An Efficient Processing Strategy to Improve the Flavor Profile of Egg Yolk: Ozone-Mediated Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Egg Yolk Samples
2.3. Ozone Treatment
2.4. Preparation of Volatile Compound Samples
2.5. Solid Phase Microextraction (SPME)
2.6. Gas Chromatography–Mass Spectrometer (GC-MS)
2.7. Pre-Treatment and Oil Extraction of Fatty Acid Determination Samples
2.8. Saponification and Methyl Esterification of Egg Yolk Oil
2.9. Determination of Fatty Acids
2.10. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.11. Scanning Electron Microscope (SEM)
2.12. Data Analysis
3. Results and Discussion
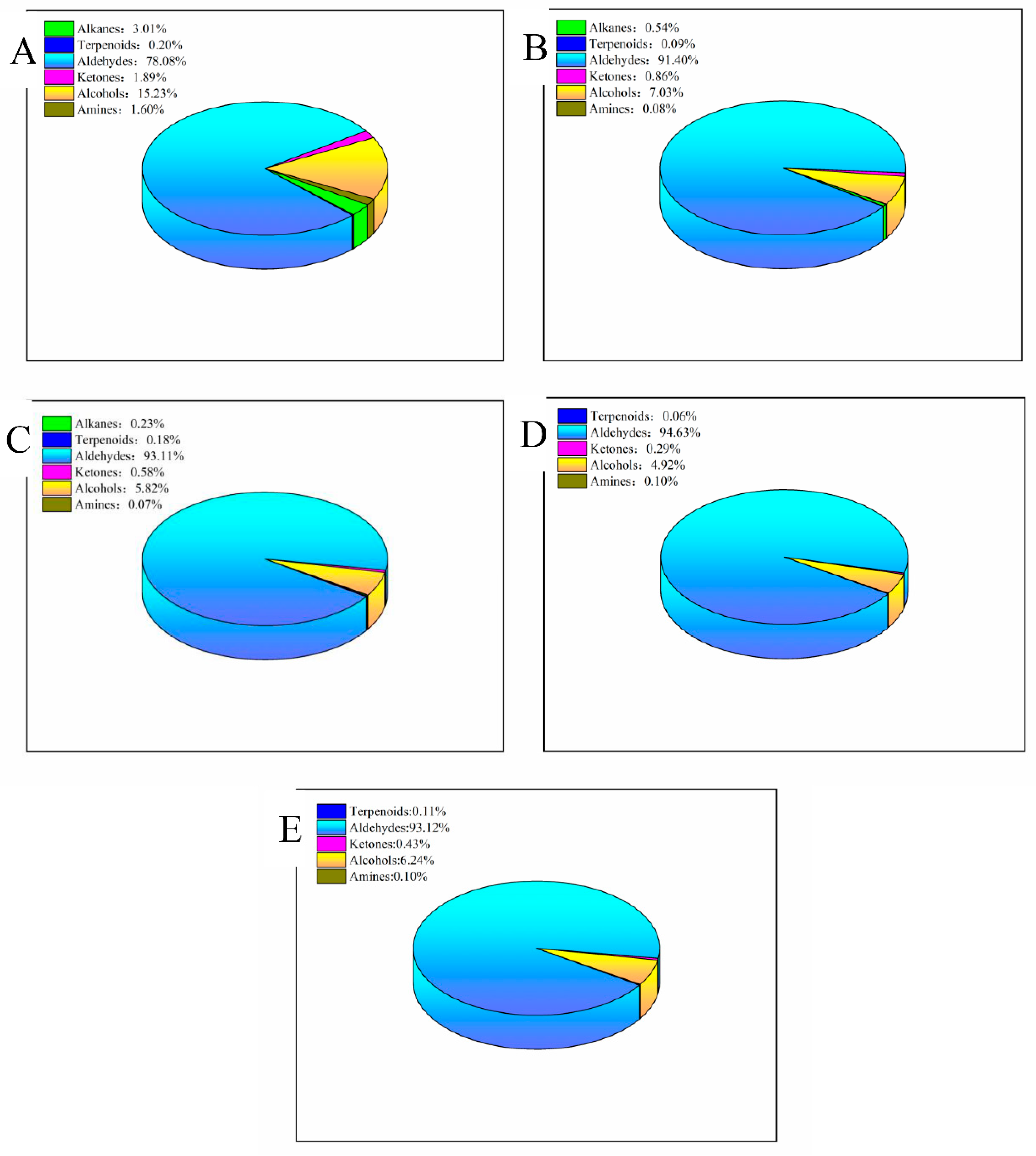
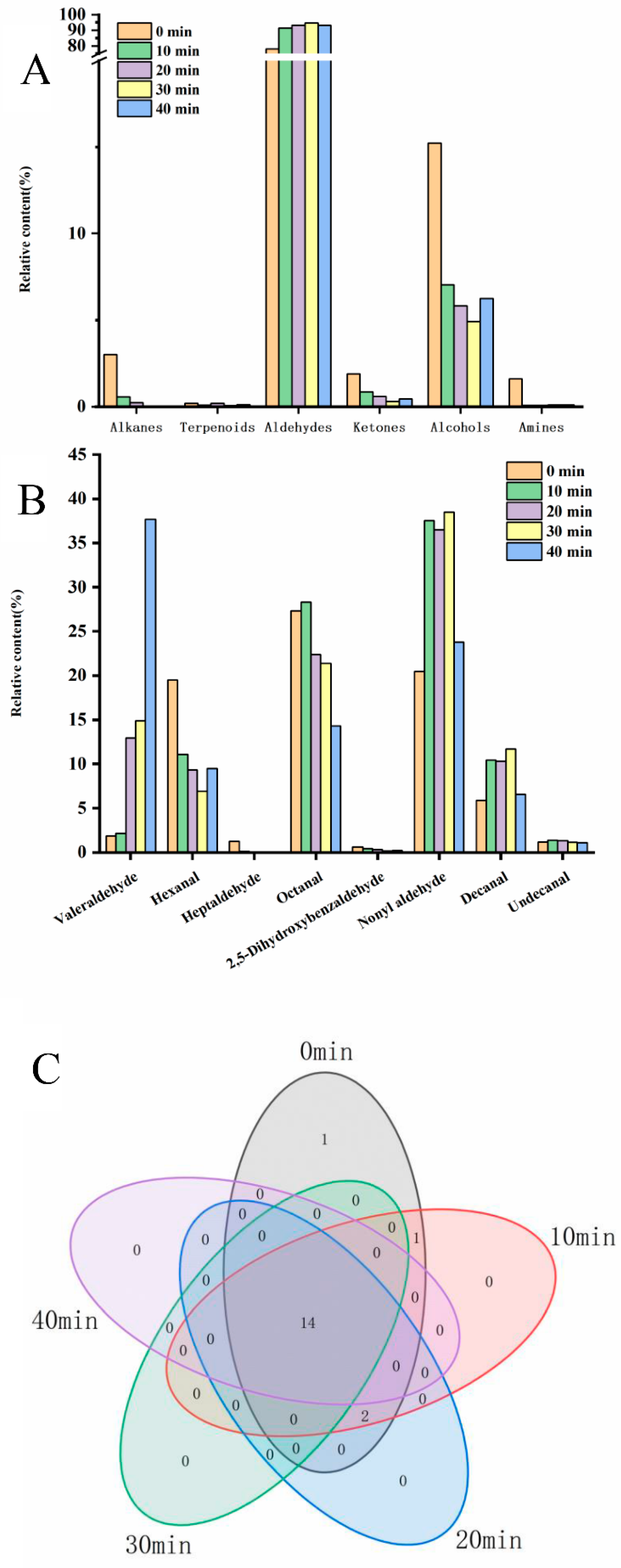
3.1. Volatile Substance Analysis
3.2. Comparative Analysis of Egg Yolk Volatiles
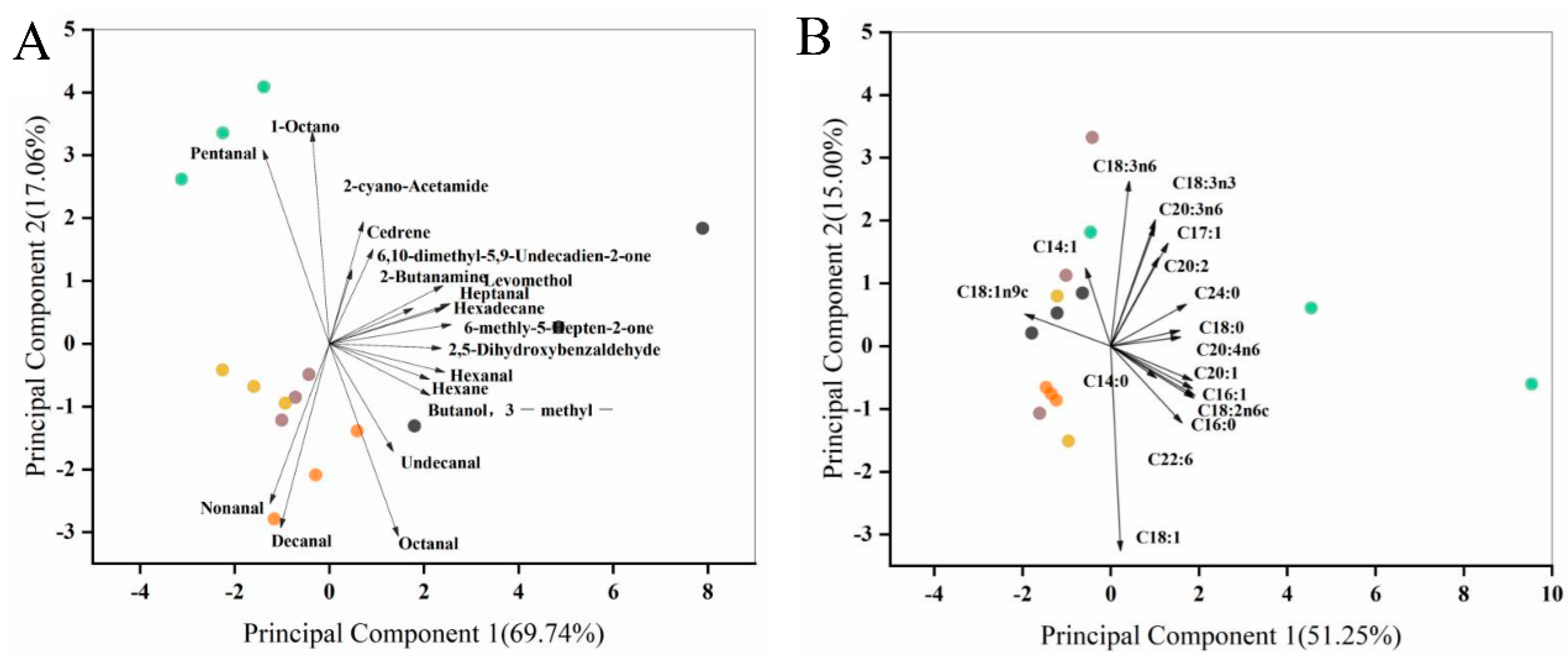
3.3. Principal Component Analysis of Egg Yolk Volatiles
3.4. Cluster Analysis of Egg Yolk Volatiles
3.5. Fatty Acid Analysis
3.6. Principal Components and Clustering Analysis of Egg Yolk Fatty Acids
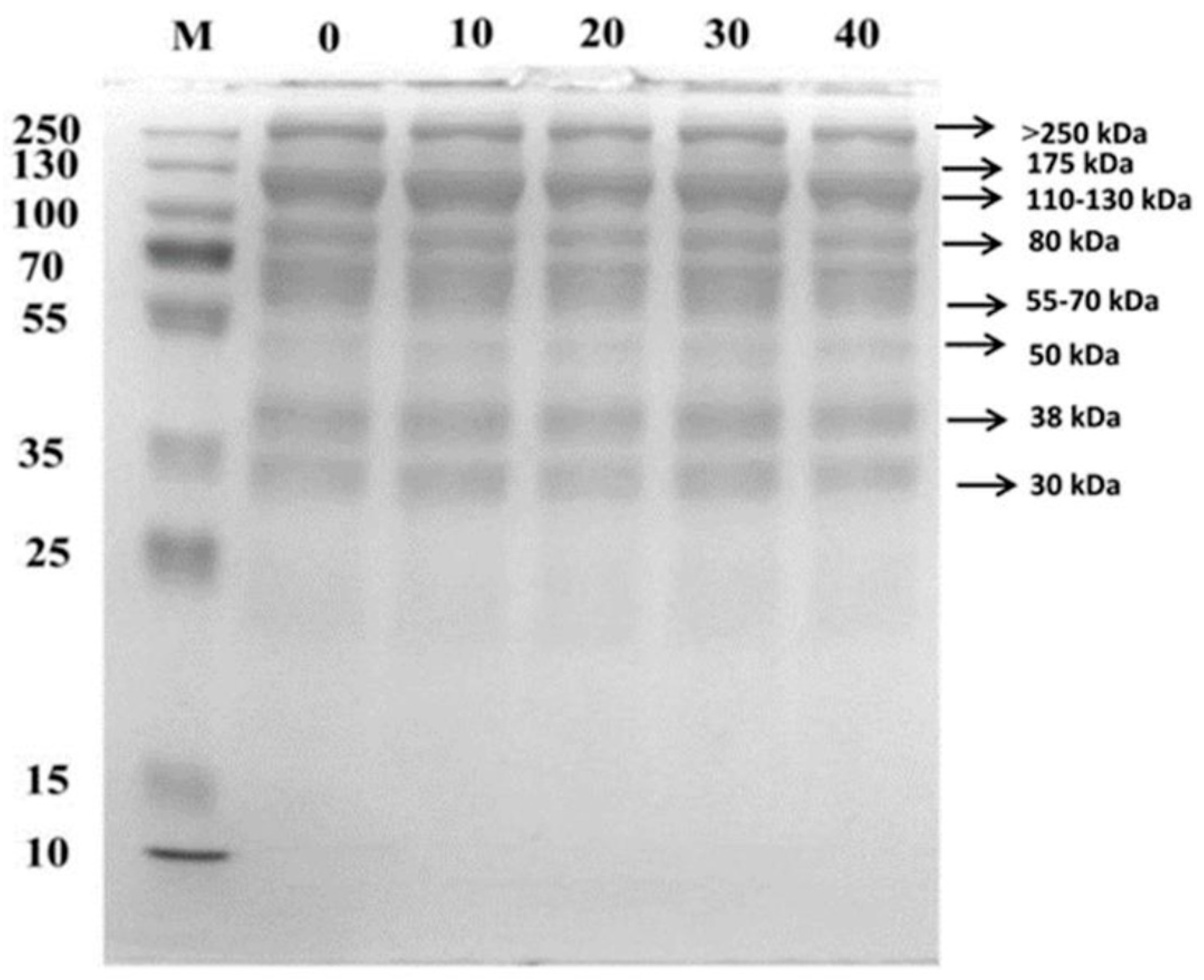
3.7. SDS-PAGE Analysis
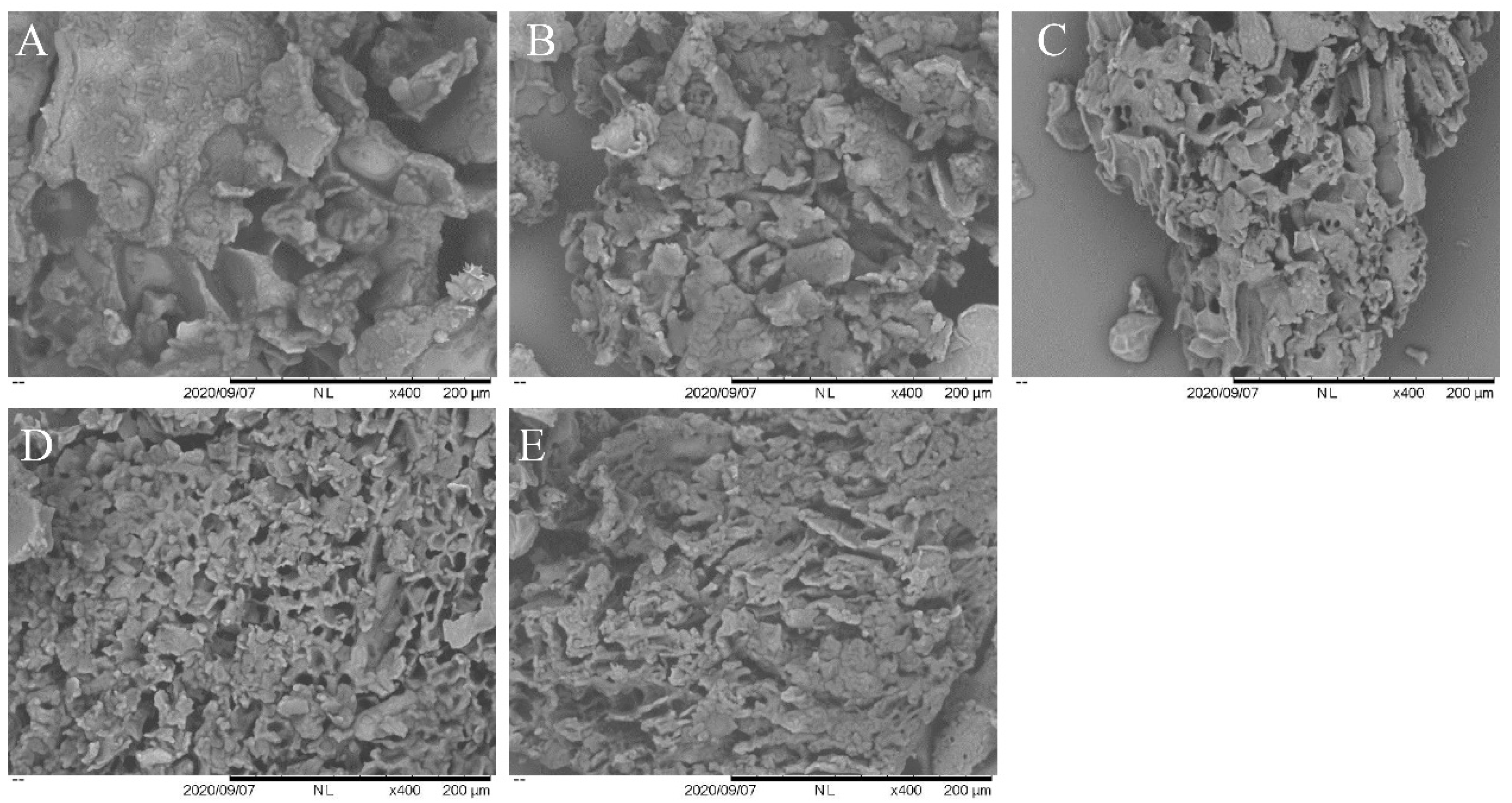
3.8. SEM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sakai, S.; Ikeda, N. A Numerical Analysis to Evaluate the Emulsifying Activity of Pasteurized Egg Yolk. Food Hydrocolloid. 2021, 4, 107087. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, A.; Mu, Y.; Zhang, N.; Sun, L.; Rajput, S.A.; Qi, D. Effects of dietary cottonseed oil and cottonseed meal supplementation on the structure, nutritional composition of egg yolk and gossypol residue in eggs. Poultry Sci. 2019, 98, 381–392. [Google Scholar] [CrossRef]
