Determination of Hg(II) and Methylmercury by Electrothermal Atomic Absorption Spectrometry after Dispersive Solid-Phase Microextraction with a Graphene Oxide Magnetic Material
Abstract
1. Introduction
2. Results and Discussion
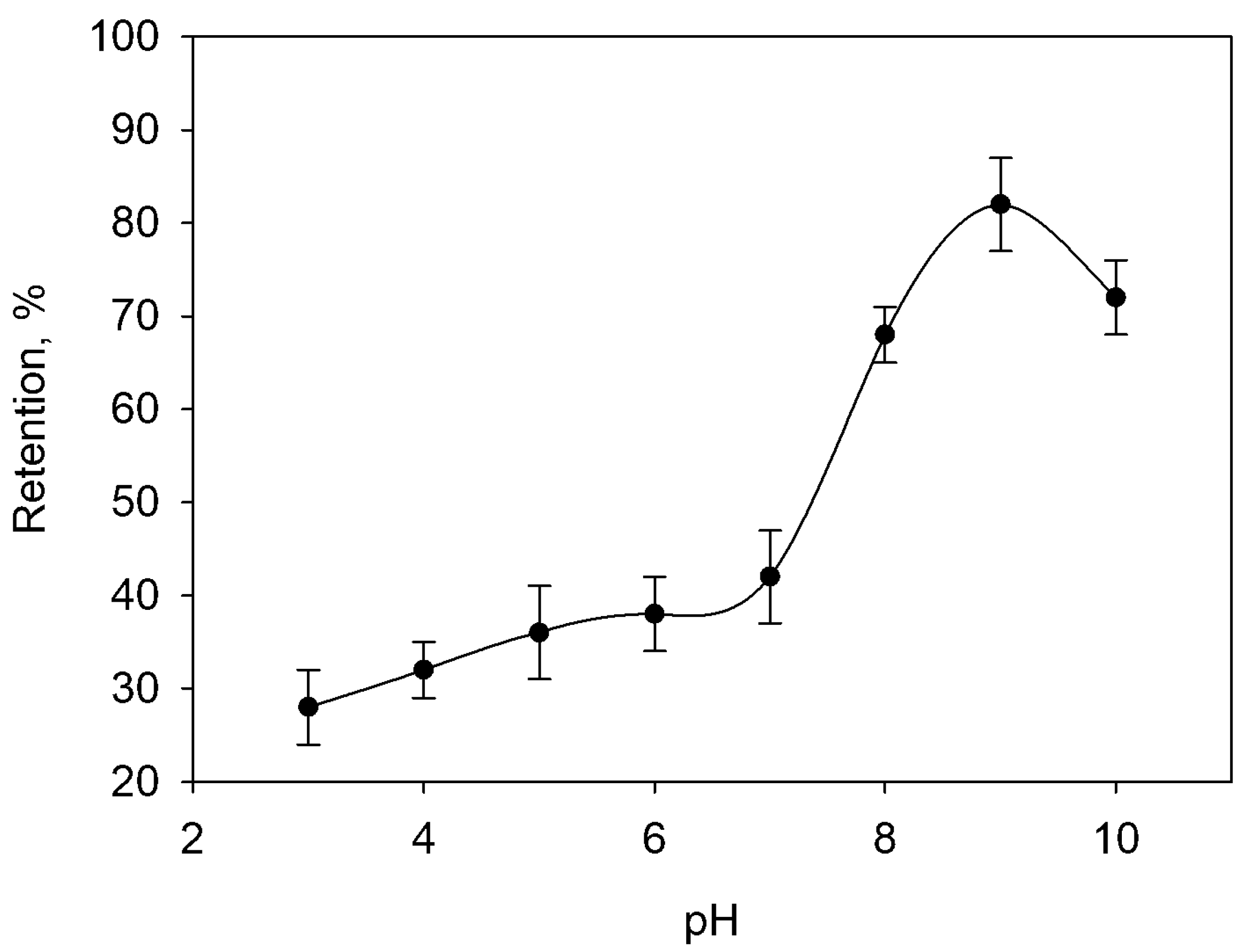
2.1. Effect of pH
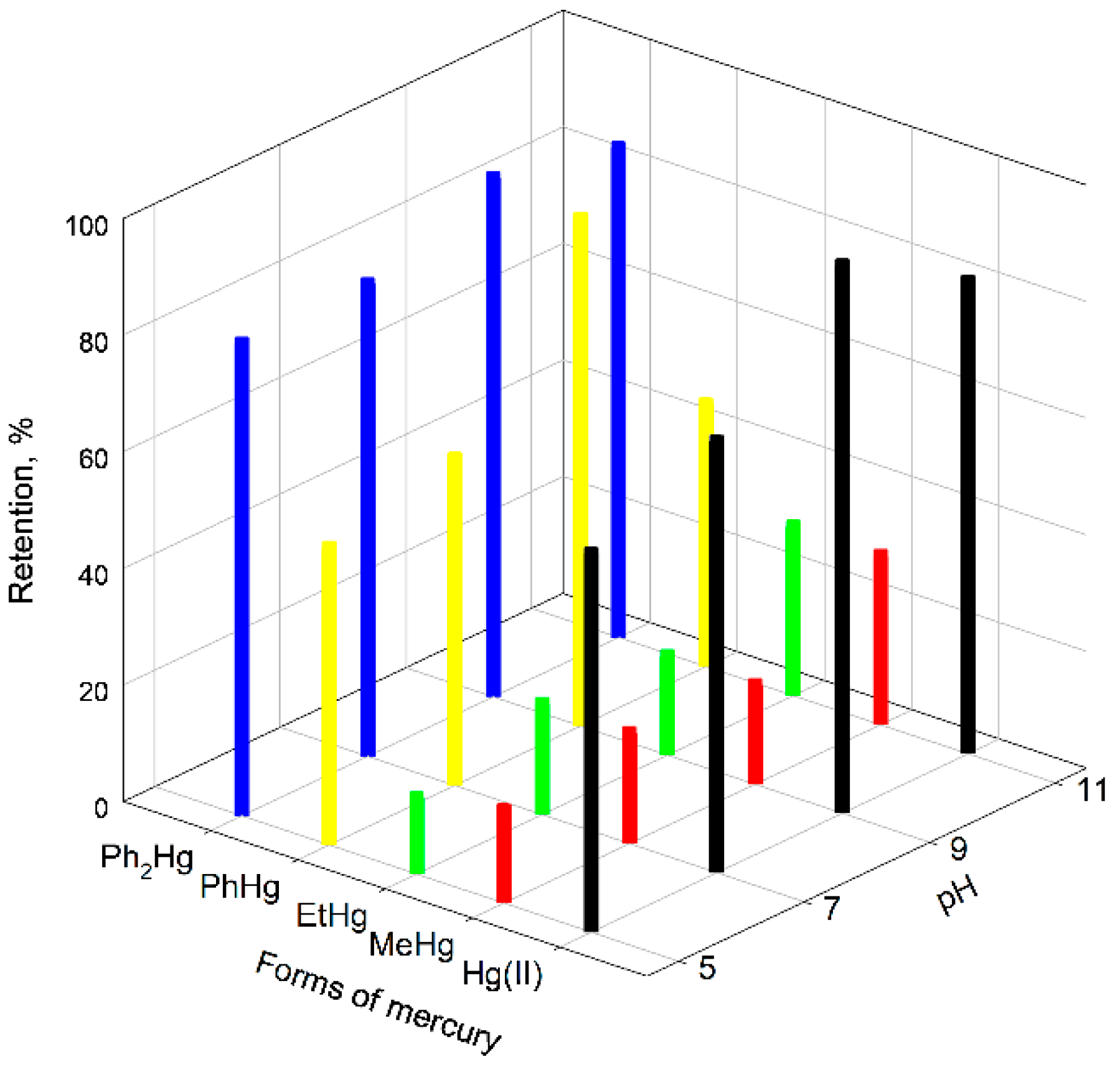
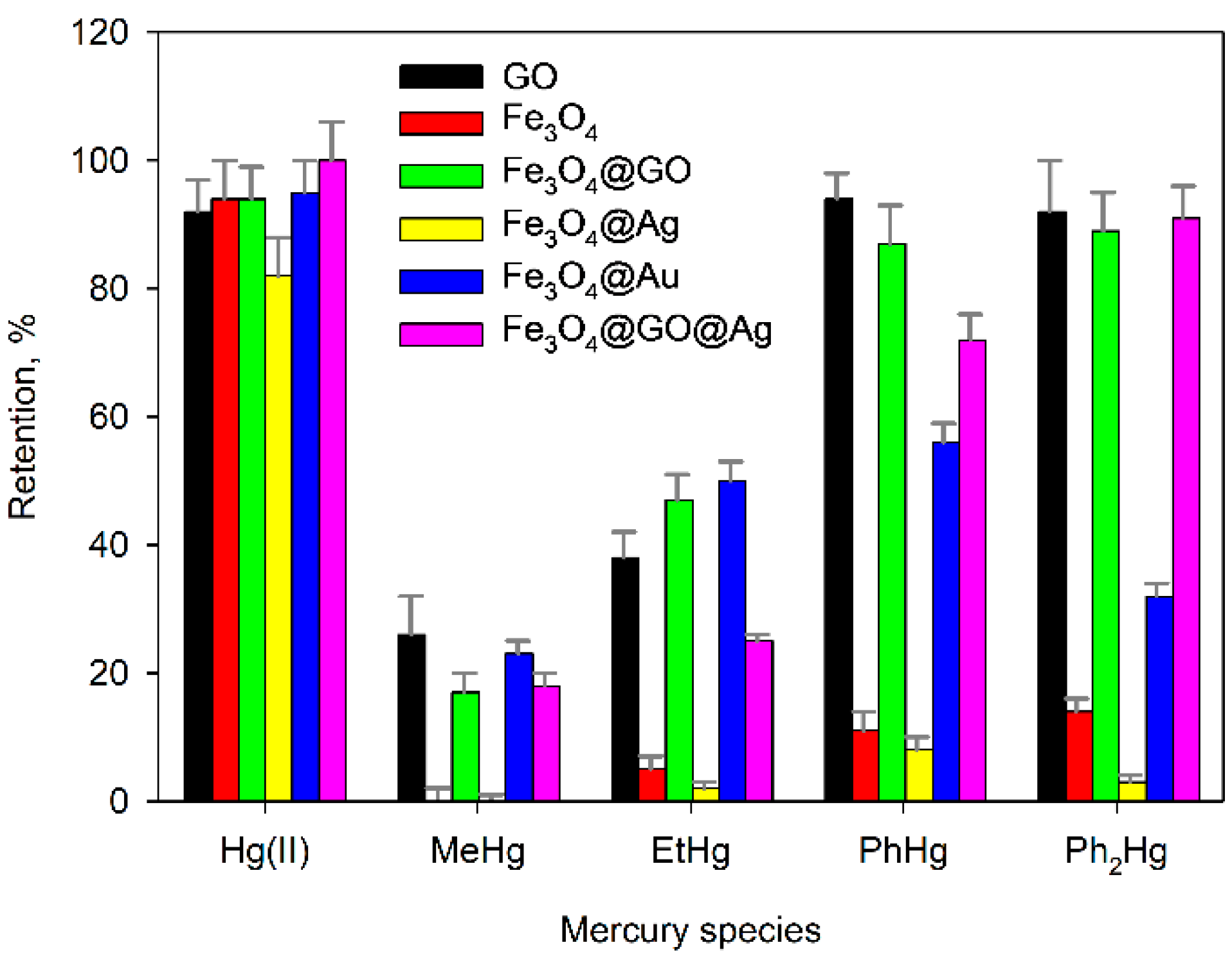
2.2. Retention of the Different Forms of Mercury
2.3. Effect of the Presence of Complexing Agents of Mercury Species
2.4. Study of Desorption Conditions
2.5. Optimization of Analysis Conditions by ETAAS
2.6. Calibration, Validation and Application to Real Samples
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Preparation of Fe3O4@GO
