Asymmetric Push–Pull Type Co(II) Porphyrin for Enhanced Electrocatalytic CO2 Reduction Activity
Abstract
1. Introduction
2. Results
2.1. Synthesis Description
2.2. Structural Characterization
2.3. Electronic Structures
2.4. DFT Calculation
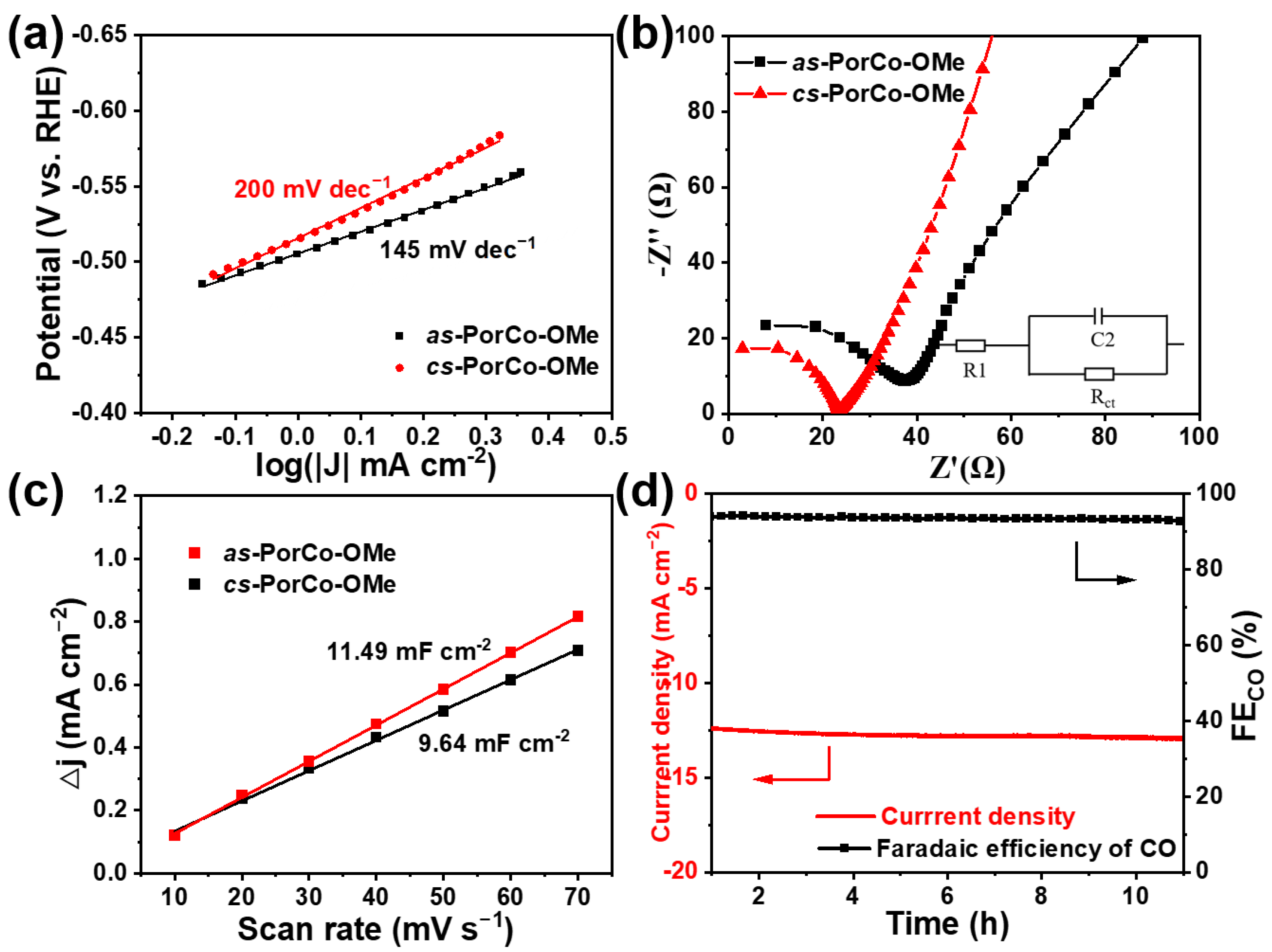
2.5. Electrocatalytic CO2RR
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis Procedures
4.3. Characterizations
4.4. Electrode Preparation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ren, S.; Joulié, D.; Salvatore, D.; Torbensen, K.; Wang, M.; Robert, M.; Berlinguette, C.P. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 2019, 365, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, G.; Du, L.; Zheng, Y.; Sun, L.; Sun, S. Nanostructured Cobalt-Based Electrocatalysts for CO2 Reduction: Recent Progress, Challenges, and Perspectives. Small 2020, 16, 2004158. [Google Scholar] [CrossRef] [PubMed]
- Nam, D.-H.; De Luna, P.; Rosas-Hernández, A.; Thevenon, A.; Li, F.; Agapie, T.; Peters, J.C.; Shekhah, O.; Eddaoudi, M.; Sargent, E.H. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 2020, 19, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Marianov, A.N.; Jiang, Y. Mechanism-Driven Design of Heterogeneous Molecular Electrocatalysts for CO2 Reduction. Acc. Mater. Res. 2022, 3, 620–633. [Google Scholar] [CrossRef]
- Maurin, A.; Robert, M. Noncovalent Immobilization of a Molecular Iron-Based Electrocatalyst on Carbon Electrodes for Selective, Efficient CO2-to-CO Conversion in Water. J. Am. Chem. Soc. 2016, 138, 2492–2495. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Zhang, X.; Li, L.; Li, Y.; Xu, H.; Li, X.; Yu, X.; Zhang, Z.; Liang, Y.; et al. Highly selective and active CO2 reduction electrocatalysts based on cobalt phthalocyanine/carbon nanotube hybrid structures. Nat. Commun. 2017, 8, 14675. [Google Scholar] [CrossRef]
- Leung, J.J.; Vigil, J.A.; Warnan, J.; Edwardes Moore, E.; Reisner, E. Rational Design of Polymers for Selective CO2 Reduction Catalysis. Angew. Chem. Int. Ed. 2019, 58, 7697–7701. [Google Scholar] [CrossRef]
- Huang, S.; Chen, K.; Li, T.-T. Porphyrin and phthalocyanine based covalent organic frameworks for electrocatalysis. Coordin. Chem. Rev. 2022, 464, 214563. [Google Scholar] [CrossRef]
- Du, J.; Ouyang, H.; Tan, B. Porous Organic Polymers for Catalytic Conversion of Carbon Dioxide. Chem.—Asian J. 2021, 16, 3833–3850. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef]
