2. Results and Discussion
The HPLC-MS analysis of the extract of
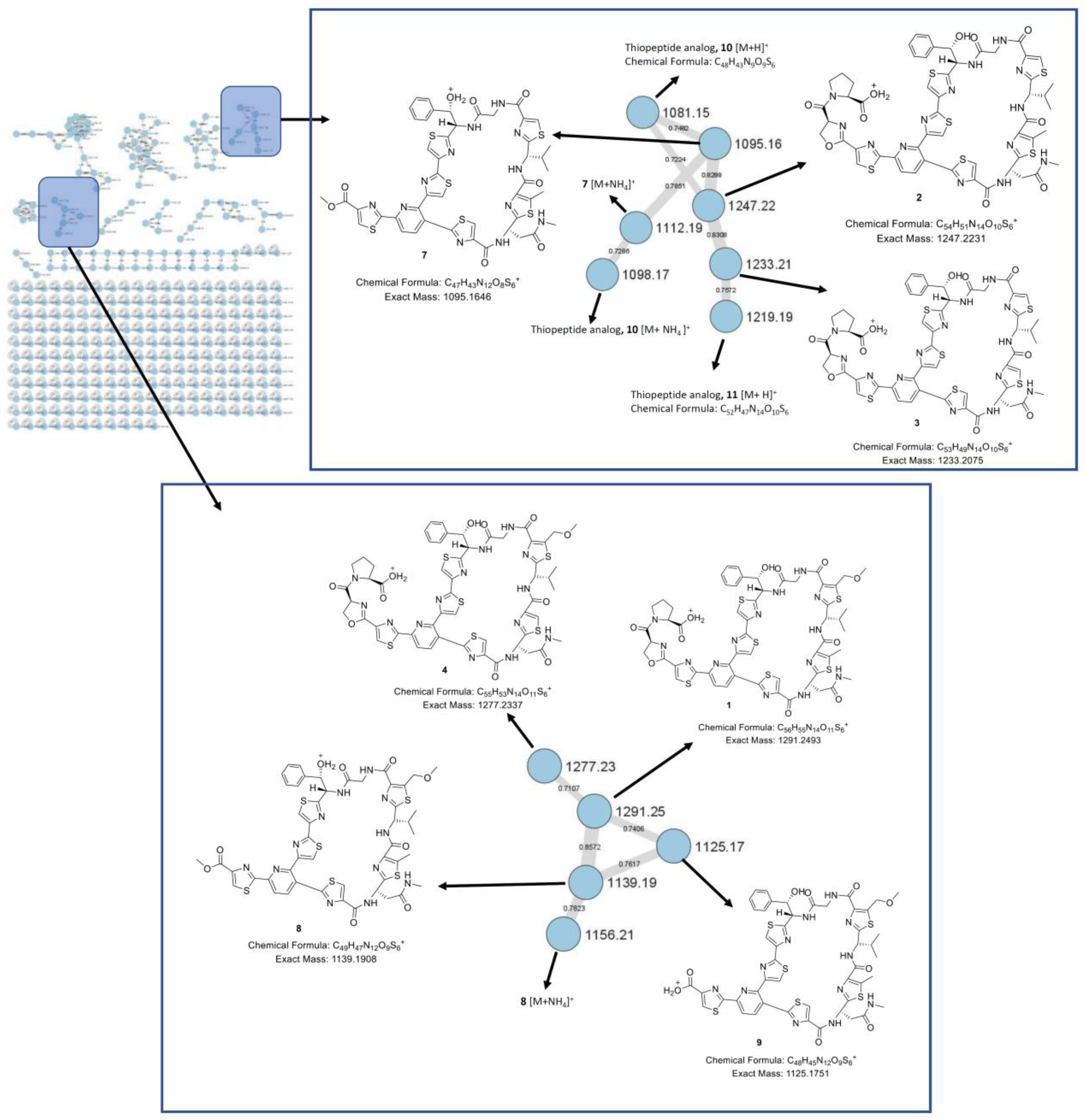
Nonomuraea jiangxiensis strain A7611 found several masses of sulfur and nitrogen containing compounds, indicating the presence of thiopeptide. To visualize the overall chemical space in the extract, a HPLC-MS/MS experiment was done. The MS/MS data were used to generate a consolidated GNPS molecular network [
12]. In this molecular network, specific clusters containing potentially new thiopeptides were found (
Figure 2). To identify the thiopeptides, a large-scale fermentation was performed, and the CH
2Cl
2 extract was subjected to RP-HPLC and PTLC to obtain compounds
1–
9 (
Figure 3).
