNRs, the study of electronic, magnetic and topological properties, GNRs continue to pose a challenge owing to their multidimensionality. In this study, we obtain the topological and electronic properties of a series of wave-like nanoribbons comprising nanographene units with zigzag-shaped edges. The edge partition techniques based on the convex components are employed to compute the mathematical formulae of molecular descriptors for the wave-like zigzag GNRs. We have also obtained the spectral and energetic properties including HOMO-LUMO gaps, bond delocalization energies, resonance energies, C NMR and ESR patterns for the GNRs. All of these computations reveal zero to very low HOMO-LUMO gaps that make these nanoribbons potential candidates for topological spintronics.
1. Introduction
Polynuclear/polycyclic aromatic hydrocarbons (PNA/PCAH) and their heterocyclic derivatives are of considerable interest in environmental pollution, carcinogenicity, and other potential toxicological applications pertinent to public health [,,,]. However, the 2D materials derived from these compounds exhibit distinct electronic properties, including their macrocyclic conjugation, making them attractive candidates for devices such as organic semiconductors, solar cells, and transistors [,,], and hence they are of interest in opto-electronics, photographic products, pharmaceuticals, functional plastics, and liquid crystals []. Henceforth, these polycyclic compounds have been the subject of experimental and theoretical investigations over the past decades [,,,]. Graphene, a two-dimensional PCAH derivative, possesses high strength, supreme thermal conductivity, gas impermeability, giant charge carrier mobility, and the ability to transport without scattering even at ambient temperatures [,,,,,]. The rate of electron transfer of around m/s through the bond and the structural configuration of graphene contribute to electrical and mechanical properties that make them suitable as biological sensors []. Its low weight, outstanding robustness, high conductivity, and chemical stability, make it suitable as an anode material in lithium-ion batteries [], whereas its nanoelectronic conductance and ambipolar behavior play a vital role in graphene-based field effect transistors, in the fabrication of ion exchange membranes, and in several other chemical and biological applications [,,,,,,,].
Graphene nanoribbons are unzipped, segmented, quasi-one-dimensional, planar, and elongated thin strips of graphene sheets with repeated hexagonal components of carbon configured in a nanoscale dimension []. On the basis of their edge symmetries and structures, the edge sets of nanoribbons are categorized as armchair, zigzag or intermediate edge characters [,], and these distinct edge classes are observed to have a strong influence on the electronic ground state and reactivity of the material []. For instance, non-zero bandgaps of GNRs with hydrogen passivation arise from quantum confinement of edges for zigzag ribbon whereas from staggered sublattice potential owing to edge magnetization for the armchair type edges [,,]. Furthermore, it is possible to realize GNRs of varied widths through patterning epitaxial graphenes [,,], which thus provides control of their structural geometry, making them a potential candidate for the development of new nanoelectronic devices [,]. GNR derivatives such as graphene-oxide nanoribbons (GONRs) have been discovered to have effective functionalities in biomedical applications, including anti-cancer and gene therapies []. The applicability of GNRs and their derivatives as biomedical and electronic materials is thus a growing research area and these structures still require further studies in order to gain insights into their properties as a function of their structures.
Stimulated by several ongoing experimental studies on GNRs, in the present study we have investigated topological, spectral and energetic studies to characterize a series of graphene nanoribbons that are employed in designing the magnetic topological graphene-based nanoribbons (MT-GNRs) with non-trivial topology []. The wave-like zigzag series WNR considered in this study is obtained by chaining n units of the trans-coupled zigzag-edged nanographene unit ZNR, where ZNR consists of two copies of parallelogram-type benzenoid systems [], as shown in Figure 1. As physico-chemical properties of these materials are correlated with the underlying molecular structure [,,,], there is a clear and compelling need to characterize the underlying topological connectivity of GNRs as a function of their width and length. Topological descriptors are structure-based numeric entities that capture the underlying network connectivity through graph theory. These techniques together with energy spectra, delocalization energies, bond resonance energies, aromatic sextets, etc., provide considerable insight into their stabilities, reactivities, aromaticity, ring currents, and so forth [,,,,,,,]. As these topological descriptors are invariant to labelings, they play a vital role in the quantitative analysis of structural activity, property, and toxicity relationships (QSAR/QSPR/QSTR) []. A wide range of topological descriptors have been developed to date for the characterization of chemical structures and nanomaterials [,,,,,,,,,,,].
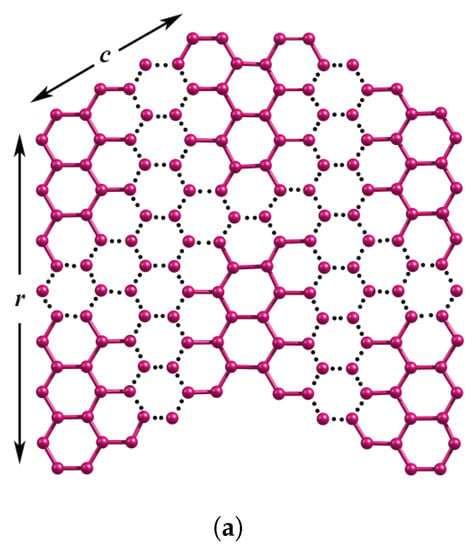
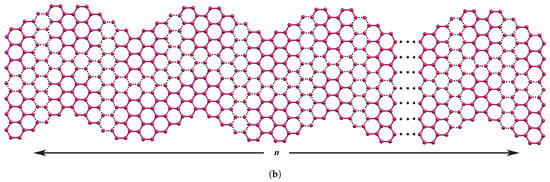
Figure 1.