- Liu, Y.; McCrory, C.C.L. Modulating the mechanism of electrocatalytic CO2 reduction by cobalt phthalocyanine through polymer coordination and encapsulation. Nat. Commun. 2019, 10, 1683. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-J.; Lu, M.; Wang, Y.-R.; Yao, S.-J.; Zhang, M.; Kan, Y.-H.; Liu, J.; Chen, Y.; Li, S.-L.; Lan, Y.-Q. Efficient electron transmission in covalent organic framework nanosheets for highly active electrocatalytic carbon dioxide reduction. Nat. Commun. 2020, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Yang, C.; Chen, Y.; Huang, N.; Duan, S.; Zhang, Z.; Hu, W.; Jiang, D. Bottom-Up Interfacial Design of Covalent Organic Frameworks for Highly Efficient and Selective Electrocatalysis of CO2. Adv. Mater. 2022, 34, 2205186. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.N.; Abdulhamied, E.; Nyayachavadi, A.; Selivanova, M.; Eichhorn, S.H.; Rondeau-Gagné, S. Topochemical Polymerization of a Nematic Tetraazaporphyrin Derivative To Generate Soluble Polydiacetylene Nanowires. Langmuir 2019, 35, 15158–15167. [Google Scholar] [CrossRef]
- He, Q.; Kang, J.; Zhu, J.; Huang, S.; Lu, C.; Liang, H.; Su, Y.; Zhuang, X. N-confused porphyrin-based conjugated microporous polymers. Chem. Commun. 2022, 58, 2339–2342. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, T.; Cao, P.; Yoshida, T.; Tang, W.; Wang, X.; Zuo, Y.; Tang, P.; Heggen, M.; Dunin-Borkowski, R.E.; et al. A novel π-d conjugated cobalt tetraaza[14]annulene based atomically dispersed electrocatalyst for efficient CO2 reduction. Chem. Eng. J. 2022, 442, 136129. [Google Scholar] [CrossRef]
- Sun, L.; Huang, Z.; Reddu, V.; Su, T.; Fisher, A.C.; Wang, X. A Planar, Conjugated N4-Macrocyclic Cobalt Complex for Heterogeneous Electrocatalytic CO2 Reduction with High Activity. Angew. Chem. Int. Ed. 2020, 59, 17104–17109. [Google Scholar] [CrossRef]
- Xuan, X.; Jiang, K.; Huang, S.; Feng, B.; Qiu, F.; Han, S.; Zhu, J.; Zhuang, X. Tertiary amine-functionalized Co(II) porphyrin to enhance the electrochemical CO2 reduction activity. J. Mater. Sci. 2022, 57, 10129–10140. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Y.; Yang, S.; Han, S.; Lu, C.; Ji, H.; Wang, T.; Ke, C.; Xu, Q.; Zhu, J.; et al. Modulating intramolecular electron and proton transfer kinetics for promoting carbon dioxide conversion. Chem. Commun. 2022, 58, 1966–1969. [Google Scholar] [CrossRef]
- Dou, S.; Sun, L.; Xi, S.; Li, X.; Su, T.; Fan, H.J.; Wang, X. Enlarging the π-Conjugation of Cobalt Porphyrin for Highly Active and Selective CO2 Electroreduction. ChemSusChem 2021, 14, 2126–2132. [Google Scholar] [CrossRef]