Compound
1 (
Figure 2) was isolated as a white amorphous powder and its molecular formula was established as C
56H
54N
14O
11S
6 based on HR-ESIMS measurements. The
1H NMR data (
Table 1) revealed features of a peptide-derived compound, including five amide
1H signals (δ
H 9.29, 8.69, 8.69, 8.45, 7.41). The
13C NMR data (
Table 1) were also consistent with a peptide-derived compound, comprising of oxazoline and thiazole units including five amide carbonyls and one carboxylic acid carbonyl signals (δ
C 169.4, 169.3, 163.2, 161.2, 161.0, 160.2), six thiazole (δ
C 170.8, 168.3, 167.9, 165.4, 164.5, 160.3) and oxazoline (δ
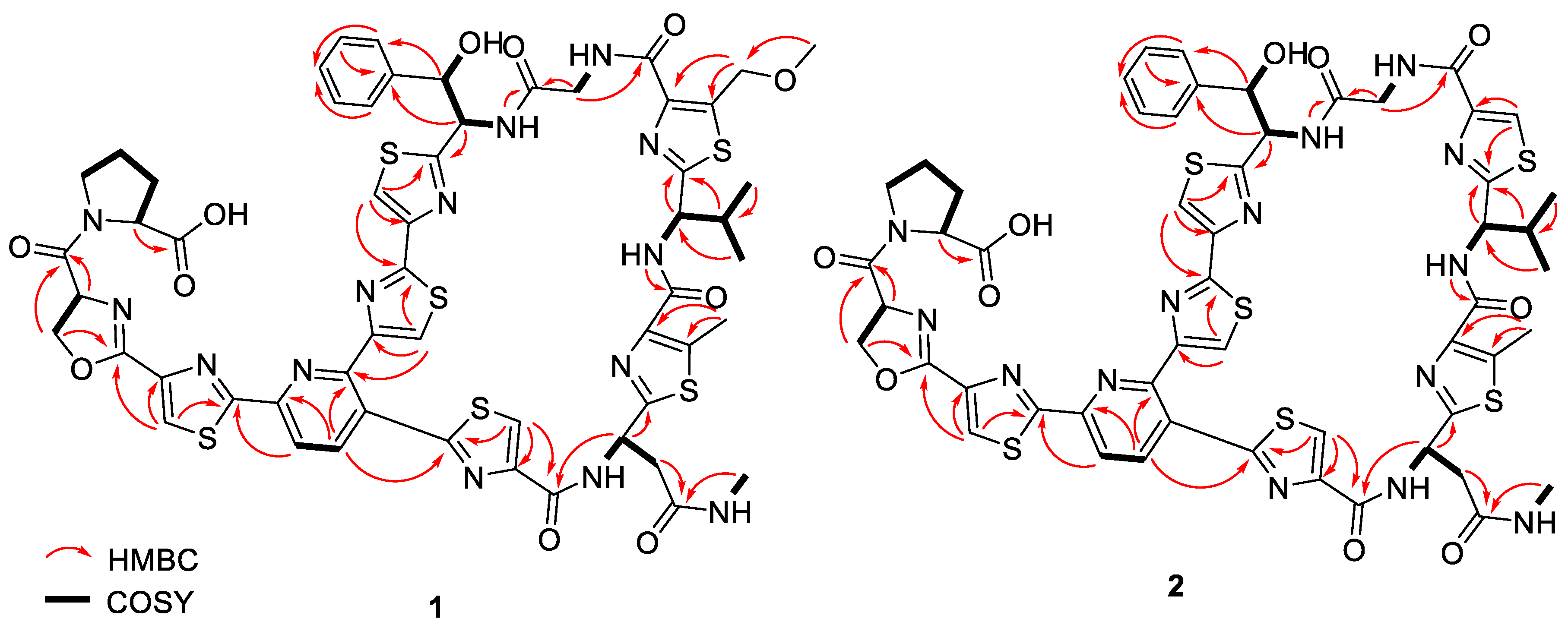
C 160.1) moieties. The presence of thiazole rings A, B, C, D, E and F, pyridine (Py), phenylserine (PheSer), valine (Val), glycine (Gly), asparagine (Asn), oxazoline (Oxa) and proline (Pro) residues in
1 was supported by the observed COSY and HMBC correlations (
Figure 4). A further analysis of the 2D-NMR data revealed that the structure of
1 was similar to that of GE2270A, a thiopeptide isolated from
Planobispora rosea. The NMR data comparison between
1 and those of GE2270A showed a high degree of similarity, implying their structural analogy [
13,
14]. The 1D NMR resonances of the aromatic protons of Py and of the thiazole rings A, B, C, D, E and F for GE2270A and
1 were coincided [
13,
14]. This showed that the building blocks of the thiazolyl peptide backbone and the sequence was conserved. Even though the structure of
1 was previously reported from a biosynthetic gene cluster and heterologous expression studies, the NMR spectroscopic data of
1 were not reported [
15]. A detailed inspection of the
13C NMR data of
1 (
Table 1) together with a comparison of the reported NMR data of GE2270A revealed a missing signal of δ
C 173.5 that corresponded to the C-terminal amide/carbonyl of proline (Pro) residue in GE2270A, which was replaced by a carboxylic acid group (δ
C 169.3) in
1 [
13,
14]. Based on these data, the structure of
1 was established to be an analog of GE2270A possessing a C-terminal carboxylic acid. Of note, this was the first report on the isolation of
1 from a native strain.
Compound
2 (
Figure 3) was isolated as white amorphous powders. The HR-ESIMS measurements determined the molecular formula of
2 as C
54H
50N
14O
10S
6. Furthermore, compounds
3 and
4 (
Figure 3) were also isolated as white amorphous powders and their molecular formulae were established as C
