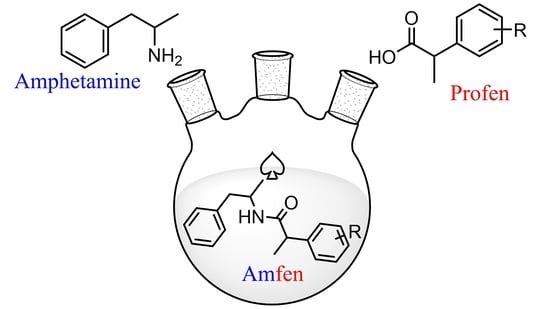
Synthesis, In Vitro Anti-Inflammatory Activity, and HRMS Analysis of New Amphetamine Derivatives
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
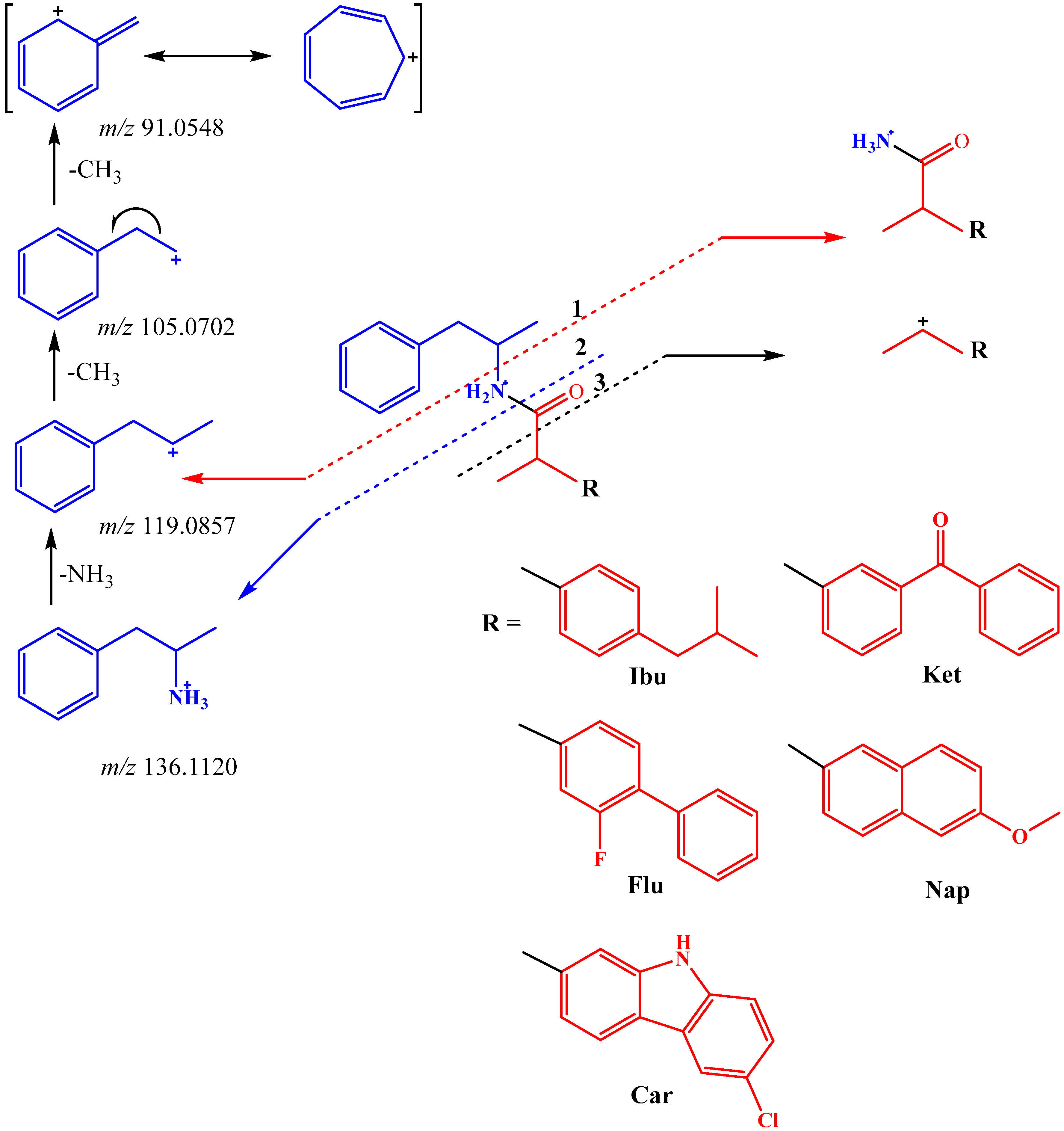
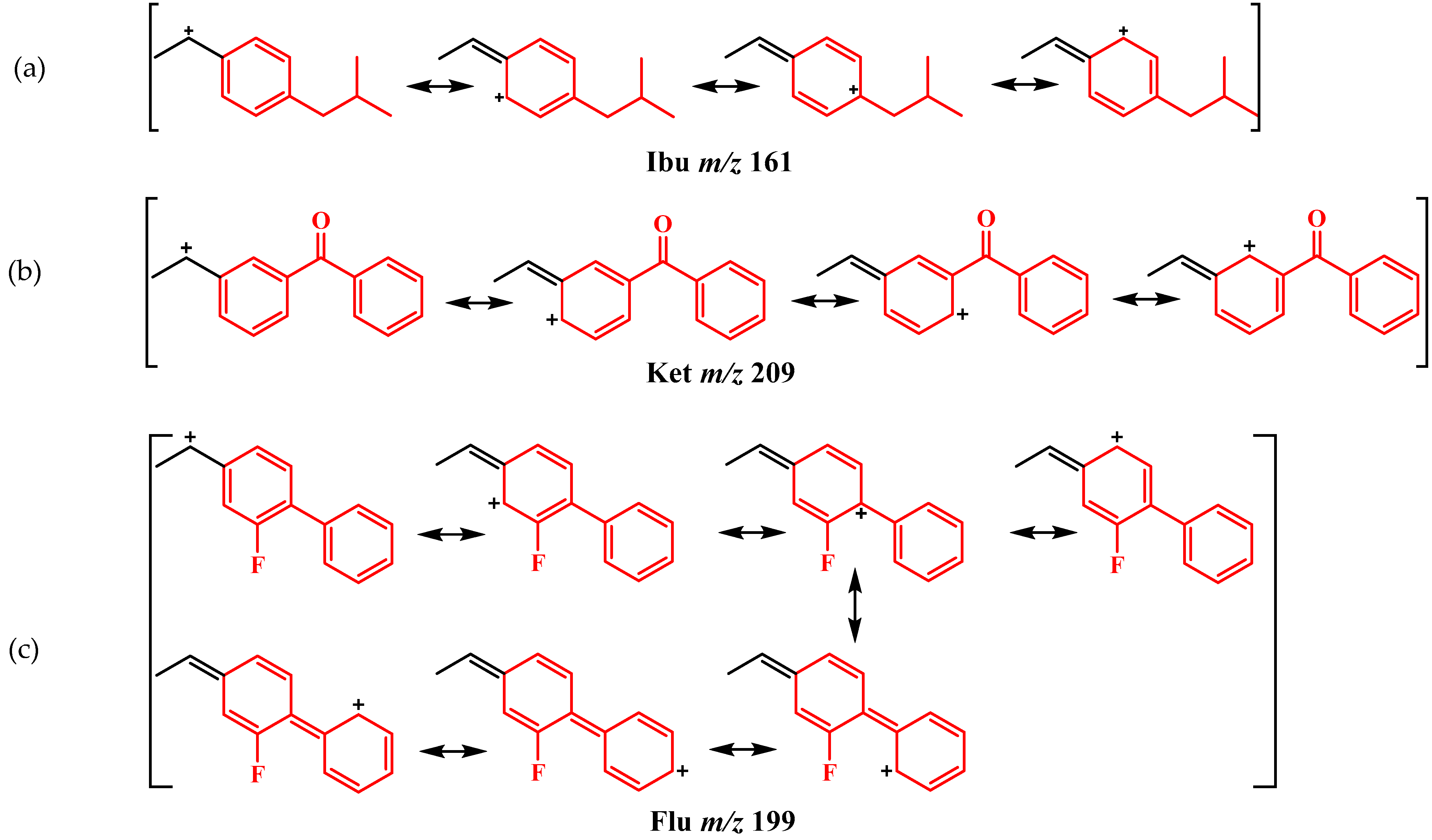
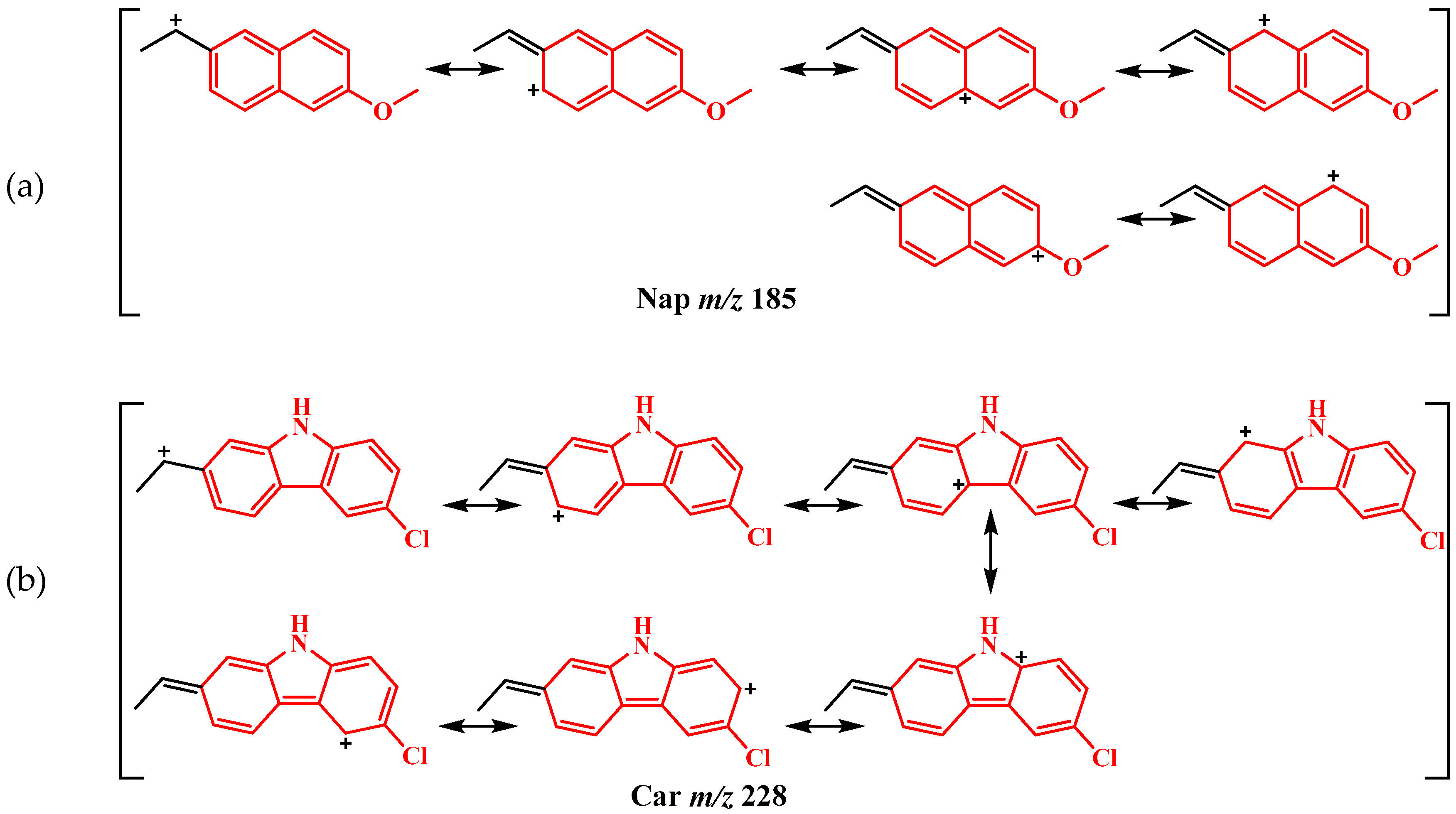
2.2. Mass Analysis
2.3. Anti-Inflammatory Activity
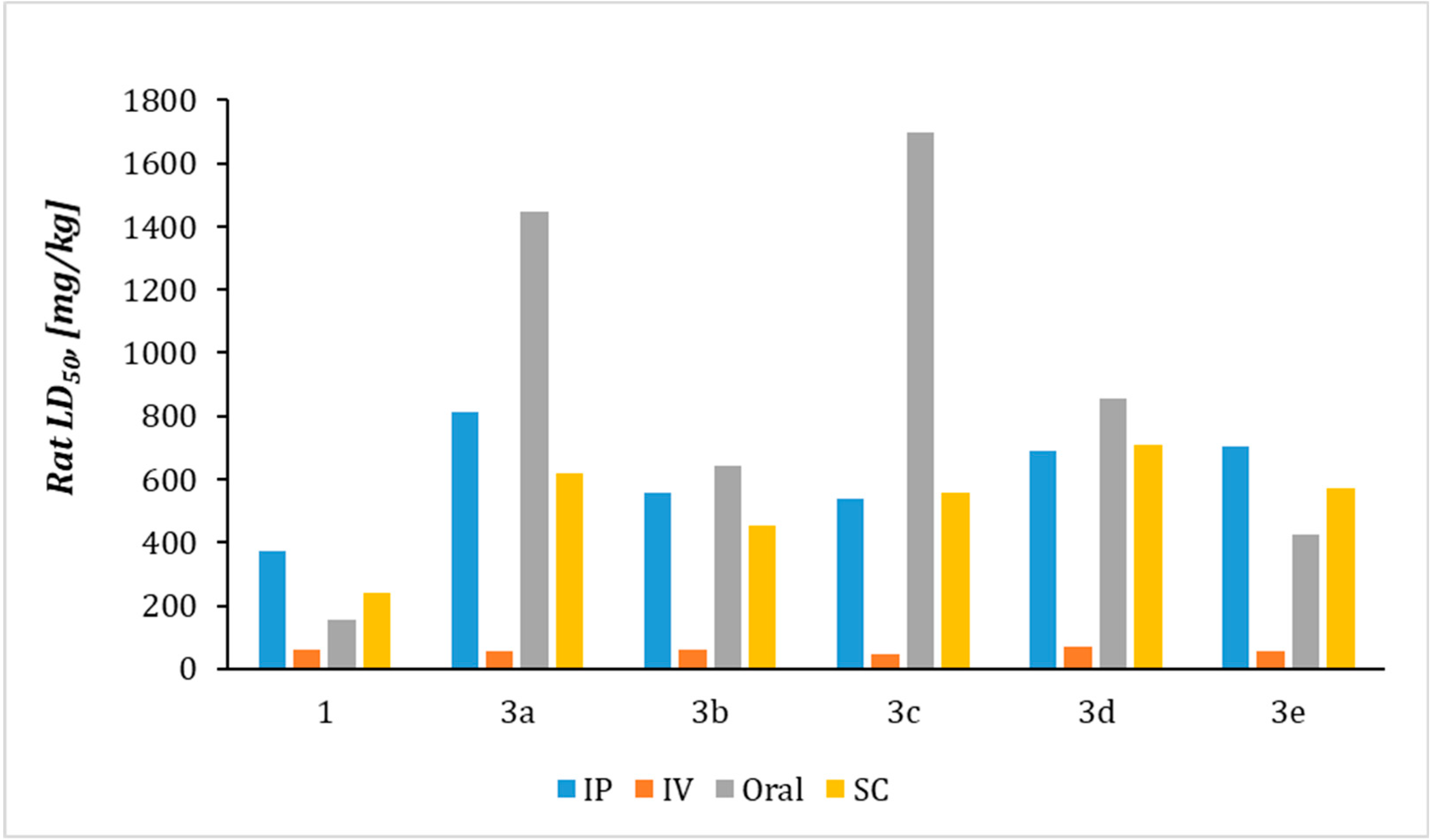
2.4. Prediction of Acute Rat Toxicity by Gusar Software
3. Materials and Methods
3.1. Synthesis
3.2. In Vitro Analysis
Inhibition of Albumin Denaturation (IAD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Allen, A.; Ely, R. Synthetic methods for amphetamine. Crime Scene Mag. 2011, 15–25. [Google Scholar]