3.3. Sample Treatment
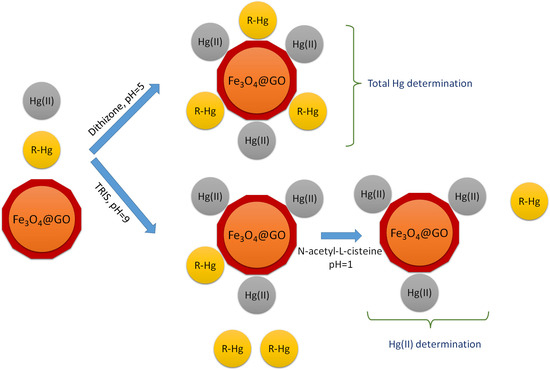
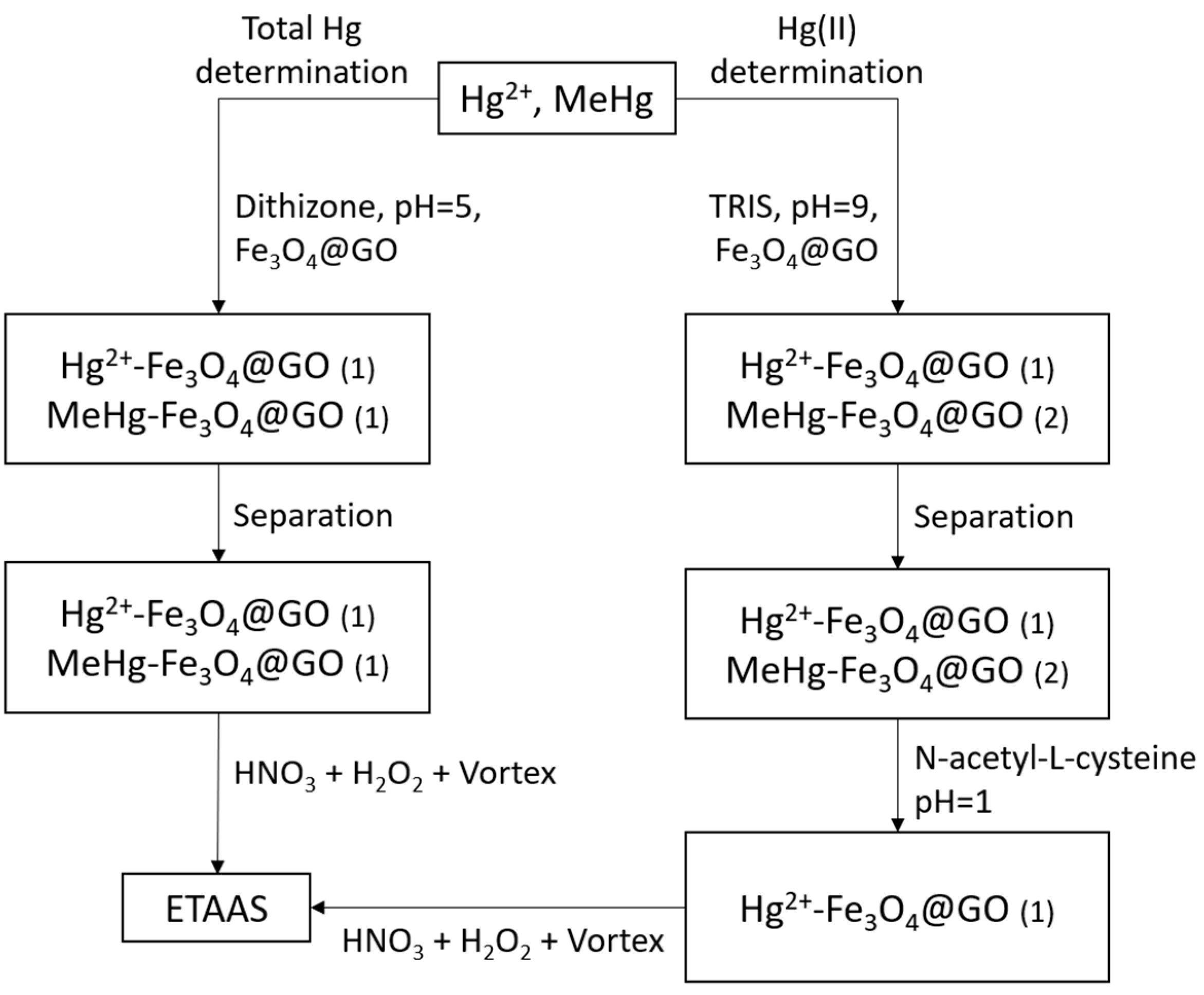
3.4. Procedure for the Determination of Total Mercury
3.5. Procedure for the Determination of Hg(II)
3.6. Procedure for the Determination of MeHg
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, M.; Li, Y.; Wang, Z. Mercury and Mercury-Containing Preparations: History of Use, Clinical Applications, Pharmacology, Toxicology, and Pharmacokinetics in Traditional Chinese Medicine. Front. Pharmacol. 2022, 13, 807807. [Google Scholar] [CrossRef] [PubMed]
- da Conceicao Nascimento Pinheiro, M.; do Nascimento, J.L.M.; de Lima Silveira, L.C.; da Rocha, J.B.T.; Aschner, M. Mercury and Selenium—A Review on Aspects Related to the Health of Human Populations in the Amazon. Environ. Bioindic. 2009, 4, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Enrico, M.; Balcom, P.; Johnston, D.T.; Foriel, J.; Sunderland, E.M. Simultaneous combustion preparation for mercury isotope analysis and detection of total mercury using a direct mercury analyzer. Anal. Chim. Acta 2021, 1154, 338327. [Google Scholar] [CrossRef] [PubMed]
- Report, Utilizing Direct Mercury Analysis for Mercury Detection in Botanical Extracts, Vitamins, Minerals and Dietary Supplements. Braz. J. Anal. Chem. 2021, 8, 237–240.