53H
48N
14O
10S
6 and C
55H
52N
14O
11S
6, respectively.
1H NMR data of
2–
4 (
Table 1 and
Table S1) were consistent with that of
1 with minor differences. For instance, the methyl singlet (δ
H 2.59) on thiazole ring E in
1 was not observed in the
1H NMR spectra of
3 and
4. This implied that C-5 of thiazole ring E in
3 and
4 was unsubstituted, which was further supported by an additional singlet at around δ
H 8.10 in the
1H NMR spectra of
3 and
4. On the other hand, the singlets at δ
H 3.39 and 4.99 of the methoxymethyl substituent at the C-5 position of thiazole ring D were not detected in
2 and
3. This observation suggested that thiazole ring D was unsubstituted at the C-5 position supported by an additional singlet at δ
H 8.29 in the
1H NMR spectra of
2 and
3. The remaining structural moieties in compounds
2–
4 were established by COSY and HMBC correlations (
Figure 4 and
Figure S4). Compounds
1–
4 shared the same thiazolyl peptide backbone and C-terminal carboxylic acid group while possessing altered decorations at thiazole rings D and E, they are grouped together and named GE2270F
1, GE2270F
2, GE2270F
3 and GE2270F
4, respectively.
Compounds
5 and
6 (
Figure 3) were isolated as white amorphous powders and their molecular formulae were established as C
54H
54N
15O
10S
6 and C
56H
58N
15O
11S
6, respectively, based on HR-ESIMS measurements. Interpretation of the COSY, HSQC and HMBC spectra of
5 and
6 together with the NMR data comparison with those of GE2270A revealed the replacement of Oxa group with a serine (Ser) moiety, which was indicated by an amide carbonyl at δ
C 173.5 (
Figure S5 and Table S2) [
13,
14]. The presence of a Ser residue was evident from the COSY correlations between NH (δ
H 8.47)/H-α (δ
H 4.90), H-α (δ
H 4.90)/H
2-β (δ
H 3.76, 3.82), and H
2-β (δ
H 3.76, 3.82)/OH (δ
H 5.28), as well as the HMBC correlation from H-α to a carbonyl carbon at δ
C 168.9 (
Figure S5). Compounds
5 and
6 were likely to be intermediates or precursors in the biosynthesis of GE2270C
1 and GE2270A, respectively, as reported in the literature through the cyclization of Ser into an Oxa group catalyzed by a YcaO-like cyclodehydratase enzyme [
15]. Compound
6 was previously synthesized and tested for antibacterial activity in the search of a lead thiazole peptide that has an enhanced aqueous solubility, and the activity against
S. aureus was equivalent to that of GE2270A but with a poor aqueous solubility [
16].