- Fleckenstein, A.; Volz, T.; Riddle, E.; Gibb, J.; Hanson, G. New Insights into the Mechanism of Action of Amphetamines. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Kristen, G.; Schaefer, A.; Schlichtegroll, A. Fenetylline: Therapeutic use, misuse and/or abuse. Drug. Alcohol. Depend. 1986, 17, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Katselou, M.; Papoutsis, I.; Nikolaou, P.; Qammaz, S.; Spiliopoulou, C.; Athanaselis, S. Fenethylline (Captagon) abuse—Local problems from an old drug become universal. Basic Clin. Pharmacol. Toxicol. 2016, 119, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Parada, M.; Iturriaga-Vasquez, P.; Cassels, B. Amphetamine derivatives as monoamine oxidase inhibitors. Front. Pharmacol. 2020, 10, 1590. [Google Scholar] [CrossRef] [PubMed]
- Farkouh, M.; Greenberg, B. An Evidence-Based Review of the Cardiovascular Risks of Nonsteroidal Anti-Inflammatory Drugs. Am. J. Cardiol. 2009, 103, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.; Beshr, E.; Almalki, F.; Halawah, H.; Taj, B.; Alnafaei, A.; Alharazi, R.; Kazi, W.; AlMatrafi, M. Arylpropionic acid-derived NSAIDs: New insights on derivatization, anticancer activity and potential mechanism of action. Bioorg. Chem. 2019, 92, 103224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Imm, S.; Bähn, S.; Neubert, L.; Neumann, H.; Beller, M. Angew. Chem. Int. Ed. 2012, 51, 3905. [Google Scholar]
- Berman, S.M.; Kuczenski, R.; McCracken, J.T.; London, E.D. Potential adverse effects of amphetamine treatment on brain and behavior: A review. Mol. Psychiatry 2009, 14, 123–142. [Google Scholar] [CrossRef] [PubMed]