- Lee, S.; Oh, S.; Shin, S.; Kim, D.; Lee, J.; Kang, H.; Jo, G. Quantitative analysis and risk assessment on total mercury and methyl mercury in seafood distributed in Daejeon. J. Biomed. Transl. Res. 2022, 23, 7–16. [Google Scholar] [CrossRef]
- Matusiewicz, H.; Sturgeon, R.E. Chemical Vapor Generation with Slurry Sampling: A Review of Applications to Atomic and Mass Spectrometry. Appl. Spectros. Rev. 2012, 47, 41–82. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Lemos, V.A.; Silva, L.O.B.; Queiroz, A.F.S.; Souza, A.S.; da Silva, E.G.P.; dos Santos, W.N.L.; das Virgens, C.F. Analytical strategies of sample preparation for the determination of mercury in food matrices—A review. Microchem. J. 2015, 121, 227–236. [Google Scholar] [CrossRef]
- Suvarapu, L.N.; Baek, S.-O. Recent Developments in the Speciation and Determination of Mercury Using Various Analytical Techniques. J. Anal. Methods Chem. 2015, 2015, 372459. [Google Scholar] [CrossRef]
- Kallithrakas-Kontos, N.; Foteinis, S. Recent Advances in the Analysis of Mercury in Water—Review. Curr. Anal. Chem. 2016, 12, 22–36. [Google Scholar] [CrossRef]
- Volynkin, S.S.; Demakov, P.A.; Shuvaeva, O.V.; Kovalenko, K.A. Metal-organic framework application for mercury speciation using solid phase extraction followed by direct thermal release-electrothermal atomization atomic absorption spectrophotometric detection (ETA AAS). Anal. Chim. Acta 2021, 1177, 338795. [Google Scholar] [CrossRef]
- Burylin, M.Y.; Romanovskiy, K.A.; Temerdashev, Z.A.; Galai, E.F. Determination of Mercury in Sediments by Slurry Sampling Electrothermal Atomic Absorption Spectrometry. J. Anal. Chem. 2019, 74, 1184–1191. [Google Scholar] [CrossRef]
- Lopez-Garcia, I.; Rivas, R.E.; Hernandez-Cordoba, M. Hollow fiber based liquid-phase microextraction for the determination of mercury traces in water samples by electrothermal atomic absorption spectrometry. Anal. Chim. Acta 2012, 743, 69–74. [Google Scholar] [CrossRef]
- Amico, D.; Tassone, A.; Pirrone, N.; Sprovieri, F.; Naccarato, A. Recent applications and novel strategies for mercury determination in environmental samples using microextraction-based approaches: A review. J. Hazard Mater. 2022, 433, 128823. [Google Scholar] [CrossRef]
- Ahmad, H.; Koo, B.H.; Khan, R.A. Preconcentration and determination of trace Hg(II) using ultrasound-assisted dispersive solid phase microextraction. RSC Adv. 2021, 12, 53–61. [Google Scholar] [CrossRef]
- Bahar, S.; Zakerian, R. Determination of Hg(II) in Environmental Water Samples by Dispersive Liquid Phase Microextraction Combined with Flame Atomic Absorption Spectrometry. J. Chin. Chem. Soc.-Taip. 2013, 60, 179–184. [Google Scholar] [CrossRef]
- Martinis, E.M.; Escudero, L.B.; Salvarezza, R.; Calderon, M.F.; Ibanez, F.J.; Wuilloud, R.G. Liquid-liquid microextraction based on a dispersion of Pd nanoparticles combined with ETAAS for sensitive Hg determination in water samples. Talanta 2013, 108, 46–52. [Google Scholar] [CrossRef]
- Lopez-Garcia, I.; Vicente-Martinez, Y.; Hernandez-Cordoba, M. Determination of ultratraces of mercury species using separation with magnetic core-modified silver nanoparticles and electrothermal atomic absorption spectrometry. J. Anal. Atom. Spectrom. 2015, 30, 1980–1987. [Google Scholar] [CrossRef]
- Ito, R.; Kawaguchi, M.; Sakui, N.; Okanouchi, N.; Saito, K.; Seto, Y.; Nakazawa, H. Stir bar sorptive extraction with in situ derivatization and thermal desorption-gas chromatography-mass spectrometry for trace analysis of methylmercury and mercury(II) in water sample. Talanta 2009, 77, 1295–1298. [Google Scholar] [CrossRef]
- Fabbri, D.; Lombardo, M.; Trombini, C.; Vassura, I. A new procedure for the speciation of mercury in water based on the transformation of mercury(II) and methylmercury(II) into stable acetylides followed by HPLC analysis. Appl. Organomet. Chem. 1995, 9, 713–718. [Google Scholar] [CrossRef]
- Cruz Sotolongo, A.; Messina, M.M.; Ibanez, F.J.; Wuilloud, R.G. Hybrid ionic liquid-3D graphene-Ni foam for on-line preconcentration and separation of Hg species in water with atomic fluorescence spectrometry detection. Talanta 2020, 210, 120614. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.-T.; Yang, X.-A.; Zhang, W.-B. Magnetic graphitic carbon nitride nano-composite for ultrasound-assisted dispersive micro-solid-phase extraction of Hg(II) prior to quantitation by atomic fluorescence spectroscopy. Anal. Chim. Acta 2019, 1074, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, L.; Xu, W.; Guo, X.; Cui, L.; Gao, L.; Wei, Q.; Du, B. Adsorption of Pb(II) and Hg(II) from aqueous solution using magnetic CoFe2O4-reduced graphene oxide. J. Mol. Liq. 2014, 191, 177–182. [Google Scholar] [CrossRef]