Compounds
7,
8 and
9 (
Figure 3) were isolated as white amorphous powders and their molecular formulae were determined as C
47H
42N
12O
8S
6, C
49H
46N
12O
9S
6 and C
48H
44N
12O
9S
6, respectively. Compounds
7–
9 possessed the same thiazolyl peptide backbone as in
1 with the loss of Pro and Oxa groups as indicated by the missing proton and carbon signals that correspond to Pro and Oxa groups (
Figure 3 and
Table S3). Thiopeptides
7 and
8 were analogs of one another with or without the substitution at the C-5 position of thiazole ring D, and both possessed the terminal methyl–ester functionality, which is indicated by an additional singlet at δ
H 3.91 (
Table S3). On the other hand,
9 was the free carboxylic acid form of
8. These compounds were likely to be intermediates or precursors in the biosynthesis of GE2270A. Compounds
8 and
9 were previously reported and synthesized as intermediates in the total synthesis of thiopeptide GE2270 analogs [
17,
18]. This was the first report of the isolation of thiopeptides
8 and
9 from Nature. Notably, one close structural analog of compounds
8 and
9, which possessed a terminal amide group in place of either an ester or a carboxylic acid group was previously synthesized and tested for antibacterial activity and was found to be inactive against Gram-positive
S. aureus [
17].
Although the thiopeptide GNPS molecular clusters showed several related thiopeptides, it was not possible to isolate and acquire the NMR spectroscopic data for two minor compounds (
m/
z 1081.15 for
10, and
m/
z 1219.19 for
11) due to their minute amounts in the extract. Nevertheless, their molecular formulae were determined by exact mass calculation (
Table 2). Due to the small amount of the samples, we were not able to assign an absolute configuration using Marfey’s reagent. However, the structures of thiopeptides
1–
9 are closely related to a series of known thiopeptides, GE2270. Further manual observation of the HPLC-HRESIMS/MS of the crude extract indeed revealed the presence of GE2270A as shown by a molecular ion
m/
z 1290.2663 [M+H]
+ consistent with that of GE2270A (
Figure S3) [
15]. Thus,
1–
9 were most likely to be biosynthetically related to GE2270A, a thiopeptide initially isolated from
Planobispora rosea [
19]. Although the structures of compounds
1–
9 and GE2270A contain several typical characteristics of a nonribosomal peptide (NRP), GE2270A is a ribosomally synthesized, post-translationally modified peptide (RiPP), which was also observed in other known thiopeptides [
15]. Therefore,
1–
9 was presumed to occur in the configuration, as shown in
Figure 3, and determined to be new members of GE2270 thiopeptides [
20]. This was further supported by a comparison of the specific rotation and the
1H and
13C NMR data of
1–
9 with those of GE2270A [
19,
21], whose configuration was confirmed by total synthesis [
18,
22].
Compounds
1–
9 were evaluated for their antibacterial activity against a panel of bacterial strains, namely
A. baumannii (ATCC
® 19606™),
K. aerogenes (ATCC
® 13048™),
P. aeruginosa (ATCC
® 9027™) and
S. aureus Rosenbach (ATCC
® 25923™). Thiopeptides
1–
9 were found to be inactive against Gram-negative bacterial strains (
Figure S43), while
1,
2,
6,
7,
8 and
9 displayed activities against
S. aureus Rosenbach (ATCC
® 25923™), the only Gram-positive bacterial strain tested (
Table 3 and
Figure 5). Interestingly,
3,
4 and
5 were found to be inactive against
S. aureus. In addition, the antifungal effects of thiopeptides
1–
9 were also determined against
Aspergillus fumigatus (ATCC
® 46645™), and no antifungal activity was observed (
Figure S44). Furthermore, all the compounds were tested for their cytotoxicity against the human lung carcinoma cell line A549 (ATCC
® CCL-185™); none of the compounds were cytotoxic towards A549 cells (
Figure S45). Notably, it was well documented that thiopeptides exhibited a wide range of biological properties and are strong antibiotics against Gram-positive bacteria [
1], including contemporary strains of methicillin-resistant
Staphylococcus aureus (MRSA).