- Hu, M.-K.; Wang, N.; Ma, D.-D.; Zhu, Q.-L. Surveying the electrocatalytic CO2-to-CO activity of heterogenized metallomacrocycles via accurate clipping at the molecular level. Nano Res. 2022, 15, 10070–10077. [Google Scholar] [CrossRef]
- Diercks, C.S.; Lin, S.; Kornienko, N.; Kapustin, E.A.; Nichols, E.M.; Zhu, C.; Zhao, Y.; Chang, C.J.; Yaghi, O.M. Reticular Electronic Tuning of Porphyrin Active Sites in Covalent Organic Frameworks for Electrocatalytic Carbon Dioxide Reduction. J. Am. Chem. Soc. 2018, 140, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Q.-X.; Chi, S.-Y.; Li, H.-F.; Huang, Y.-B.; Cao, R. Hydrophobic perfluoroalkane modified metal-organic frameworks for the enhanced electrocatalytic reduction of CO2. SmartMat 2022, 3, 163–172. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.-J.; Dou, Y.; Zhu, Z.; Su, J.; Huang, L.; Guo, W.; Cao, X.; Cheng, L.; Zhu, Z.; et al. Atomically Thin, Ionic–Covalent Organic Nanosheets for Stable, High-Performance Carbon Dioxide Electroreduction. Adv. Mater. 2022, 34, 2110496. [Google Scholar] [CrossRef] [PubMed]
- Costentin, C.; Drouet, S.; Robert, M.; Savéant, J.-M. A Local Proton Source Enhances CO2 Electroreduction to CO by a Molecular Fe Catalyst. Science 2012, 338, 90–94. [Google Scholar] [CrossRef]
- Centane, S.; Sekhosana, E.K.; Matshitse, R.; Nyokong, T. Electrocatalytic activity of a push-pull phthalocyanine in the presence of reduced and amino functionalized graphene quantum dots towards the electrooxidation of hydrazine. J. Electroanal. Chem. 2018, 820, 146–160. [Google Scholar] [CrossRef]
- Abdinejad, M.; Dao, C.; Deng, B.; Dinic, F.; Voznyy, O.; Zhang, X.-a.; Kraatz, H.-B. Electrocatalytic Reduction of CO2 to CH4 and CO in Aqueous Solution Using Pyridine-Porphyrins Immobilized onto Carbon Nanotubes. ACS Sustain. Chem. Eng. 2020, 8, 9549–9557. [Google Scholar] [CrossRef]
- Yu, K.; Bi, S.; Ming, W.; Wei, W.; Zhang, Y.; Xu, J.; Qiang, P.; Qiu, F.; Wu, D.; Zhang, F. Side-chain-tuned π-extended porous polymers for visible light-activated hydrogen evolution. Polym. Chem. 2019, 10, 3758–3763. [Google Scholar] [CrossRef]
- Bao, W.; Huang, S.; Tranca, D.; Feng, B.; Qiu, F.; Rodríguez-Hernández, F.; Ke, C.; Han, S.; Zhuang, X. Molecular Engineering of CoII Porphyrins with Asymmetric Architecture for Improved Electrochemical CO2 Reduction. ChemSusChem 2022, 15, e202200090. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Y.; Jiang, D. CMPs as Scaffolds for Constructing Porous Catalytic Frameworks: A Built-in Heterogeneous Catalyst with High Activity and Selectivity Based on Nanoporous Metalloporphyrin Polymers. J. Am. Chem. Soc. 2010, 132, 9138–9143. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Chen, L.; Liu, J.; Parvez, K.; Liang, H.; Shu, J.; Sachdev, H.; Graf, R.; Feng, X.; Müllen, K. High-Performance Electrocatalysts for Oxygen Reduction Derived from Cobalt Porphyrin-Based Conjugated Mesoporous Polymers. Adv. Mater. 2014, 26, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Chambon, S.; Rivaton, A.; Gardette, J.-L.; Firon, M.; Lutsen, L. Aging of a donor conjugated polymer: Photochemical studies of the degradation of poly[2-methoxy-5-(3′,7′-dimethyloctyloxy)-1,4-phenylenevinylene]. J. Polym. Sci. A Polym. Chem. 2007, 45, 317–331. [Google Scholar] [CrossRef]
- Atreya, M.; Li, S.; Kang, E.T.; Neoh, K.G.; Ma, Z.H.; Tan, K.L.; Huang, W. Stability studies of poly(2-methoxy-5-(2′-ethyl hexyloxy)-p- (phenylene vinylene) [MEH-PPV]. Polym. Degrad. Stab. 1999, 65, 287–296. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Shen, Q.; Guo, C.; Zhao, G. A biomimetic photoelectrocatalyst of Co–porphyrin combined with a g-C3N4 nanosheet based on π–π supramolecular interaction for high-efficiency CO2 reduction in water medium. Green Chem. 2017, 19, 5900–5910. [Google Scholar] [CrossRef]
- Li, S.; Kang, E.T.; Neoh, K.G.; Ma, Z.H.; Tan, K.L.; Huang, W. In situ XPS studies of thermally deposited potassium on poly(p-phenylene vinylene) and its ring-substituted derivatives. Appl. Surf. Sci. 2001, 181, 201–210. [Google Scholar] [CrossRef]
- Zheng, W.; Shan, N.; Yu, L.; Wang, X. UV–visible, fluorescence and EPR properties of porphyrins and metalloporphyrins. Dyes Pigment. 2008, 77, 153–157. [Google Scholar] [CrossRef]
- Tsuda, A.; Osuka, A. Fully Conjugated Porphyrin Tapes with Electronic Absorption Bands That Reach into Infrared. Science 2001, 293, 79–82. [Google Scholar] [CrossRef]
- Qiu, F.; Zhang, F.; Tang, R.; Fu, Y.; Wang, X.; Han, S.; Zhuang, X.; Feng, X. Triple Boron-Cored Chromophores Bearing Discotic 5,11,17-Triazatrinaphthylene-Based Ligands. Org. Lett. 2016, 18, 1398–1401. [Google Scholar] [CrossRef]
- Hu, X.-M.; Rønne, M.H.; Pedersen, S.U.; Skrydstrup, T.; Daasbjerg, K. Enhanced Catalytic Activity of Cobalt Porphyrin in CO2 Electroreduction upon Immobilization on Carbon Materials. Angew. Chem. Int. Ed. 2017, 56, 6468–6472. [Google Scholar] [CrossRef]
- Liu, K.; Qiu, F.; Yang, C.; Tang, R.; Fu, Y.; Han, S.; Zhuang, X.; Mai, Y.; Zhang, F.; Feng, X. Nonplanar Ladder-Type Polycyclic Conjugated Molecules: Structures and Solid-State Properties. Cryst. Growth Des. 2015, 15, 3332–3338. [Google Scholar] [CrossRef]