- Sreeprasad, T.S.; Maliyekkal, S.M.; Lisha, K.P.; Pradeep, T. Reduced graphene oxide-metal/metal oxide composites: Facile synthesis and application in water purification. J. Hazard Mater. 2011, 186, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Jinadasa, K.K.; Pena-Vazquez, E.; Bermejo-Barrera, P.; Moreda-Pineiro, A. Smart materials for mercury and arsenic determination in food and beverages. Microchem. J. 2022, 179, 107472. [Google Scholar] [CrossRef]
- Jamshidi, S.; Rofouei, M.K.; Seidi, S.; Emmer, Å. Applicability of a magnetic bucky gel for microextraction of mercury from complicated matrices followed by cold vapor atomic absorption spectroscopy. Sep. Sci. Technol. 2020, 55, 1505–1514. [Google Scholar] [CrossRef]
- Seidi, S.; Fotouhi, M. Magnetic dispersive solid phase extraction based on polythiophene modified magnetic graphene oxide for mercury determination in seafood followed by flow-injection cold vapor atomic absorption spectrometry. Anal. Methods 2017, 9, 803–813. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Shi, J.; Zou, X.; Li, Y.; Tahir, H.E.; Huang, X.; Li, Z.; Zhai, X.; Hu, X. Electrodeposition of gold nanoparticles and reduced graphene oxide on an electrode for fast and sensitive determination of methylmercury in fish. Food Chem. 2017, 237, 423–430. [Google Scholar] [CrossRef]
- Keramat, A.; Zare-Dorabei, R. Ultrasound-assisted dispersive magnetic solid phase extraction for preconcentration and determination of trace amount of Hg (II) ions from food samples and aqueous solution by magnetic graphene oxide (Fe3O4@ GO/2-PTSC): Central composite design optimization. Ultrason. Sonochem. 2017, 38, 421–429. [Google Scholar]
- Akbar, M.; Manoochehri, M. An efficient 2-mercapto-5-phenylamino-1,3,4-thiadiazole functionalized magnetic graphene oxide nanocomposite for preconcentrative determination of mercury in water and seafood samples. Inorg. Chem. Commun. 2019, 103, 37–42. [Google Scholar] [CrossRef]
- Li, L.; Bi, R.; Wang, Z.; Xu, C.; Li, B.; Luan, L.; Chen, X.; Xue, F.; Zhang, S.; Zhao, N. Speciation of mercury using high-performance liquid chromatography-inductively coupled plasma mass spectrometry following enrichment by dithizone functionalized magnetite-reduced graphene oxide. Spectrochim. Acta B 2019, 159, 105653. [Google Scholar] [CrossRef]
- Boszke, L.; Głosińska, G.; Siepak, J. Some Aspects of Speciation of Mercury in Water Environment. Pol. J. Environ. Stud. 2002, 11, 285–298. [Google Scholar]
- Si, L.; Branfireun, B.A.; Fierro, J. Chemical Oxidation and Reduction Pathways of Mercury Relevant to Natural Waters: A Review. Water 2022, 14, 1891. [Google Scholar] [CrossRef]
- Powell, K.J.; Brown, P.L.; Byrne, R.H.; Gajda, T.; Hefter, G.; Sjoberg, S.; Wanner, H. Chemical speciation of environmentally significant heavy metals with inorganic ligands—Part 1: The Hg2+-Cl−, OH−, CO32−, SO42−, and PO43− aqueous systems—(IUPAC technical report). Pure Appl. Chem. 2005, 77, 739–800. [Google Scholar] [CrossRef]
- Golding, G.R.; Sparling, R.; Kelly, C.A. Effect of pH on intracellular accumulation of trace concentrations of Hg(II) in Escherichia coli under anaerobic conditions, as measured using a mer-lux bioreporter. Appl. Environ, Microb. 2008, 74, 667–675. [Google Scholar] [CrossRef]
- Dong, W.; Liang, L.; Brooks, S.; Southworth, G.; Gu, B. Roles of dissolved organic matter in the speciation of mercury and methylmercury in a contaminated ecosystem in Oak Ridge, Tennessee. Environ. Chem. 2010, 7, 94–102. [Google Scholar] [CrossRef]
- Bittrich, D.R.; Chadwick, S.P.; Babiarz, C.L.; Manolopoulos, H.; Rutter, A.P.; Schauer, J.J.; Armstrong, D.E.; Collett, J.; Herckes, P. Speciation of Mercury (II) and Methylmercury in Cloud and Fog Water. Aerosol Air Qual. Res. 2011, 11, 161–169. [Google Scholar] [CrossRef]
- Lopez-Garcia, I.; Rivas, R.E.; Hernandez-Cordoba, M. Liquid-phase microextraction with solidification of the organic floating drop for the preconcentration and determination of mercury traces by electrothermal atomic absorption spectrometry. Anal. Bioanal. Chem. 2010, 396, 3097–3102. [Google Scholar] [CrossRef]