Compounds
1–
4 possessed the same R1 group on thiazole ring A, but various substitutions, i.e., the R2 and R3 groups on thiazole rings D and E, respectively. As shown in
Table 3,
1 was three-fold more active (i.e., minimal inhibitory concentration (MIC
90) of 2.63 µM and minimal bactericidal concentration (MBC
90) of 18.48 µM) against
S. aureus Rosenbach (ATCC
® 25923™) than
2 (i.e., MIC
90 of 6.94 µM and MBC
90 of 67.93 µM). This was likely attributed to the presence of both the R2 (i.e., methylene-oxy-methyl) and the R3 (i.e., methyl) groups in
1, resulting in a significant increase in antibacterial activity. In addition, the presence of the R3 group and the absence of the R2 group in
2 resulted in a weaker antibacterial activity. The absence of both R2 and R3 groups in
3, and the absence of the R3 group in
4 resulted in no bioactivity. Compounds
5 and
6 shared similar chemical structures, each possessing a terminal serine–proline group (R1) on thiazole ring A. Compound
6 possessing both R2 and R3 groups demonstrated antibacterial activity against
S. aureus Rosenbach (ATCC
® 25923™), while
5 possessing only the R3 group displayed no activity against
S. aureus Rosenbach (ATCC
® 25923™). This indicated that the presence of both R2 and R3 groups was important for the bioactivity of this series of thiopeptides.
Both compounds
7 and
8 possessed a terminal ester group (R1) on thiazole ring A, with
7 possessing only the R3 group, whilst
8 possessed both R2 and R3 groups. A slight increase in the antibacterial activity (i.e., MIC
90 of 3.17 µM) was observed in
8 when compared to
7 (i.e., MIC
90 of 4.71 µM) when both R2 and R3 groups were present. Compound
9 is the only compound with a terminal carboxylic acid group (R1) on thiazole ring A. Interestingly, at least a three-fold reduction in the antibacterial activity (i.e., MIC
90 of 10.27 µM) was observed in
9 when compared to
8 when the ester group was changed to a carboxylic acid. This could be due to the poor cell membrane permeability of the carboxylic acid group, thus resulting in a poorer antibacterial activity. The mechanism of action of thiopeptides was previously studied, and it was well established that thiopeptides exert their antibacterial function in the bacterial cell via the inhibition of ribosomal protein synthesis [