- Su, Y.; Ji, M.; Li, J.; Chang, C.; Dong, S.; Deng, Y.; Yang, Y.; Gu, L. Subcritical fluid extraction treatment on egg yolk: Product characterization. J. Food Eng. 2020, 274, 109805. [Google Scholar] [CrossRef]
- Derbassi, N.B.; Pedrosa, M.C.; Heleno, S.; Carocho, M.; Ferreira, I.C.F.R.; Barros, L. Plant volatiles: Using Scented molecules as food additives. Trends Food Sci. Technol. 2022, 122, 97–103. [Google Scholar] [CrossRef]
- Gonzalez-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhai, J.; Gu, L.; Su, Y.; Gong, L.; Yang, Y.; Chang, C. Hen egg yolk in food industry—A review of emerging functional modifications and applications. Trends Food Sci. Technol. 2021, 115, 12–21. [Google Scholar] [CrossRef]
- Niveditha, A.; Pandiselvam, R.; Prasath, V.A.; Singh, S.K.; Gul, K.; Kothakota, A. Application of cold plasma and ozone technology for decontamination of Escherichia coli in foods—a review. Food Control. 2021, 130, 108338. [Google Scholar] [CrossRef]
- Jin-Gab, K.; Yousef, A.E.; Sandhya, D. Application of ozone for enhancing the microbiological safety and quality of foods: A review. J. Food Protect. 1999, 62, 1071–1087. [Google Scholar] [CrossRef]