(a) Zigzag edges of nanographene ZNR (b) n-wave series of WNR.
In this paper, we compute two different classes of topological descriptors for wave-like graphene-based nanoribbons. Furthermore we employ graph theory based techniques for machine learning of C NMR and ESR spectral patterns of the GNRs. We also compute their HOMO-LUMO gaps, bond delocalization energies, total -electronic energies and resonance energies/bond as a function of lengths and widths to gain insights into their stabilities and reactivities as a function of their ribbon widths and lengths.
2. Computational Techniques
The computation of topological descriptors can theoretically provide quantitative relationships to the physico-chemical properties of chemical structures under study. The technique for such computation is predominately based on the classification of chemical structures under the subclass of hypercubes. A hypercube is an undirected graph of dimension n consisting of vertices labelled from 0 to and edges connecting any two vertices if and only if their binary representations differs exactly by one bit. Partial cubes are isometric subgraphs of hypercubes. Many chemical graphs such has trees, phenylenes, and benzenoid graphs are comes under partial cube family. In our research, we considered the partial cube family with a class having and respectively as vertex and the edge sets.
The characterization of due to Djokovi−Winkler was examined on the edges of using a special relation known as , where defines that two edges and are -related if ≠+, where represents the distance between and . As we can see, the relation is equivalence in , the different classes of denoted by [,]. Thus, the class , , splits into 2-components graph. Let , , and . Consequently, we have and .
The distance-based topological descriptors are derived from the above-defined -classes and their component parameters, which are formally given below.
- Vertex Wiener =
- Edge Wiener
- Vertex-edge Wiener
- Vertex Szeged =
- Edge Szeged =
- Edge-vertex Szeged =
- Padmakar-Ivan
- Schultz
- Gutman
- Vertex Mostar =
- Edge Mostar =
Since the computation of degree-based indices required edge classification of , we denote to represent the degree of that counts the edges that are incident to . A collection of vertices that are adjacent to are referred to as the neighbors of and are represented by . The sum of degrees to the neighbors of is denoted by and computed by . It is clear that and . Let .
Let and and and . For , we denote to represent the degree/degree sum based indices [,,], where defined for the first Zagreb index , Sombor index , second Zagreb index , sum-connectivity index , geometric-arithmetic index , augmented Zagreb index , inverse sum indeg index , atom-bond connectivity index , forgotten index , symmetric degree division index =, and harmonic index .
3. Results and Discussion
As the wave-like nanoribbons are the most challenging material for the forthcoming generation, we now investigate the topological characteristics in this section.
3.1. Distance Based Descriptors
The wave series of WNR is composed of n-units of trans-coupled zigzag-edged nanographene units ZNR, where each ZNR contains vertices and edges, and thus WNR has vertices and edges as and respectively. Since the topological properties of WNR behave differently for the cases and , we first considered the case . In this section, we use to denote the quantitative values emerging from the components with respect to the class of -relation for the index function in which .
Theorem 1.
Let G be a wave-like nanoribbons WNR, where , , and .
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
Proof.
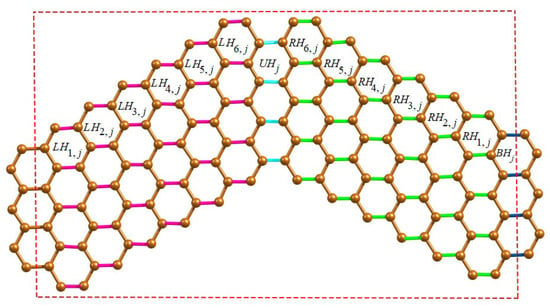

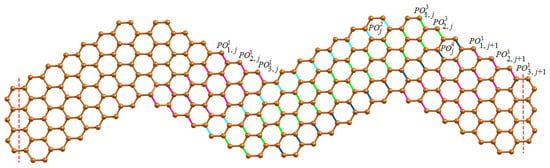

We first present the -classes of wave-like nanoribbons WNR and their associated component parameters. Since WNR is a linear arrangement of n units of ZNR, we now classify various -classes arising from the unit of ZNR where . We now consider the horizontal edges (H-type) of WNR and group them into four categories of -classes. The first category of H-type -classes starting from left to right of ZNR denoted by , while the second category runs from right to left which is the mirror image of first category. The third and fourth categories of H-type -classes are denoted by , respectively, which consists of coupling edges in the upper and bottom wave series. The H-type -classes are shown in Figure 2 and graph parameters are given in Table 1.

Figure 2.
H-type -classes for the jth unit of WNR.

Table 1.
H-type -classes of WNR.
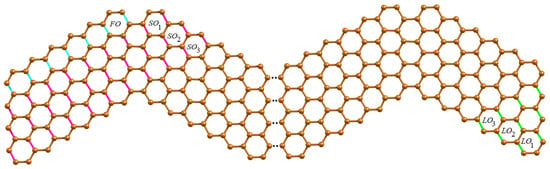
The obtuse edges (O-type) of WNR are now taken into consideration, and they are divided into seven categories of -classes. The first category of O-type -class consists of a unique class composing of obtuse edges in the top-left corner. Then, we have = , = and = . The second category of O-type -classes follow the class which are denoted by as drawn in Figure 3. The third to sixth categories of O-type -classes covering the edges in the lower area of wave series under parallelogram shapes, which are denoted by , , and respectively. These -classes are shown in Figure 4.

Figure 3.
O-type -classes on the initial and final unit of WNR.

Figure 4.
O-type parallelogram -classes on two consecutive units of WNR.
Finally, we define the seventh category of O-type -classes to be covered on the last unit of WNR, as illustrated in Figure 3. The graph parameters for O-type classes are given in Table 2.