- Madras, B.K.; Miller, G.M.; Fischman, A.J. The dopamine transporter and attention-deficit/hyperactivity disorder. Biol. Psychiatry 2005, 57, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. New insights into the mode of action of anti-inflammatory drugs. Inflamm. Res. 1995, 44, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, V.; Bagyalakshmi, S.; Manjula Devi, K.; Richard Daniel, D. In-vitro anti-inflammatory activity of 4-benzylpiperidine. Asian J. Pharm.Clin. Res. 2016, 9, 108–110. [Google Scholar] [CrossRef]
- Carvalho, M.; Carmo, H.; Costa, V.; Capela, J.; Pontes, H.; Remião, F.; Carvalho, F.; de Lourdes Bastos, M. Toxicity of amphetamines: An update. Arch. Toxicol. 2012, 86, 1167–1231. [Google Scholar] [CrossRef] [PubMed]
- Lagunin, A.; Zakharov, A.; Filimonov, D.; Poroikov, V. QSAR modelling of rat acute toxicity on the basis of PASS prediction. Mol. Inform. 2011, 30, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Manolov, S.; Ivanov, I.; Bojilov, D. (±)–2-(2-Fluoro-[1,1′-biphenyl]-4-yl)–N-(1-phenylpropan-2-yl) propanamide. Molbank 2022, 1, M1319. [Google Scholar] [CrossRef]
- Sakat, S.S.; Juvekar, A.R.; Gambhire, M.N. In-vitro antioxidant and anti-inflammatory activity of methanol extract of Oxalis corniculata linn. Int. J. Pharm. Pharm. Sci. 2010, 2, 146–155. [Google Scholar]
- Manolov, S.; Ivanov, I.; Bojilov, D. Microwave-assisted synthesis of 1,2,3,4-tetrahydroisoquinoline sulfonamide derivatives and their biological evaluation. J. Serb. Chem. Soc. 2021, 86, 139–151. [Google Scholar] [CrossRef]