- Lopez-Garcia, I.; Sanchez-Merlos, M.; Hernandez-Cordoba, M. Determination of mercury in soils and sediments by graphite furnace atomic absorption spectrometry with slurry sampling. Spectrochim. Acta B 1997, 52, 2085–2092. [Google Scholar] [CrossRef]
- Lopez-Garcia, I.; Sanchez-Merlos, M.; Arroyo-Cortez, J.; Hernandez-Cordoba, M. Determination of mercury in sewage sludges by slurry sampling electrothermal atomic absorption spectrometry. J. AOAC Int. 2002, 85, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Vinas, P.; Pardo-Martinez, M.; Lopez-Garcia, I.; Hernandez-Cordoba, M. Determination of mercury in baby food and seafood samples using electrothermal atomic absorption spectrometry and slurry atomization. J. Anal. Atom. Spectrom. 2001, 16, 633–637. [Google Scholar] [CrossRef]
- Vinas, P.; Pardo-Martinez, M.; Lopez-Garcia, I.; Hernandez-Cordoba, M. Rapid determination of mercury in food colorants using electrothermal atomic absorption spectrometry with slurry sample introduction. J. Agr. Food Chem. 2002, 50, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Vereda Alonso, E.; del Mar Guerrero, M.; Colorado Cueto, P.; Barreno Benitez, J.; Cano Pavon, J.M.; Garcia de Torres, A. Development of an on-line solid phase extraction method based on new functionalized magnetic nanoparticles. Use in the determination of mercury in biological and sea-water samples. Talanta 2016, 153, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.G.M.; de Oliveira, E.; Pedrotti, J.J.; Oliveira, P.V. An electrochemical flow-cell for permanent modification of graphite tube with palladium for mercury determination by electrothermal atomic absorption spectrometry. Spectrochim. Acta B 2002, 57, 769–778. [Google Scholar] [CrossRef]
- Thongsaw, A.; Sananmuang, R.; Udnan, Y.; Ross, G.M.; Chaiyasith, W.C. Dual-cloud point extraction for speciation of mercury in water and fish samples by electrothermal atomic absorption spectrometry. Spectrochim. Acta B 2019, 160, 105685. [Google Scholar] [CrossRef]
- Thongsaw, A.; Sananmuang, R.; Udnan, Y.; Ross, G.M.; Chaiyasith, W.C. Speciation of mercury in water and freshwater fish samples using two-step hollow fiber liquid phase microextraction with electrothermal atomic absorption spectrometry. Spectrochim. Acta B 2019, 152, 102–108. [Google Scholar] [CrossRef]
- Sakanupongkul, A.; Sananmuang, R.; Udnan, Y.; Ampiah-Bonney, R.J.; Chaiyasith, W.C. Speciation of mercury in water and freshwater fish samples by a two-step solidified floating organic drop microextraction with electrothermal atomic absorption spectrometry. Food Chem. 2019, 277, 496–503. [Google Scholar] [CrossRef]
- Akramipour, R.; Golpayegani, M.R.; Ghasemi, M.; Noori, N.; Fattahi, N. Optimization of a methodology for speciation of arsenic, selenium and mercury in blood samples based on the deep eutectic solvent. Methodsx 2019, 6, 2141–2147. [Google Scholar] [CrossRef]
- Cruz Sotolongo, A.; Martinis, E.M.; Wuilloud, R.G. An easily prepared graphene oxide-ionic liquid hybrid nanomaterial for micro-solid phase extraction and preconcentration of Hg in water samples. Anal. Methods 2018, 10, 338–346. [Google Scholar] [CrossRef]
- Krawczyk, M.; Stanisz, E. Silver nanoparticles as a solid sorbent in ultrasound-assisted dispersive micro solid-phase extraction for the atomic absorption spectrometric determination of mercury in water samples. J. Anal. Atom. Spectrom. 2015, 30, 2353–2358. [Google Scholar] [CrossRef]
- Mitani, C.; Kotzamanidou, A.; Anthemidis, A.N. Automated headspace single-drop microextraction via a lab-in-syringe platform for mercury electrothermal atomic absorption spectrometric determination after in situ vapor generation. J. Anal. Atom. Spectrom. 2014, 29, 1491–1498. [Google Scholar] [CrossRef]
- Sarica, D.Y.; Turker, A.R. Speciation and Determination of Inorganic Mercury and Methylmercury by Headspace Single Drop Microextraction and Electrothermal Atomic Absorption Spectrometry in Water and Fish. Clean-Soil Air Water 2012, 40, 523–530. [Google Scholar] [CrossRef]
- United States Enronmental Protection Agency (EPA). Health Effects of Exposures to Mercury. Available online: https://www.epa.gov/mercury/health-effects-exposures-mercury (accessed on 1 December 2022).