Table 2.
O-type -classes of WNR.
The acute edges (A-type) consist of -classes and which have symmetry with respect to O-type -classes and . Thus, for computing the topological descriptors in the case of A-type classes, we count the numerical quantities of O-type classes twice. Therefore, we obtain the topological expressions by the graph theoretical parameters given in Table 1 and Table 2 using the equation given below.
□
Now consider the case of WNR in which the subcase holds for the Theorem 1, but the other subcases do not. Furthermore, the -classes behave strangely in cases where , making it difficult to identify common patterns. Therefore, we discuss the case and other cases can be dealt with in a similar way.
Theorem 2.
Let G be a wave-like nanoribbons WNR, where , and .
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
Proof.
To compute the topological expression for the case of WNR, we use the same H-type -classes of Theorem 1, and the O-type -classes, and do not exist. In addition, the ranges of and have been changed to , the graph parameters of have been changed to , , and an inclusion of new -class with parameters = , = and = . Consequently, such changes are applicable to A-type -classes and the computation of descriptors is performed as in Theorem 1. □
3.2. Degree Based Topological Descriptors
We have adopted the edge partition approach to compute some of the degree based topological descriptors for wave-like nanoribbons WNR in this section.
The analytical formulas of topological descriptors for degree and degree-sum types are derived using the following equations and Table 3 and Table 4, where

Table 3.
Edge degree partition of wave-like nanoribbons.

Table 4.
Edge degree-sum partition of wave-like nanoribbons.
Theorem 3.
The additive degree descriptors are given by
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
Theorem 4.
The additive degree-sum descriptors are given by
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
3.3. Numerical Values
Considering the topological parameters with equal case of WNR, we present the numerical data of distance and degree-type topological descriptors for the wave-like nanoribbons in Table 5, Table 6 and Table 7. Further, we performed the plotting using MATLAB interface, as shown in Figure 5.

Table 5.
Distance descriptors of wave-like nanoribbons WNR.

Table 6.
Degree descriptors of wave-like nanoribbons WNR.

Table 7.
Degree-sum descriptors of wave-like nanoribbons WNR.
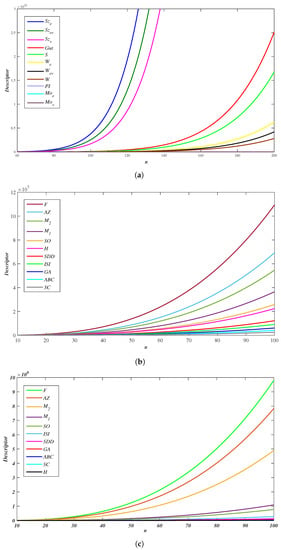
Figure 5.
Comparative analysis of descriptors in WNR (a) Distances (b) Degree (c) Degree-sum.
4. Applications to Stabilities and Spectroscopy of Wavy Zigzag Nanoribbons
The topological indices developed in the previous section can be applied to characterize the structures of wavy zigzag nanoribbons, and hence in QSAR predictions of the properties of these nanomaterials as a function of their structures. The adjacency and distance matrices of these structures also contain rich topological information that can be used in machine learning of stabilities and spectroscopies of these WNRs. The distance matrices of these structures can be used to generate the distance degree sequence vectors (DDSV) for each vertex of GNR, which in turn provide a technique for the vertex partitioning of GNRs. The technique is purely graph theoretical in nature and does not use any experimental reference parameters to construct the vertex partitions. Quintas and coworkers [] have shown the use of DDSV as a means to characterize the vertices of graphs although the equivalence of DDSV is not always isomorphic with the automorphic equivalence of vertices. In this technique, we associate with each vertex in the GNR a p-tuple vector where is the number of vertices at distance j from . The DDSV of each vertex in any GNR can be easily generated by an algorithm that generates the distance matrix, and it needs iterations up to the maximal eccentricity of the vertices or the radius of the graph. In this algorithm, the distance matrix is generated from the adjacency matrix A where th element of the distance matrix is the topological distance of the shortest path from the vertex to . Thus, the DDSVs of various GNRs considered here are computed using the computer code developed previously by one of the authors []. The same TopoChemie-2020 package [] was also used to validate the numerical results derived from the analytical expressions for the topological descriptors of GNRs considered here. The DDSV for each vertex is a vector of variable length with the length of DDSV of each vertex given by its eccentricity. The maximal length of the DDSV is the radius of the GNR under consideration.
Table 8 shows the machine-generated numbers of C NMR signals thus computed for both the composing ZNR and WNR for various values of r, c, and n. As the constituting units ZNR possess symmetry, the same symmetry is reflected in the composed wavy zigzag nanoribbons. The number of NMR signals and their intensity patterns are generated from the vertex partitioning and do not require any experimental parameters. Thus all GNRs exhibit C NMR signal and intensity pattern consistent with 1:1:...:1:1 ratios with the number of signals enumerated by the DDSV method as shown on Table 8. As all of the wavy zigzag nanoribbons considered here are diradicals the equivalence classes enumerated thus can be used for the machine generation of the ESR hyperfine structures of nanoribbons. Consider for example, the wavy nanoribbon WNR shown in Table 8. There are 380 carbon nuclei in this nanoribbon which are partitioned into 190 classes, with each class containing two nuclei as derived from the DDSV algorithm. Thus, the ESR hyperfine structure generating function for such a case is determined by the polynomials GF shown below, where represents spin up and represents spin down for the C spin-1/2 nucleus.

Table 8.
Spectral and energetic properties of GNRs.