- Lima, D.C.; Villar, J.; Castanha, N.; Maniglia, B.C.; Matta Junior, M.D.; Duarte Augusto, P.E. Ozone modification of arracacha starch: Effect on structure and functional properties. Food Hydrocolloid. 2020, 108, 106066. [Google Scholar] [CrossRef]
- Yang, Y.; Demeestere, K.; Van Hulle, S. Ozone-based advanced oxidation of biologically treated landfill leachate: Oxidation efficiency, mechanisms, and surrogate-based monitoring for bulk organics. J. Environ. Chem. Eng. 2021, 9, 106459. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry and Reactions of Reactive Oxygen Species in Foods. Crit. Rev. Food Sci. 2006, 46, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L. The reaction stoichiometry between ozone and unsaturated fatty acids in an aqueous environment. Chem. Phys. Lipids 1978, 22, 25–37. [Google Scholar] [CrossRef]
- Nickhil, C.; Mohapatra, D.; Kar, A.; Giri, S.K.; Tripathi, M.K.; Sharma, Y. Gaseous ozone treatment of chickpea grains, part I: Effect on protein, amino acid, fatty acid, mineral content, and microstructure. Food Chem. 2021, 345, 128850. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, Y.; Jin, H.; Wang, Q.; Jin, Y.; Huang, X.; Sheng, L. Improvement and mechanism of emulsifying properties of liquid egg yolk by ozonation technology. Lwt-Food Sci. Technol. 2022, 156, 113038. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Q.; Jin, H.; Li, Z.; Sheng, L. Impact of ozone-induced oxidation on the textural, moisture, micro-rheology and structural properties of egg yolk gels. Food Chem. 2021, 361, 130075. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ma, J.; Lv, Y.; Tong, Q.; Guo, H. Characterization of key off-odor compounds in thermal duck egg gels by GC-olfactometry-MS, odor activity values, and aroma recombination. Lwt-Food Sci. Technol. 2021, 143, 111182. [Google Scholar] [CrossRef]
- Gouda, M.; Ma, M.; Sheng, L.; Xiang, X. SPME-GC-MS & metal oxide E-Nose 18 sensors to validate the possible interactions between bio-active terpenes and egg yolk volatiles. Food Res. Int. 2019, 125, 108611. [Google Scholar] [CrossRef]
- Warren, M.W.; Larick, D.K.; Jr, H. Volatiles and Sensory Characteristics of Cooked Egg Yolk, White and Their Combinations. J. Food Sci. 2010, 60, 79–84. [Google Scholar] [CrossRef]
- Qi, J.; Liu, D.Y.; Zhou, C.H.; Xu, X.L. Characteristic flavor of traditional soup made by stewing Chinese yellow-feather chickens. J. Food Sci. 2017, 82, 2031–2040. [Google Scholar] [CrossRef]
- Leng, P.; Hu, H.-W.; Cui, A.-H.; Tang, H.-J.; Liu, Y.-G. HS-GC-IMS with PCA to analyze volatile flavor compounds of honey peach packaged with different preservation methods during storage. Lwt-Food Sci. Technol. 2021, 149, 111963. [Google Scholar] [CrossRef]
- Paraskevopoulou, A.; Amvrosiadou, S.; Biliaderis, C.G.; Kiosseoglou, V. Mixed whey protein isolate-egg yolk or yolk plasma heat-set gels: Rheological and volatile compounds characterisation. Food Res. Int. 2014, 62, 492–499. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, X.; Huang, Y.; Ma, L. The role of hydroxylation on ·OH generation for enhanced ozonation of benzoic acids: Reactivity, ozonation efficiency and radical formation mechanism. J. Hazard. Mater. 2022, 431, 128620. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Xiong, S.; Yin, T.; Hu, Y.; Liu, R.; Du, H.; Liu, Y.; You, J. Proteomic profiling and oxidation site analysis of gaseous ozone oxidized myosin from silver carp (Hypophthalmichthys molitrix) with different oxidation degrees. Food Chem. 2021, 363, 130307. [Google Scholar] [CrossRef]
- Yuan, X.; Feng, Z.; Hu, C.; Zhang, K.; Qu, L.; Paoletti, E. Effects of elevated ozone on the emission of volatile isoprenoids from flowers and leaves of rose (Rosa sp.) varieties. Environ. Pollut. 2021, 291, 118141. [Google Scholar] [CrossRef]
- Cerny, C.; Guntz, R. Evaluation of potent odorants in heated egg yolk by aroma extract dilution analysis. Eur. Food Res. Technol. 2004, 219, 452–454. [Google Scholar] [CrossRef]
- Jo, S.H.; Kim, K.H.; Kim, Y.H.; Lee, M.H.; Kim, Y. Study of odor from boiled eggs over time using gas chromatography. Microchem. J. 2013, 110, 517–529. [Google Scholar] [CrossRef]
- Boubchir, M.; Boubchir, R.; Aourag, H. The Principal Component Analysis as a tool for predicting the mechanical properties of Perovskites and Inverse Perovskites. Chem. Phys. Lett. 2022, 798, 139615. [Google Scholar] [CrossRef]