2].
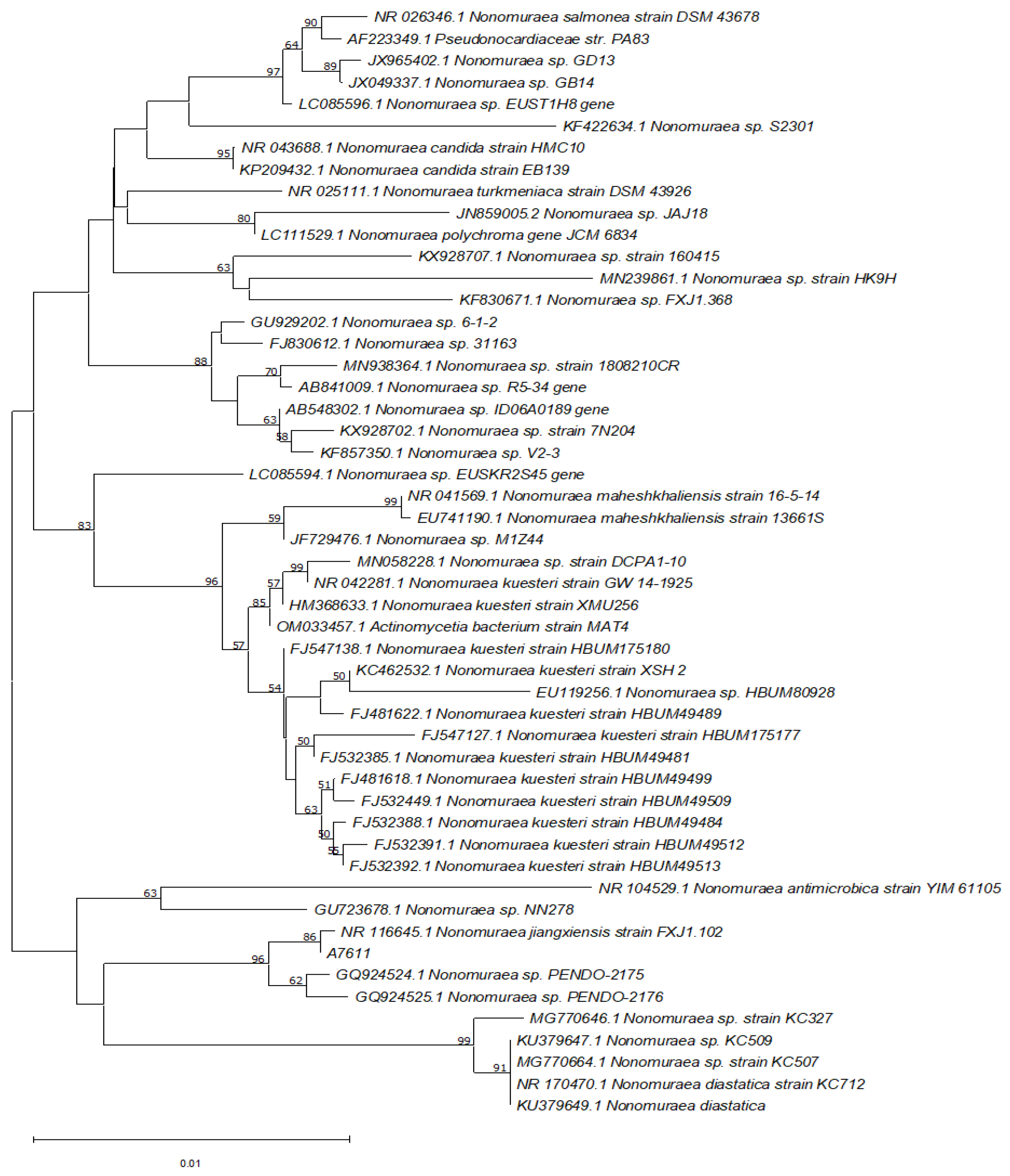
A nucleotide BLAST search of the 16S rRNA gene sequence of A7611 was performed against the NCBI 16S ribosomal RNA database revealed that the isolate shared 99.93% sequence identity (E-value = 0.0) to 16S rRNA of
Nonomuraea jiangxiensis with accession number NR_116645.1. The phylogenetic relatedness using the neighbour-joining analysis method of the isolated strain and its closely related species was obtained from the GenBank database and is shown in
Figure 6.
3. Materials and Methods
3.1. General Experimental Procedures
A JASCO P-2000 digital polarimeter was utilized to measure the specific rotations of the compounds. A Bruker DRX-400 NMR spectrometer was utilized to obtain the NMR spectra of the compounds. Specifications of the NMR spectrometer include a Cryoprobe, and a 5 mm BBI (1H, G-COSY, multiplicity-edited G-HSQC, and G-HMBC spectra) or BBO (13C spectra) probe heads equipped with z-gradients. Residual solvent peaks for DMSO-
d6 were set at δ
H 2.50 and δ
C 39.5 ppm as reference signals in the
1H and
13C NMR spectra, respectively. A preparative HPLC experiment was performed using Agilent 1260 Infinity Preparative-scale LC/MS Purification System coupled to an Agilent 6130B single quadrupole mass spectrometer with an XTerra Prep MS C
18 column (19 × 300 mm, 10 µm). The detection wavelength used in the preparative HPLC was 254 nm. An Agilent UHPLC 1290 Infinity, coupled with an Agilent 6540 accurate–mass quadrupole time-of-flight (QTOF) mass spectrometer, equipped with an ESI source was utilized to conduct the HPLC-MS experiment. The analyses were conducted with an Acquity UPLC BEH C18 column (2.1 × 50 mm, 1.7 µm), at a flow rate of 0.5 mL/min under standard gradient conditions of 2% MeCN (0.1% formic acid) to 100% MeCN (0.1% formic acid) over 8.6 min. The operating parameters for QTOF were the same as previously reported [
8].