In the above ESR generating function, when expanded and simplified, the coefficient of each term gives the intensity of the ESR hyperfine line. This is a purely combinatorial generating function method and does not require any experimental reference standards or other experimental inputs. As the above GF generates far too many lines for illustration purposes, we show in Figure 6 the machine-generated ESR hyperfine pattern arising from the composing units of wavy zigzag nanoribbon diradicals. The ESR hyperfine patterns get composed one into the other for the wavy nanoribbons similar to the operator methods that invoke the wreath product structures considered earlier in the context of NMR spectroscopy []. It should be noted that the -e coupling depends on the Euclidean proximity of the unpaired electron and the C nucleus, and thus those nuclei close to the electron would have stronger interaction reducing complexity of the hyperfine pattern, although we expect the machine-generated pattern shown in Figure 6 to be a motif that will repeat or compose within the final ESR hyperfine pattern.
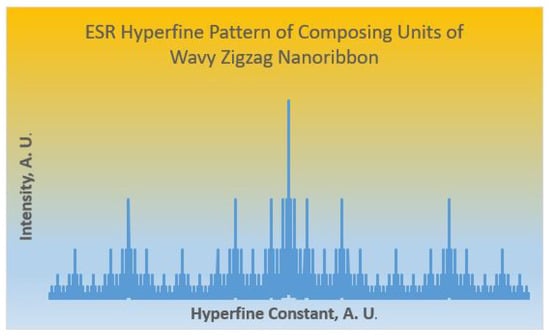
Figure 6.
ESR hyperfine pattern arising from the composing units of wavy zigzag nanoribbon diradicals.
Thermodynamic and kinetic stabilities can be estimated for the GNRs considered using the graph spectra and other measures, such as resonance energy measures per bond which are computed from the combinatorial counts of the Kekulé structures. The parameters thus derived are shown in Table 8 for both the composing ZNR and the nanoribbons WNR. As seen from Table 8, the HOMO-LUMO gaps, which measure the kinetic stability, decrease as a r or c increases for the ZNR (see Table 8). Indeed, the gap approaches zero quite rapidly as r and c increase. Consequently, the HOMO-LUMO gaps of WNR are nearly zero for most of the ribbons. This, in turn, suggests that the wavy zigzag nanoribbons exist in triplet spin ground states, making them attractive candidates for topological spintronics.
Table 8 shows graph-theoretically derived energy measures such as delocalization energies/bond, total -electron energy and the resonance energy per bond. The total binding energies and delocalization energies were obtained from the spectra of the graphs of WNRs. Let the spectra be denoted by the set , where m is the number of vertices of WNR. For the most general case, the total -electron energy is defined as
where is the occupancy of the orbital i. For an alternant WNR, the resulting graph is bipartite, and hence the total -electron energy and delocalization energy/bond, are defined from its spectra as follows:
The total -electron energy and delocalization energies/-bond shown in Table 8 were computed from the above expressions. The resonance energy, on the other hand, is derived from the constant coefficient of the matching polynomials of WNR graphs, which is the number of resonance structures, or commonly called the Kekulé count, K. Consequently, if K is the coefficient of the constant term of the matching polynomial of the WNR then the resonance energy is given by Herndon’s definition []:
In Table 8, we have provided the resonance energies/bond computed with the above formula for several WNRs.
Aihara as well as Dias and coworkers [,,,,] have carried out pioneering studies on polycyclic aromatic molecules derived from graphenes. These studies have revealed fascinating trends on aromaticity, -electron delocalization energies, bond resonance energies, and topological resonance energies of these species [,,,,]. It is clear that hybrid techniques through machine learning are warranted for these systems as pure ab initio quantum chemical techniques could become intractable for such nanoribbons and derivatives of graphenes. Hence, Table 8 provides some interesting trends regarding the thermodynamic stabilities and the extent of delocalization energies for these GNRs. As can be seen from Table 8 for the composing unit ZNR, the total -electron energy/electron and p-delocalization energies uniformly increase as the composing zigzag unit increases in dimension in either direction. The resonance energy per bond exhibits an opposite trend as a function of the dimensions, suggesting less contribution from resonance stabilization as the size increases. A similar trend is seen in Table 8 for the WNR in that as the size increases in width, length or breadth the -delocalization energy increases accompanied by a decrease in the resonance energy trend. In particular, the thermodynamic stability of the ribbon increases with the ribbon length while the kinetic stability decreases, suggesting that the longer ribbons are highly reactive yet exhibit good thermodynamic stabilities as suggested by their total -electron energies/electron. These features suggest that the wavy zigzag nanoribbons exhibit highly reactive diradical triplet states with interesting topological features as a function of the dimensions of the ribbons, making them suitable candidates for topological spintronics, a topic of considerable interest in recent years.