- Nami, Y.; Panahi, B.; Mohammadzadeh Jalaly, H.; Vaseghi Bakhshayesh, R.; Hejazi, M.A. Application of unsupervised clustering algorithm and heat-map analysis for selection of lactic acid bacteria isolated from dairy samples based on desired probiotic properties. Lwt-Food Sci. Technol. 2020, 118, 108839. [Google Scholar] [CrossRef]
- Khandakar, A.; Chowdhury, M.E.H.; Ibne Reaz, M.B.; Md Ali, S.H.; Hasan, M.A.; Kiranyaz, S.; Rahman, T.; Alfkey, R.; Bakar, A.A.A.; Malik, R.A. A machine learning model for early detection of diabetic foot using thermogram images. Comput. Biol. Med. 2021, 137, 104838. [Google Scholar] [CrossRef]
- Aguillon-Paez, Y.J.; Romero, L.A.; Diaz, G.J. Effect of full-fat sunflower or flaxseed seeds dietary inclusion on performance, egg yolk fatty acid profile and egg quality in laying hens. Anim. Nutr. 2020, 6, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Leng, C.; Zeng, G.; Fu, D.; Zhang, Y.; Liu, Y. Ozone initiated heterogeneous oxidation of unsaturated carboxylic acids by ATR-FTIR spectroscopy. Spectrochim. Acta A 2019, 214, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Desai, S.S.; Mane, V.K.; Enman, J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Futuristic food fortification with a balanced ratio of dietary ω-3/ω-6 omega fatty acids for the prevention of lifestyle diseases. Trends Food Sci. Technol. 2022, 120, 140–153. [Google Scholar] [CrossRef]
- Liu, Q.; Gui, Z.; Xiong, S.; Zhan, M. A principal component analysis dominance mechanism based many-objective scheduling optimization. Appl. Soft. Comput. 2021, 113, 107931. [Google Scholar] [CrossRef]