3.2. Molecular Identification and Phylogenetic Analysis of the Bacteria Isolate A7611
The bacterial strain A7611 was isolated from terrestrial soil in Singapore. The isolated bacteria was grown on Bennett Agar for 5–7 days at 28 °C. The DNA of the strain was extracted from the plate using the DNeasy PowerSoil Pro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol where the cells underwent a beat-beating step for cell disruption using an automated tissue homogenizer and cell lyser 1600 MiniG (SPEX SamplePrep, Metuchen, NJ, USa) at 1500 rpm for 3 min. The NanoDrop2000 spectroscopy system (ThermoFisher Scientific, Waltham, MA, USA) was used to measure the DNA purity and yield extracted. Bacterial 16S rRNA genes were amplified from the DNA extracted from the isolated actinobacteria with universal 16S primers 27F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and 1492R (5′-TAC GGY TAC CTT GTT ACG ACT T-3′) [
23,
24]. The PCR amplification reactions were performed using Applied Biosystems ProFlex Thermocycler (ThermoFisher Scientific, Waltham, MA, USA) with a total reaction of 20 µL that comprised of 2.0 µL of 10× PCR buffer with 20 mM MgCl
2, 2.0 µL of 2 mM dNTPs, 1 unit of Taq polymerase (ThermoFisher Scientific, Waltham, MA, USA), 1.0 µL of 10 µM of each primer and 1.0 μL of purified DNA templates. A non-template and a negative control using sterile resuspension buffer were included in the run. The reactions were subjected to the following temperature cycling profile of initial denaturation at 95 °C for 5 min; 30 cycles each of 30 s at 95 °C for denaturation; 50 s at 60 °C for annealing and 1 min at 72 °C for extension, with a final extension of 5 min at 72 °C. The commercial service of forward and reverse Sanger Sequencing was performed on the PCR amplified DNA fragment (1st BASE, Singapore, Singapore). The sequences obtained were aligned using Benchling and further analyzed using BLAST (National Center for Biotechnology Information (NCBI)). Related actinobacteria strains, retrieved from the GenBank databases, were aligned with the sequence of the I6S rRNA region of the isolated strain A7611 using ClustalW. A neighbor-joining tree algorithm method was used to determine the genetic relationship between the strains. The phylogenetic tree was constructed with a bootstrapped database containing 1000 replicates in MEGA 11.0 software (Mega, State College, USA). The DNA sequence for the sample A7611, reported in the present study, were deposited with GenBank database of NCBI under the accession numbers OM967343.
3.3. Fermentation and Extraction of Bacterial Crude Extract
Nonomuraea jiangxiensis strain A7611 was cultured in 5 mL SV2 media, (For 1 L, add 15 g glucose (1st BASE, Singapore, Singapore), 15 g glycerol (VWR, Radnor, PA, USA), 15 g soya peptone (Oxoid, Basingstoke, Hampshire, UK), and 1 g calcium carbonate (Sigma-Aldrich, St. Louis, MO, USA), pH was adjusted to 7.0) for 3 days at 28 °C with shaking performed at 200 rpm. Saturated seed cultures were diluted in fresh fermentation media: CA09LB (For 1 L, add 10 g meat extract (Sigma-Aldrich, St. Louis, MO, USA), 4 g yeast extract (BD Biosciences, Franklin Lakes, NJ, USA), 20 g glucose (1st BASE, Singapore, Singapore), and glycerol 3 g (VWR, Radnor, PA, USA), pH was adjusted to 7.0) in a 1:20 volume ratio and fermented with 200 rpm shook at 28 °C in the dark. The cultures were pelleted after 9 days followed by lyophilization of the separated biomass and supernatant. The dried samples were extracted by MeOH then filtered through filter paper (Whatman Grade 4, Maidstone, Kent, UK). MeOH was removed under reduced pressure to give a crude extract of a combined weight of 15.70 g. The crude extract consists of broth extract 84.4% (13.25 g) and biomass extract 15.6% (2.45 g).