A number of experimental and computational studies have been carried out earlier on WNRs of various shapes and lengths [,,,,]. Most of these works have focused on the electronic and magnetic properties of WNRs, and in particular the HOMO-LUMO gaps, stabilities, and reactivities as functions of the length of the ribbons. Zdetsis et al. [] have observed that the HOMO-LUMO gaps of WNRs rapidly decrease as a function of the ribbon length. This is corroborated by our results in Table 8, for the HOMO-LUMO gaps of WNRs as well as an independent study by El Abbassi et al. [], who have observed rapid approach of metallic characters by longer WNRs. On the other hand, Jiang et al. [] have observed enhanced reactivity of longer WNRs at the edges fully consistent with our energy gaps presented in Table 8 where zero to small HOMO-LUMO gaps suggest high kinetic instability and the reactive nature of WNRs. Moreover, Jiang and Dai [] have shown that as the WNR increases in length, it undergoes a metamorphosis from a nonmagnetic to a magnetic ground state. This observation is fully consistent with our computed ground states of larger WNRs which exhibit diradical triplet ground states for larger nanoribbons compared to WNRs of smaller lengths which exhibit singlet ground states owing to larger HOMO-LUMO gaps for the WNRs for smaller ribbon lengths. Chopra and Maidich [] have carried out density functional computations on WNRs for different lengths and they have noted that the binding energy increases as a function of ribbon length. As can be seen from Table 8, the column that contains the total -electron binding energy per bond uniformly increases as a function of the ribbon length fully consistent with the DFT studies []. Furthermore, our computed bond resonance energies and delocalization energies support these findings. Kimouche et al. [] have studied ultra-narrow armchair WNRs and note that the HOMO-LUMO gaps of the ribbons decrease in proportion to , where n is the ribbon length. Our computed data in Table 8 support the same trend. Furthermore, our other topological properties computed here exhibit very promising QSAR relations with other properties of such polycyclic aromatic compounds such as toxicity and biological activities as shown in a recent study on a large set of polycyclic aromatic compounds [,]. At present, there are no experimental data pertinent to toxicity or measurable bioactivities of WNRs. When such data become available in the future, our computed topological properties can be harnessed to develop QSAR/QSPR relations for WNRs and related nanomaterials.
Although graph theoretical techniques by themselves do not always provide accurate energetics, they facilitate hybrid techniques. In hybrid techniques, the parameters needed for the -electron treatments, say by the PPP method, can be derived from the DFT computations on constituent ZNR or through the CASSCF/CI techniques [] on a series of smaller zigzag strips with the active space restricted to -electron molecular orbitals. Subsequently, machine learning techniques can be employed on a small combinatorial library of zigzag strips in order to generate the PPP parameters needed for larger GNRs. There are limitations in the CASSCF method as the number of configuration spin functions explode combinatorially as a function of the number of electrons and total number of active orbitals included in the CASSCF. The present computational limit appears to be 18 electrons distributed in all possible ways among 18 orbitals. Even then, one could carry out CASSCF with CASPT2 on several polyacenes up to tetracene (naphtacene) which contains 18 -electrons and 18 -orbitals. For example, a training set for such a CASPT2 could include naphthalene, antracene, phenanthrene, pyrene, triphenylene, tetracene, chrysene, benzo[a]anthracene, benco[c]anthracene, and so forth. The results of such a CASPT2 scheme on a small combinatorial library of polyacenes could provide suitable fit for the PPP integrals. On the other hand, a restricted CASSCF (RASSCF/RASPT2) technique could also be used to expand the combinatorial library. We note that a similar CASPT2 technique on ethylene was employed by Zhang et al. [] to parameterize the 2-electron PPP integrals for polyenes. They have demonstrated that the accuracy of the results obtained by this hybrid PPP method is quite comparable to the CASPT2 method. Consequently, the graph theoretical techniques presented here provide promising pathways for the development of novel hybrid techniques to treat such large graphene-based nanomaterials.
5. Conclusions
We have computed the exact mathematical expressions for Wiener, Szeged-type, Schultz, and Gutman topological descriptors; additionally, a catalogue of degree-based descriptors are demonstrated for the wave-like graphene-based nanomaterial series WNR, through graph-theoretical techniques. The derived topological entities of WNR presented in this study are believed to play a predominant role in addressing the robustness and complexity of their underlying structure and thus provide significant mathematical functions that can be related to the QSAR/QSPR studies of these systems. We have also developed combinatorial tools based on distance degree sequences for the machine learning of C NMR and ESR hyperfine spectra of GNRs. Furthermore, our computed HOMO-LUMO gaps and various -electron delocalization and resonance energies clearly reveal the highly reactive and yet thermodynamically stable nature of the GNRs. It is predicted that GNRs exhibit triplet ground states and are very reactive diradicals, making them ideal candidates for topological spintronics. As graphene is considered to be a highly adaptable material with significant industrial value, the GNRs are highly desirable candidates for interesting applications in spintronics, quantum physics, and information technology. Consequently, the formulated structural analysis will enhance the understanding of their geometries, stabilities, electronic and magnetic properties.