- Han, N.; Wang, Y.; Ma, L.; Wen, J.; Li, J.; Zheng, H.; Nie, K.; Wang, X.; Zhao, F.; Li, Y.; et al. Supported Cobalt Polyphthalocyanine for High-Performance Electrocatalytic CO2 Reduction. Chem 2017, 3, 652–664. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Huang, Q.; He, C.-T.; Chen, Y.; Liu, J.; Shen, F.-C.; Lan, Y.-Q. Oriented electron transmission in polyoxometalate-metalloporphyrin organic framework for highly selective electroreduction of CO2. Nat. Commun. 2018, 9, 4466. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Qiu, Y.; Zhang, T.; Su, P.; Li, X.; Zhang, H. N-Doped Nanoporous Carbon from Biomass as a Highly Efficient Electrocatalyst for the CO2 Reduction Reaction. ACS Sustain. Chem. Eng. 2019, 7, 5249–5255. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhao, S.; Johannessen, B.; Veder, J.-P.; Saunders, M.; Rowles, M.R.; Cheng, M.; Liu, C.; Chisholm, M.F.; De Marco, R.; et al. Atomically Dispersed Transition Metals on Carbon Nanotubes with Ultrahigh Loading for Selective Electrochemical Carbon Dioxide Reduction. Adv. Mater. 2018, 30, 1706287. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal–Organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef]
- Shen, J.; Kortlever, R.; Kas, R.; Birdja, Y.Y.; Diaz-Morales, O.; Kwon, Y.; Ledezma-Yanez, I.; Schouten, K.J.P.; Mul, G.; Koper, M.T.M. Electrocatalytic reduction of carbon dioxide to carbon monoxide and methane at an immobilized cobalt protoporphyrin. Nat. Commun. 2015, 6, 8177. [Google Scholar] [CrossRef]
- Dong, B.-X.; Qian, S.-L.; Bu, F.-Y.; Wu, Y.-C.; Feng, L.-G.; Teng, Y.-L.; Liu, W.-L.; Li, Z.-W. Electrochemical Reduction of CO2 to CO by a Heterogeneous Catalyst of Fe–Porphyrin-Based Metal–Organic Framework. ACS Appl. Energy Mater. 2018, 1, 4662–4669. [Google Scholar] [CrossRef]
- Tang, J.-K.; Zhu, C.-Y.; Jiang, T.-W.; Wei, L.; Wang, H.; Yu, K.; Yang, C.-L.; Zhang, Y.-B.; Chen, C.; Li, Z.-T.; et al. Anion exchange-induced single-molecule dispersion of cobalt porphyrins in a cationic porous organic polymer for enhanced electrochemical CO2 reduction via secondary-coordination sphere interactions. J. Mater. Chem. A 2020, 8, 18677–18686. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, D.-T.; Ye, R.; Zeng, J.; Corbin, N.; Manthiram, K. Inductive and electrostatic effects on cobalt porphyrins for heterogeneous electrocatalytic carbon dioxide reduction. Catal. Sci. Technol. 2019, 9, 974–980. [Google Scholar] [CrossRef]