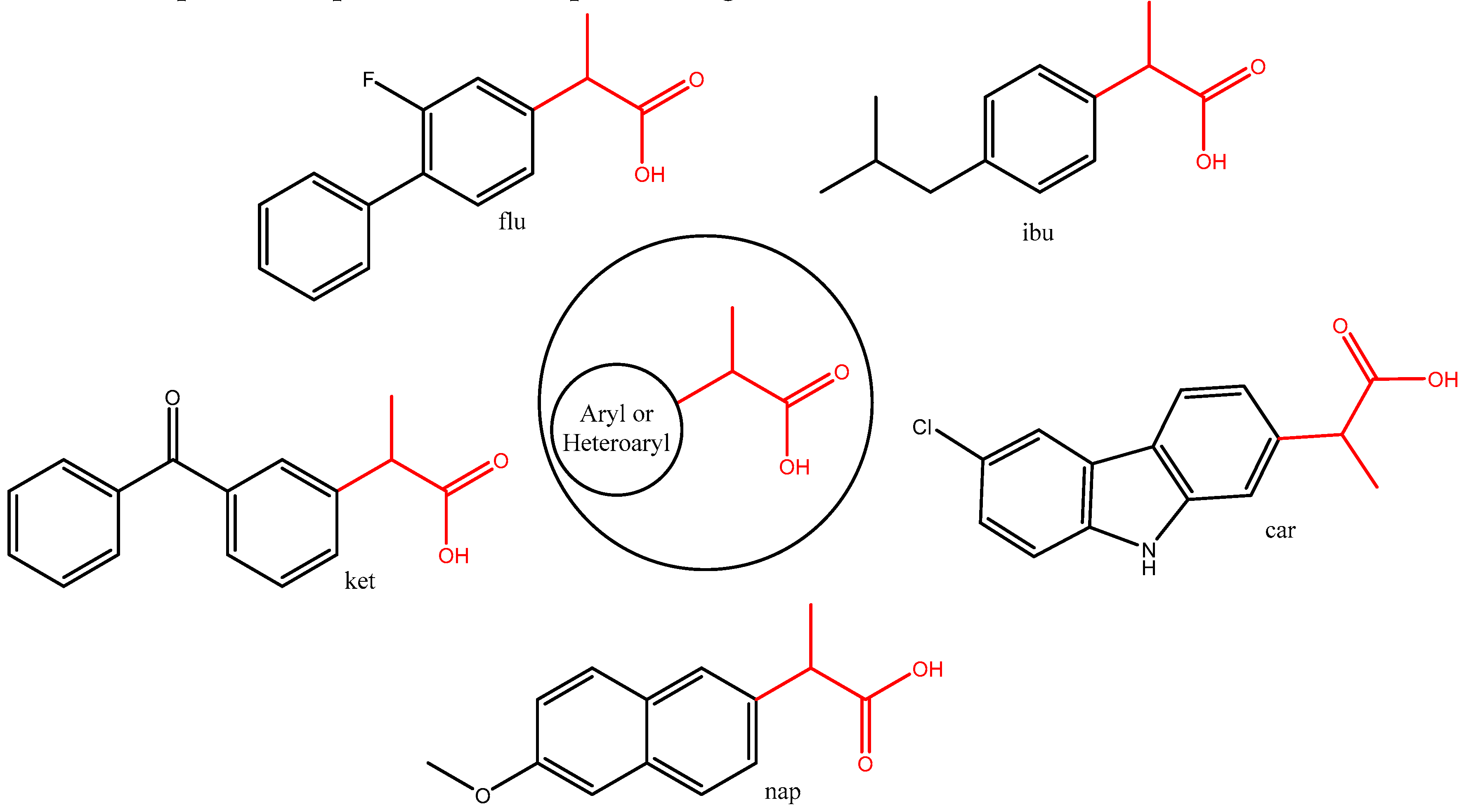
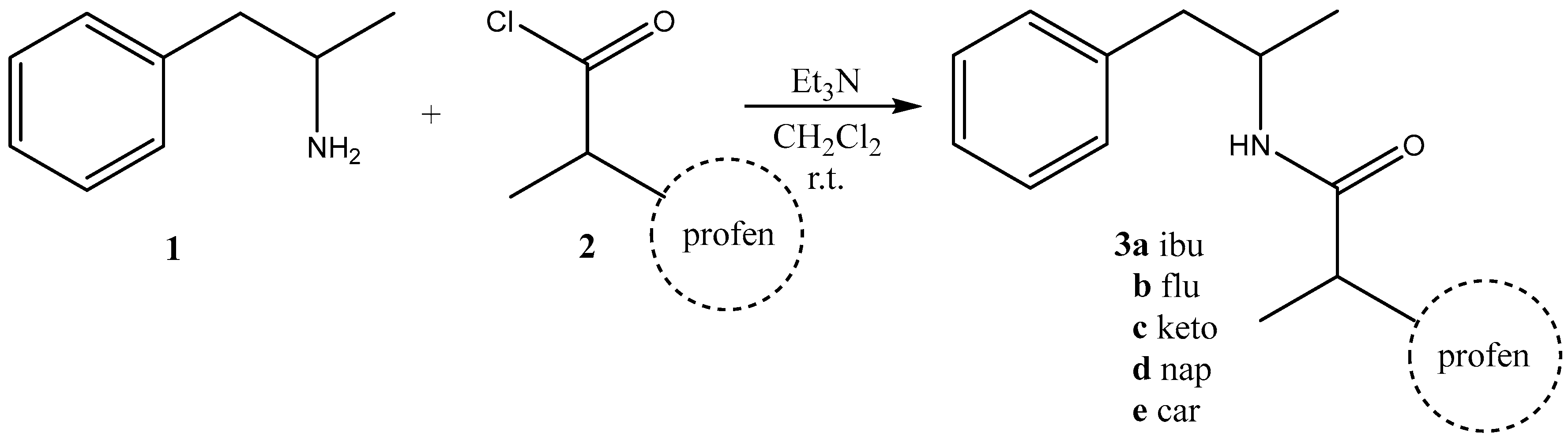


| Compound 3 | Yield, % | mp, oC |
|---|---|---|
| a | 94 | 46–49 |
| b | 90 | 112–115 |
| c | 90 | Oil |
| d | 95 | 112–114 |
| e | 85 | 126–127 |
| Compound | Rat LD50 [mg/kg] | |||
|---|---|---|---|---|
| IP | IV | Oral | SC | |
| 1 | 370.74a | 60.254a | 153.43b | 238.54a |
| 3a | 813.65a | 56.994a | 14484a | 617.34a |
| 3b | 557.95a | 59.794a | 641.84a | 454.64a |
| 3c | 539.65a | 47.64a | 16964a | 555.74a |
| 3d | 691.75a | 70.674a | 856.54a | 706.94a |
| 3e | 702.15a | 54.64a | 4264a | 572.54a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolov, S.; Ivanov, I.; Bojilov, D.; Nedialkov, P. Synthesis, In Vitro Anti-Inflammatory Activity, and HRMS Analysis of New Amphetamine Derivatives. Molecules 2023, 28, 151. https://doi.org/10.3390/molecules28010151
Manolov S, Ivanov I, Bojilov D, Nedialkov P. Synthesis, In Vitro Anti-Inflammatory Activity, and HRMS Analysis of New Amphetamine Derivatives. Molecules. 2023; 28(1):151. https://doi.org/10.3390/molecules28010151
Chicago/Turabian StyleManolov, Stanimir, Iliyan Ivanov, Dimitar Bojilov, and Paraskev Nedialkov. 2023. "Synthesis, In Vitro Anti-Inflammatory Activity, and HRMS Analysis of New Amphetamine Derivatives" Molecules 28, no. 1: 151. https://doi.org/10.3390/molecules28010151
APA StyleManolov, S., Ivanov, I., Bojilov, D., & Nedialkov, P. (2023). Synthesis, In Vitro Anti-Inflammatory Activity, and HRMS Analysis of New Amphetamine Derivatives. Molecules, 28(1), 151. https://doi.org/10.3390/molecules28010151