- Selim, S.; Hussein, E. Production performance, egg quality, blood biochemical constituents, egg yolk lipid profile and lipid peroxidation of laying hens fed sugar beet pulp. Food Chem. 2022, 310, 125864. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhou, X.; Gouda, M.; Cai, Z.; Jin, Y. Effect of enzymatic hydrolysis on the solubility of egg yolk powder from the changes in structure and functional properties. Lwt-Food Sci. Technol. 2019, 110, 214–222. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Sun, Y.; Jin, H.; Wang, Q.; Li, Z.; Jin, Y.; Sheng, L. An Efficient Processing Strategy to Improve the Flavor Profile of Egg Yolk: Ozone-Mediated Oxidation. Molecules 2023, 28, 124. https://doi.org/10.3390/molecules28010124
Chen B, Sun Y, Jin H, Wang Q, Li Z, Jin Y, Sheng L. An Efficient Processing Strategy to Improve the Flavor Profile of Egg Yolk: Ozone-Mediated Oxidation. Molecules. 2023; 28(1):124. https://doi.org/10.3390/molecules28010124
Chicago/Turabian StyleChen, Bao, Yi Sun, Haobo Jin, Qi Wang, Zhe Li, Yongguo Jin, and Long Sheng. 2023. "An Efficient Processing Strategy to Improve the Flavor Profile of Egg Yolk: Ozone-Mediated Oxidation" Molecules 28, no. 1: 124. https://doi.org/10.3390/molecules28010124
APA StyleChen, B., Sun, Y., Jin, H., Wang, Q., Li, Z., Jin, Y., & Sheng, L. (2023). An Efficient Processing Strategy to Improve the Flavor Profile of Egg Yolk: Ozone-Mediated Oxidation. Molecules, 28(1), 124. https://doi.org/10.3390/molecules28010124