- Lu, C.; Yang, J.; Wei, S.; Bi, S.; Xia, Y.; Chen, M.; Hou, Y.; Qiu, M.; Yuan, C.; Su, Y.; et al. Atomic Ni Anchored Covalent Triazine Framework as High Efficient Electrocatalyst for Carbon Dioxide Conversion. Adv. Funct. Mater. 2019, 29, 1806884. [Google Scholar] [CrossRef]
- Kang, J.; Wang, M.; Lu, C.; Ke, C.; Liu, P.; Zhu, J.; Qiu, F.; Zhuang, X. Platinum Atoms and Nanoparticles Embedded Porous Carbons for Hydrogen Evolution Reaction. Materials 2020, 13, 1513. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Wang, Y.-R.; Chen, Y.; Li, W.-L.; Dong, L.-Z.; Lan, Y.-Q. Metallocene implanted metalloporphyrin organic framework for highly selective CO2 electroreduction. Nano Energy 2020, 67, 104233. [Google Scholar] [CrossRef]
- Xin, Z.; Liu, J.; Wang, X.; Shen, K.; Yuan, Z.; Chen, Y.; Lan, Y.-Q. Implanting Polypyrrole in Metal-Porphyrin MOFs: Enhanced Electrocatalytic Performance for CO2RR. ACS Appl. Mater. Inter. 2021, 13, 54959–54966. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Liu, F.; Kang, J.; Qiu, F.; Ke, C.; Huang, Y.; Han, S.; Zhang, F.; Zhuang, X. Self-Assembly Approach Towards MoS2-Embedded Hierarchical Porous Carbons for Enhanced Electrocatalytic Hydrogen Evolution. Chem.—Eur. J. 2021, 27, 2155–2164. [Google Scholar] [CrossRef] [PubMed]





| Entry 1 | Ecv.red (V) [a] | Ecv,LUMO (eV) [b] | Ecv.HOMO (eV) [c] | Eopt.gap (eV) [d] | EDFT,LOMO (eV) [e] | EDFT,HOMO (eV) [e] | EDFT,gap (eV) [e] |
|---|---|---|---|---|---|---|---|
| as-PorCo-OMe | −0.95 | −3.39 | −5.57 | 2.18 | −2.05 | −5.13 | 3.08 |
| cs-PorCo-OMe | −0.71 | −3.63 | −5.80 | 2.17 | −2.08 | −5.12 | 3.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Bao, W.; Huang, S.; Wang, B.; Wang, C.; Han, S.; Lu, C.; Qiu, F. Asymmetric Push–Pull Type Co(II) Porphyrin for Enhanced Electrocatalytic CO2 Reduction Activity. Molecules 2023, 28, 150. https://doi.org/10.3390/molecules28010150
Huang C, Bao W, Huang S, Wang B, Wang C, Han S, Lu C, Qiu F. Asymmetric Push–Pull Type Co(II) Porphyrin for Enhanced Electrocatalytic CO2 Reduction Activity. Molecules. 2023; 28(1):150. https://doi.org/10.3390/molecules28010150
Chicago/Turabian StyleHuang, Chenjiao, Wenwen Bao, Senhe Huang, Bin Wang, Chenchen Wang, Sheng Han, Chenbao Lu, and Feng Qiu. 2023. "Asymmetric Push–Pull Type Co(II) Porphyrin for Enhanced Electrocatalytic CO2 Reduction Activity" Molecules 28, no. 1: 150. https://doi.org/10.3390/molecules28010150
APA StyleHuang, C., Bao, W., Huang, S., Wang, B., Wang, C., Han, S., Lu, C., & Qiu, F. (2023). Asymmetric Push–Pull Type Co(II) Porphyrin for Enhanced Electrocatalytic CO2 Reduction Activity. Molecules, 28(1), 150. https://doi.org/10.3390/molecules28010150