Author Contributions
Conceptualization, M.A., J.C.F. and K.B.; methodology, M.A., J.C.F., S.R.J.K., A.J.S. and K.B.; validation, J.C.F. and K.B.; formal analysis, M.A., S.R.J.K. and A.J.S.; writing—original draft preparation, J.C.F.; writing-review and editing, M.A., J.C.F. and K.B.; visualization, S.R.J.K., A.J.S. and K.B.; supervision, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, F.; Chen, J.; Hu, X.; He, F.; Bean, E.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Applications of carbonaceous adsorbents in the remediation of polycyclic aromatic hydrocarbon-contaminated sediments: A review. J. Clean. Prod. 2020, 225, 120263. [Google Scholar] [CrossRef]
- Gan, S.; Lau, E.V.; Ng, H.K. Remediation of soils contaminated with polycyclic aromatic hydrocarbons (PAHs). J. Hazard. Mater. 2009, 172, 532–549. [Google Scholar] [CrossRef] [PubMed]
- Bansal, V.; Kim, K.-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, R.N.; Prediger, P.; Vieira, M.G.A. Adsorption of polycyclic aromatic hydrocarbons from wastewater using graphene-based nanomaterials synthesized by conventional chemistry and green synthesis: A critical review. J. Hazard. Mater. 2022, 422, 126904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cao, Y.; Colella, N.S.; Liang, Y.; Brédas, J.-L.; Houk, K.N.; Briseno, A.L. Unconventional, chemically stable, and soluble two-dimensional angular polycyclic aromatic hydrocarbons: From molecular design to device applications. Acc. Chem. Res. 2015, 48, 500–509. [Google Scholar] [CrossRef]
- Aumaitre, C.; Morin, J.-F. Polycyclic aromatic hydrocarbons as potential building blocks for organic solar cells. Chem. Rec. 2019, 19, 1142–1154. [Google Scholar] [CrossRef]
- Shafy, H.I.A.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Menzie, C.A.; Potocki, B.B.; Santodonato, J. Exposure to carcinogenic PAHs in the environment. Environ. Sci. Technol. 1992, 26, 1278–1284. [Google Scholar] [CrossRef]
- Masih, J.; Singhvi, R.; Kumar, K.; Jain, V.K.; Taneja, A. Seasonal variation and sources of polycyclic aromatic hydrocarbons (PAHs) in indoor and outdoor air in a semi arid tract of northern india. Aerosol Air Qual. Res. 2012, 12, 515–525. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Bi, Z.; Zhang, R.; Liu, J.; Yu, X.; Li, Z. A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 2019, 373, 104–121. [Google Scholar] [CrossRef]
- Dhinakaran, V.; Stalin, B.; Sai, M.S.; Vairamuthu, J.; Marichamy, S. Recent developments of graphene composites for energy storage devices. Mater. Today Proc. 2021, 45, 1779–1782. [Google Scholar] [CrossRef]
- Hao, W.; Wang, Y.; Zhao, H.; Zhu, J.; Li, S. Strong dependence of the vertical charge carrier mobility on the π-π stacking distance in molecule/graphene heterojunctions. Phys. Chem. Chem. Phys. 2020, 22, 13802–13807. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.; Li, C.; Yang, J.; Jenkins, G.; Huang, W.; Dong, X. Graphene field-effect transistor and its application for electronic sensing. Small 2014, 10, 4042–4065. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.-S.; Kudo, T.; Honma, I. Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef]
- Sharma, B.K.; Ahn, J.-H. Graphene based field effect transistors: Efforts made towards flexible electronics. Solid-State Electron. 2013, 89, 177–188. [Google Scholar] [CrossRef]
- Alabi, A.; Cseri, L.; Hajaj, A.A.; Szekely, G.; Budd, P.; Zou, L. Graphene-PSS/l-DOPA nanocomposite cation exchange membranes for electrodialysis desalination. Environ. Sci. Nano 2020, 7, 3108–3123. [Google Scholar] [CrossRef]
- Dash, B.S.; Jose, G.; Lu, Y.-J.; Chen, J.-P. Functionalized reduced graphene oxide as a versatile tool for cancer therapy. Int. J. Mol. Sci. 2021, 22, 2989. [Google Scholar] [CrossRef]
- Dong, X.; Fu, D.; Fang, W.; Shi, Y.; Chen, P.; Li, L.-J. Doping single-layer graphene with aromatic molecules. Small 2009, 5, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H. Chemical preparation of graphene-based nanomaterials and their applications in chemical and biological sensors. Small 2011, 7, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; Lima, R.D.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Narváez, E.M.; Pires, L.B.; Gálvez, A.Z.; Merkoçi, A. Graphene-based biosensors: Going simple. Adv. Mater. 2017, 29, 1604905. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Hashmi, M.S.J. Wonder material graphene: Properties, synthesis and practical applications. Adv. Mater. Process. Technol. 2018, 4, 573–602. [Google Scholar] [CrossRef]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef]
- Pincak, R.; Smotlacha, J.; Osipov, V.A. Electronic states of zigzag graphenenanoribbons with edges reconstructed with topological defects. Phys. B Condens. Matter. 2015, 475, 61–65. [Google Scholar] [CrossRef][Green Version]
- Damasceno, D.A.; Rajapakse, R.K.N.D.N.; Mesquita, E. Atomistic modelling of size-dependent mechanical properties and fracture of pristine and defective cove-edged graphene nanoribbons. Nanomaterials 2020, 10, 1422. [Google Scholar] [CrossRef]
- Jiang, D.-E.; Sumpter, B.G.; Dai, S. Unique chemical reactivity of a graphene nanoribbon’s zigzag edge. J. Chem. Phys. 2007, 126, 134701. [Google Scholar] [CrossRef]