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef]
- De Palma, G.; Mariotti, O.; Lonati, D.; Goldoni, M.; Catalani, S.; Mutti, A.; Locatelli, C. Toxicokinetics and toxicodynamics of elemental mercury following self-administration. Clin. Toxicol. 2008, 46, 869–876. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.; Lee, Y.-I.; Wu, L.; Hou, X. Simple mercury speciation analysis by CVG-ICP-MS following TMAH pre-treatment and microwave-assisted digestion. Microchem. J. 2012, 103, 105–109. [Google Scholar] [CrossRef]
- Vieira, M.A.; Ribeiro, A.S.; Curtius, A.J.; Sturgeon, R.E. Determination of total mercury and methylmercury in biological samples by photochemical vapor generation. Anal. Bioanal. Chem. 2007, 388, 837–847. [Google Scholar] [CrossRef]
| Parameter | |||
| Wavelength, nm | 253.6519 | ||
| Slit, nm | 0.7 | ||
| Atomizer | Transversal with L’Vov platform | ||
| Background correction | Zeeman effect | ||
| Injected sample volume, µL | 10 | ||
| Chemical modifier | 20 µL of 125 mg L−1 Pd(II) solution | ||
| Sample volume, mL | 10 | ||
| Heating program | |||
| Step | Temperature, °C | Ramp, °C s−1 | Hold, s |
| 1: Dry | 110 | 10 | 30 |
| 2: Pyrolysis | 300 | 50 | 20 |
| 4: Atomization a,b | 1500 | 2200 | 5 |
| 5: Clean | 1500 | 0 | 4 |
| Sequence: Inject chemical modifier and run steps 1 to 2. Stop heating and inject sample. Then run the heating program. | |||
| Total Hg, µg g−1 | MeHg a, µg g−1 | Hg(II) a, µg g−1 | ||||
|---|---|---|---|---|---|---|
| SRM | Certified | Found | Certified | Found | Certified | Found |
| NCS DC73347 | 0.360 ± 0.050 | 0.37 ± 0.07 | -- | 0.37 ± 0.07 | -- | <LOD |
| DORM-4 | 0.410 ± 0.055 | 0.408 ± 0.06 | 0.355 ± 0.028 | 0.348 ± 0.04 | -- | 0.06 ± 0.03 |
| DORM-2 | 4.64 ± 0.26 | 4.67 ± 0.12 | 4.47 ± 0.32 | 4.42 ± 0.06 | -- | 0.26 ± 0.06 |
| DOLT-2 | 2.14 ± 0.18 | 2.18 ± 0.09 | -- | 1.49 ± 0.09 | -- | 0.693 ± 0.08 |
| Hg Total, µg L−1 | MeHg a, µg L−1 | Hg(II) a, µg L−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SRM | Added | Found | Rec. % | Added | Found | Rec. % | Added | Found | Rec. % |
| NIST SRM 1640a | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 7 | 6.9 ± 0.1 | 98 ± 3 | 2 | 1.9 ± 0.1 | 96 ± 2 | 5 | 5.0 ± 0.1 | 99 ± 3 | |
| 7 | 7.3 ± 0.1 | 105 ± 3 | 5 | 5.1 ± 0.2 | 103 ± 3 | 2 | 2.2 ± 0.1 | 110 ± 5 | |
| SPS-SW2 | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 7 | 6.9 ± 0.1 | 98 ± 3 | 2 | 1.9 ± 0.1 | 96 ± 3 | 5 | 4.9 ± 0.1 | 99 ± 3 | |
| 7 | 6.8 ± 0.2 | 97 ± 2 | 5 | 4.9 ± 0.1 | 98 ± 3 | 2 | 1.9 ± 0.1 | 95 ± 4 | |
| ERM CAO11b | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 7 | 7.1 ± 0.1 | 102 ± 3 | 2 | 2.1 ± 0.1 | 104 ± 4 | 5 | 5.0 ± 0.2 | 101 ± 3 | |
| 7 | 7.2 ± 0.1 | 103 ± 2 | 5 | 5.1 ± 0.2 | 103 ± 3 | 2 | 2.0 ± 0.2 | 101 ± 4 | |
| NASS-6 | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 7 | 6.9 ± 0.2 | 99 ± 3 | 2 | 1.9 ± 0.1 | 95 ± 3 | 5 | 5.0 ± 0.2 | 101 ± 3 | |
| 7 | 6.9 ± 0.1 | 99 ± 2 | 5 | 5.0 ± 0.1 | 100 ± 3 | 2 | 1.9 ± 0.1 | 96 ± 3 | |
| Hg Total. µg L−1 | MeHg a. µg L−1 | Hg(II) a. µg L−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RWS | Added | Found | Rec. % | Added | Found | Rec. % | Added | Found | Rec. % |
| M1 | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 4 | 3.92 ± 0.09 | 97.9 ± 5.3 | 2 | 1.97 ± 0.04 | 98.5 ± 2.3 | 2 | 1.95 ± 0.03 | 97.3 ± 1.8 | |
| M2 | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 4 | 3.80 ± 0.03 | 95.1 ± 2.3 | 2 | 1.91 ± 0.07 | 95.5 ± 4.1 | 2 | 1.89 ± 0.07 | 94.5 ± 4.1 | |
| M3 | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 4 | 4.01 ± 0.06 | 100.3 ± 4.2 | 2 | 2.00 ± 0.06 | 100.0 ± 3.2 | 2 | 2.01 ± 0.02 | 100.6 ± 1.7 | |
| M4 | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 4 | 4.02 ± 0.05 | 100.6 ± 4.0 | 2 | 2.02 ± 0.05 | 100.9 ± 2.6 | 2 | 2.01 ± 0.05 | 100.3 ± 2.6 | |
| M5 | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 4 | 3.89 ± 0.01 | 97.2 ± 2.1 | 2 | 1.89 ± 0.06 | 94.5 ± 3.1 | 2 | 2.00 ± 0.04 | 99.9 ± 2.4 | |