- Okada, S.; Oshiyama, A. Magnetic ordering in hexagonally bonded sheets with first-row elements. Phys. Rev. Lett. 2001, 87, 146803. [Google Scholar] [CrossRef]
- Lee, H.; Son, Y.-W.; Park, N.; Han, S.; Yu, J. Magnetic ordering at the edges of graphitic fragments: Magnetic tail interactions between the edge-localized states. Phys. Rev. B 2005, 72, 174431. [Google Scholar] [CrossRef]
- Son, Y.-W.; Cohen, M.L.; Louie, S.G. Half-metallic graphene nanoribbons. Nature 2006, 444, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N.; et al. Electronic confinement and coherence in patterned epitaxial graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Song, Z.; Li, T.; Li, X.; Ogbazghi, A.Y.; Feng, R.; Dai, Z.; Marchenkov, A.N.; Conrad, E.H.; First, P.N.; et al. Ultrathin epitaxial graphite: 2D Electron gas properties and a route toward graphene-based nanoelectronics. J. Phys. Chem. B 2004, 108, 19912–19916. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef]
- Rizzo, D.J.; Veber, G.; Cao, T.; Bronner, C.; Chen, T.; Zhao, F.; Rodriguez, H.; Louie, S.G.; Crommie, M.F.; Fischer, F.R. Topological band engineering of graphene nanoribbons. Nature 2018, 560, 204–208. [Google Scholar] [CrossRef]
- Ruffieux, P.; Cai, J.; Plumb, N.C.; Patthey, L.; Prezzi, D.; Ferretti, A.; Molinari, E.; Feng, X.; Müllen, K.; Pignedoli, C.A.; et al. Electronic structure of atomically precise graphene nanoribbons. ACS Nano 2012, 6, 6930–6935. [Google Scholar] [CrossRef]
- Johnson, A.P.; Gangadharappa, H.V.; Pramod, K. Graphene nanoribbons: A promising nanomaterial for biomedical applications. J. Control. Release 2020, 325, 141–162. [Google Scholar] [CrossRef]
- Song, S.; Ng, P.W.; Edalatmanesh, S.; Solé, A.P.; Peng, X.; Kolorenč, J.; Sosnová, Z.; Stetsovych, O.; Su, J.; Li, J.; et al. Designer magnetic topological graphene nanoribbons. arXiv 2022, arXiv:2204.12880. [Google Scholar]
- Arockiaraj, M.; Clement, J.; Balasubramanian, K. Analytical expressions for topological properties of polycyclic benzenoid networks. J. Chemom. 2016, 30, 682–697. [Google Scholar] [CrossRef]
- Imran, M.; Baig, A.Q.; Ali, H. On molecular topological properties of hex-derived networks. J. Chemom. 2016, 30, 121–129. [Google Scholar] [CrossRef]
- Khadikar, P.V.; Karmarkar, S.; Agrawal, V.K. A novel PI index and its applications to QSPR/QSAR studies. J. Chem. Inf. Comput. Sci. 2001, 41, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Khadikar, P.V.; Karmarkar, S.; Agrawal, V.K.; Singh, J.; Shrivastava, A.; Lukovits, I.; Diudea, M.V. Szeged index-Applications for drug modeling. Lett. Drug Des. Discov. 2005, 2, 606–624. [Google Scholar] [CrossRef]
- Todeschini, R.; Consonni, V. Molecular Descriptors for Chemoinformatics: Vol. I: Alphabetical Listing/Vol. II: Appendices, References; Methods and Principles in Medical Chemistry; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Balasubramanian, K. Combinatorial enumeration of isomers of superaromatic polysubstituted cycloarenes and coronoid hydrocarbons with applications to NMR. J. Phys. Chem. A 2018, 122, 8243–8257. [Google Scholar] [CrossRef]
- Balasubramanian, K. Computational and Artificial Intelligence Techniques for Drug Discovery and Administration; Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Aihara, J.-I. Topological resonance energy, bond resonance energy, and circuit resonance energy. J. Phys. Org. Chem. 2008, 21, 79–85. [Google Scholar] [CrossRef]
- Aihara, J.-I. Graph theory of ring-current diamagnetism. Bull. Chem. Soc. Jpn. 2018, 91, 274–303. [Google Scholar] [CrossRef]
- Dias, J.R. Valence-bond determination of diradical character of polycyclic aromatic hydrocarbons: From acenes to rectangular benzenoids. J. Phys. Chem. A 2013, 117, 4716–4725. [Google Scholar] [CrossRef]
- Aihara, J.-I.; Makino, M.; Ishida, T.; Dias, J.R. Analytical study of superaromaticity in cycloarenes and related coronoid hydrocarbons. J. Phys. Chem. A 2013, 117, 4688–4697. [Google Scholar] [CrossRef]
- Makino, M.; Dias, J.R.; Aihara, J.-I. Bond resonance energy verification of σ-aromaticity in cycloalkanes. J. Phys. Chem. A 2020, 124, 4549–4555. [Google Scholar] [CrossRef]
- Balasubramanian, K. Symmetry and combinatorial concepts for cyclopolyarenes, nanotubes and 2D-sheets: Enumerations, isomers, structures spectra & properties. Symmetry 2022, 14, 34. [Google Scholar]
- Dearden, J.C. The use of topological indices in QSAR and QSPR modeling. Adv. QSAR Model. 2017, 24, 57–88. [Google Scholar]
- Hayat, S.; Imran, M. Computation of topological indices of certain networks. Appl. Math. Comput. 2014, 240, 213–228. [Google Scholar] [CrossRef]
- Arockiaraj, M.; Klavžar, S.; Mushtaq, S. Distance-based topological indices of nanosheets, nanotubes and nanotori of SiO2. J. Math. Chem. 2019, 57, 343–369. [Google Scholar] [CrossRef]
- Arockiaraj, M.; Paul, D.; Klavžar, S.; Clement, J.; Tiggaa, S.; Balasubramanian, K. Relativistic distance based and bond additive topological descriptors of zeolite RHO materials. J. Mol. Struct. 2022, 1250, 131798. [Google Scholar] [CrossRef]
- Liu, J.-B.; Iqbal, H.; Shahzad, K. Topological properties of concealed non-kekulean benzenoid hydrocarbon. Polycycl. Aromat. Compd. 2022. [Google Scholar] [CrossRef]
- Adnan, M.; Bokhary, S.A.; Abbas, G.; Iqbal, T. Degree-based topological indices and QSPR analysis of antituberculosis drugs. J. Chem. 2022, 2022, 5748626. [Google Scholar] [CrossRef]