| M6 | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 4 | 3.67 ± 0.07 | 91.8 ± 3.9 | 2 | 1.88 ± 0.07 | 93.9 ± 4.1 | 2 | 1.79 ± 0.01 | 89.7 ± 1.1 | |
| M7 | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 4 | 3.99 ± 0.06 | 99.7 ± 4.2 | 2 | 2.07 ± 0.03 | 103.5 ± 1.9 | 2 | 1.92 ± 0.03 | 95.8 ± 1.7 | |
| M8 | 0 | <LOD | -- | 0 | <LOD | -- | 0 | <LOD | -- |
| 4 | 4.11 ± 0.02 | 102.6 ± 3.7 | 2 | 2.08 ± 0.07 | 103.8 ± 4.0 | 2 | 2.03 ± 0.09 | 101.4 ± 5.1 | |
| Specie | Microextraction Technique | Remarks | CM | T, °C | L, µg L−1 | LOD, µg L−1 | EF | Ref. |
|---|---|---|---|---|---|---|---|---|
| Hg(II), MeHg, PhHg | SPE with MOF | Thermal release. | None | 135–275/800 | 0.001–0.5 a | 0.06 | -- | [11] |
| Hg(II), MeHg | Double CPE | 1: dithizone; 2: thiourea | Pd(II) | 200/1800 | 0.4–15 | 0.23 | 17.8 | [46] |
| MeHg; Hg(II) | Double HF-LPME | 1: MeHg (S2O3=); 2: Hg(II) (DDTC). | Pd(II) | 200;120/ 1800; 1300 | 0.5–8; 0.2–12 | 0.143; 0.063 | 103; 95 | [47] |
| MeHg; Hg(II) | SFODME in two steps | 1: MeHg (1-undecanol); 2: MeHg (4-NODP). | Pd(II) | 250/1300 | 0.8–8 | 0.24; 0.25 | 32.2; 25.7 | [48] |
| Hg(II) | LPME with eutectic solvent | DDTP. | Ir(IV) | 210/1100 | 0.36–60 | 0.1 | 98 | [49] |
| Hg(II) | SPME with GO + (C4C12Im)Br | Methodology in flow and elution with HNO3. | Pd(II) | 250/1300 | 0.02–8 | 0.014 | 100 | [50] |
| Hg(II) | SPME with Fe3O4@SiO2@DPTH | Methodology in flow and elution in thiourea. | Ir(0) | 20/1200 | 0.1–10 | 0.0078 | 5.4 | [44] |
| Hg(II); MeHg, Me2Hg, EtHg, PhHg,Ph2Hg | DSPME with Fe3O4@Ag@MESNA or Fe3O4@Ag@CYS | 1: Fe3O4@Ag@MESNA for Hg(II); 2: Fe3O4@Ag@CYS for others. | Ag(I) + KMnO4 | 300/1300 | 0.03–3.5 | 0.01 | 200 | [18] |
| Hg(II) | AgNPs and DSPME | The amalgamated Hg is dissolved in HNO3. | Pd(II) + Mg(II) | 300/1300 | 0.1–20 | 0.005 | 15 | [51] |
| Hg(II) | HS-SDME | Hg(0) with SnCl2 and drop of Pd(0). | Pd(II) | 200/1300 | 1.5–40 | 0.48 | 75 | [52] |
| Hg(II) | DSPME with PdNPs functionalized with dodecanethiolate | The nanoparticles are in a mixture of toluene and chloroform. | Pd(II) | 250/1300 | 0.1–10 | 0.0075 | 95 | [17] |
| Hg(II), MeHg | HS-SDME with thiourea or APDC | Generation of Hg(0) and MeHg hydride with NaBH4. | -- | 250/1100 | 17–355 | 5 | 35 | [53] |
| Hg(II) | HF-LPME three-phase | 1: complexation of Hg(II) with PAN; 2: toluene extraction; 3: iodide back extraction. | Pd(0) | 300/1100 | 0.2–3 | 0.06 | 270 | [13] |
| Hg(II), MeHg | DSPME with Fe3O4@GO | 1: Dithizone for total Hg; 2: NAC for Hg(II). | Pd(II) | 300/1500 | 0.1–10 | 0.02 | 49 | TW |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicente-Martínez, Y.; Muñoz-Sandoval, M.J.; Hernandez-Cordoba, M.; Lopez-Garcia, I. Determination of Hg(II) and Methylmercury by Electrothermal Atomic Absorption Spectrometry after Dispersive Solid-Phase Microextraction with a Graphene Oxide Magnetic Material. Molecules 2023, 28, 14. https://doi.org/10.3390/molecules28010014
Vicente-Martínez Y, Muñoz-Sandoval MJ, Hernandez-Cordoba M, Lopez-Garcia I. Determination of Hg(II) and Methylmercury by Electrothermal Atomic Absorption Spectrometry after Dispersive Solid-Phase Microextraction with a Graphene Oxide Magnetic Material. Molecules. 2023; 28(1):14. https://doi.org/10.3390/molecules28010014
Chicago/Turabian StyleVicente-Martínez, Yesica, María Jose Muñoz-Sandoval, Manuel Hernandez-Cordoba, and Ignacio Lopez-Garcia. 2023. "Determination of Hg(II) and Methylmercury by Electrothermal Atomic Absorption Spectrometry after Dispersive Solid-Phase Microextraction with a Graphene Oxide Magnetic Material" Molecules 28, no. 1: 14. https://doi.org/10.3390/molecules28010014
APA StyleVicente-Martínez, Y., Muñoz-Sandoval, M. J., Hernandez-Cordoba, M., & Lopez-Garcia, I. (2023). Determination of Hg(II) and Methylmercury by Electrothermal Atomic Absorption Spectrometry after Dispersive Solid-Phase Microextraction with a Graphene Oxide Magnetic Material. Molecules, 28(1), 14. https://doi.org/10.3390/molecules28010014