- Hayat, S.; Khan, S.; Imran, M. Quality testing of spectrum-based distance descriptors for polycyclic aromatic hydrocarbons with applications to carbon nanotubes and nanocones. Arabian J. Chem. 2021, 14, 102994. [Google Scholar] [CrossRef]
- Arockiaraj, M.; Clement, J.; Paul, D.; Balasubramanian, K. Relativistic distance-based topological descriptors of Linde type A zeolites and their doped structures with very heavy elements. Mol. Phys. 2020, 119, e1798529. [Google Scholar] [CrossRef]
- Sarkar, P.; De, N.; Pal, A. On some topological indices and their importance in chemical sciences: A comparative study. Eur. Phys. J. Plus. 2022, 137, 195. [Google Scholar] [CrossRef]
- Arockiaraj, M.; Klavžar, S.; Mushtaq, S.; Balasubramanian, K. Topological characterization of the full k-subdivision of a family of partial cubes and their applications to α-types of novel graphyne and graphdiyne materials. Polycycl. Aromat. Compd. 2021, 41, 1902–1924. [Google Scholar] [CrossRef]
- Huilgol, M.I.; Sriram, V.; Balasubramanian, K. Structure-activity relations for antiepileptic drugs through omega polynomials and topological indices. Mol. Phys. 2022, 120, e1987542. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, Y.; Li, Y.; Furtula, B. On relations between Sombor and other degree-based indices. J. Appl. Math. Comput. 2022, 68, 1–17. [Google Scholar] [CrossRef]
- Klavžar, S.; Gutman, I.; Mohar, B. Labeling of benzenoid systems which reflects the vertex-distance relation. J. Chem. Inf. Comput. Sci. 1995, 35, 590–593. [Google Scholar] [CrossRef]
- Klavžar, S. A bird’s eye view of the cut method and a survey of its recent applications in chemical graph theory. MATCH Commun. Math. Comput. Chem. 2008, 60, 255–274. [Google Scholar]
- Kavitha, S.R.J.; Abraham, J.; Arockiaraj, M.; Jency, J.; Balasubramanian, K. Topological characterization and graph entropies of tessellations of kekulene structures: Existence of isentropic structures and applications to thermochemistry, NMR and ESR. J. Phys. Chem. A 2021, 125, 8140–8158. [Google Scholar] [CrossRef]
- Abraham, J.; Arockiaraj, M.; Jency, J.; Kavitha, S.R.J.; Balasubramanian, K. Graph entropies, enumeration of circuits, walks and topological properties of three classes of isoreticular metal organic frameworks. J. Math. Chem. 2022, 60, 695–732. [Google Scholar] [CrossRef]
- Arockiaraj, M.; Jency, J.; Abraham, J.; Kavitha, S.R.J.; Balasubramanian, K. Two-dimensional coronene fractal structures: Topological entropy measures, energetics, NMR and ESR spectroscopic patterns and existence of isentropic structures. Mol. Phys. 2022, 120, e2079568. [Google Scholar] [CrossRef]
- Bloom, G.S.; Kennedy, J.W.; Quintas, L.V. Some Problems Concerning Distance and Path Degree Sequences. In Graph Theory; Springer: Berlin/Heidelberg, Germany, 1983; pp. 179–190. [Google Scholar]
- Balasubramanian, K. Topochemie-2020-A computational package for computing topological indices, spectral polynomials, walks and distance degree sequences and combinatorial generators. J. Phys. Chem. A 2021, 125, 8140–8158. [Google Scholar]
- Balasubramanian, K. Operator and algebraic methods for NMR-spectroscopy. I. Generation of NMR Spin Species. J. Chem. Phys. 1983, 78, 6358–6368. [Google Scholar] [CrossRef]
- Henrdon, W.C. Resonance energies of aromatic hydrocarbons. Quantitative test of resonance theory. J. Am. Chem. Soc. 1973, 95, 2404–2406. [Google Scholar]
- Zdetsis, A.D.; Economou, E.N. Rationalizing and reconciling energy gaps and quantum confinement in narrow atomically precise armchair graphene nanoribbons. Carbon 2017, 116, 422–434. [Google Scholar] [CrossRef]
- El Abbassi, M.; Perrin, M.L.; Barin, G.B.; Sangtarash, S.; Overbeck, J.; Braun, O.; Lambert, C.J.; Sun, Q.; Prechtl, T.; Narita, A.; et al. Controlled quantum dot formation in atomically engineered graphene nanoribbon field-effect transistors. ACS Nano 2020, 14, 5754–5762. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Dai, S. Circumacenes versus periacenes: HOMO–LUMO gap and transition from nonmagnetic to magnetic ground state with size. Chem. Phys. Lett. 2008, 466, 72–75. [Google Scholar] [CrossRef]
- Chopra, S.; Maidich, L. Density functional theory based study of graphene nano-ribbons of various shapes and sizes. Quantum Matter 2014, 3, 559–563. [Google Scholar] [CrossRef]
- Kimouche, A.; Ervasti, M.M.; Drost, R.; Halonen, S.; Harju, A.; Joensuu, P.M.; Sainio, J.; Liljeroth, P. Ultra-narrow metallic armchair graphene nanoribbons. Nat. Commun. 2015, 6, 10177. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.M.; Narkhede, R.R.; Masand, N.; Cheke, R.S.; Balasubramanian, K. Molecular insights into resveratrol and its analogs as SARS-CoV-2 (COVID-19) protease inhibitors. Coronaviruses 2021, 2, e130921189258. [Google Scholar] [CrossRef]
- Balasubramanian, K. CASSCF/CI calculations on Si4 and Si4+. Chem. Phys. Lett. 1987, 135, 283–287. [Google Scholar] [CrossRef]
- Zhang, D.; Qu, Z.; Liu, C.; Jiang, Y. Excitation energy calculation of conjugated hydrocarbons: A new Pariser–Parr–Pople model parameterization approaching CASPT2 accuracy. J. Chem. Phys. 2011, 134, 024114. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).