3.4. Isolation and Structure Elucidation
The dried extracts obtained were combined and partitioned with CH2Cl2/MeOH/H2O in a ratio of 2:1:1. A rotary evaporator was utilized to remove the CH2Cl2 layer under a reduced pressure. The CH2Cl2 crude extract (692 mg) was redissolved in CH2Cl2 and subjected to a silica gel column chromatography (Merck, Silica gel 60, 0.040–0.063 mm). The column was eluted with a stepwise gradient of 0%, 2%, 4%, 8%, 10% and 12% MeOH in CH2Cl2 followed by 100% MeOH. The 12% MeOH in CH2Cl2 and 100% MeOH fractions were combined to obtain 350 mg of an enriched fraction of thiopeptide analogs. The dried extract was then redissolved in CH2Cl2:MeOH in a ratio of 1:1. Further separation was performed using a Sephadex LH-20 column (mobile phase: CH2Cl2:MeOH = 1:1) to obtain subfraction, containing thiopeptide analogs (53 mg). The dried mixtures were dissolved in MeOH and separated by C18 RP-HPLC (solvent A: H2O + 0.1% HCOOH, solvent B: acetonitrile + 0.1% HCOOH; flow rate: 24 mL/min, gradient conditions: 70:30 isocratic for 5 min; 30% to 60% of solvent B over 55 min, 60% to 100% of solvent B over 2 min, and finally isocratic at 100% of solvent B for 10 min to give 5.1 mg of compound 2 (RT = 30.5 min), 6.9 mg of compound 1 (RT = 37 min), 1.1 mg of compound 7 (RT = 45 min), 0.2 mg compound 8 (RT = 51 min), and 0.5 mg of compound 9 (RT = 39 min). Fractions collected between retention the times of 23.5 min and 27 min were combined. The dried combined fraction was separated by preparative normal phase TLC (Merck, TLC Silica gel 60 F254, 20 × 20 cm, 6% MeOH/CH2Cl2 for the first TLC run followed by 10% MeOH/CH2Cl2 for the second elution) to obtain 5 (2.0 mg) and 3 (1.0 mg). In addition, fractions collected between retention times of 31.5 min and 33.75 min were combined. The dried combined fraction was further separated by preparatory TLC using the same conditions used for the separation of 5 and 3, to obtain 6 (2.7 mg) and 4 (1.7 mg).
3.5. Chemical Structural Data
GE2270F
1 (
1): White amorphous powders;
+ 78 (c 1.2, CH
2Cl
2:MeOH = 1:1); UV (MeCN/H
2O) λmax (%) 221 (100%), 308 (33%) nm;
(+)-HRESIMS:
m/
z 1291.2544 [M+H]+ (calcd for C
56H
55N
14O
11S
6,
1291.2499);
1H and
13C NMR data, see
Table 1.
GE2270F
2 (
2): White amorphous powders;
+ 111 (c 0.9, CH
2Cl
2:MeOH
= 1:1); UV (MeCN/H
2O) λmax (%) 221 (100%), 308 (33%) nm;
(+)-HRESIMS:
m/
z 1247.2240 [M+H]+ (calcd for C
54H
51N
14O
10S
6,
1247.2237);
1H and
13C NMR data, see
Table 1.
GE2270F
3 (
3): White amorphous powders;
+ 58 (c 0.2, CH
2Cl
2:MeOH
= 1:1); UV (MeCN/H
2O) λmax (%) 220 (100%), 310 (33%) nm;
(+)-HRESIMS: m/z 1233.2096 [M+H]+ (calcd for C
53H
49N
14O
10S
6,
1233.2080);
1H and
13C NMR data,
Supplementary Materials Table S3.
GE2270F
4 (
4): White amorphous powders;
+ 88 (c 0.3, CH
2Cl
2:MeOH
= 1:1); UV (MeCN/H
2O) λmax (%) 220 (100%), 310 (33%) nm;
(+)-HRESIMS:
m/
z 1277.2336 [M+H]+ (calcd for C
55H
53N
14O
11S
6,
1277.2337);
1H and
13C NMR data,
Supplementary Materials Table S3.
Compound
5: White amorphous powders;
+ 102 (c 0.3, CH
2Cl
2:MeOH
= 1:1); UV (MeCN/H
2O) λmax (%) 221 (100%), 310 (33%) nm;
(+)-HRESIMS:
m/
z 1264.2495 [M+H]+ (calcd for C
54H
54N
15O
10S
6,
1264.2502);
1H and
13C NMR data, see
Supplementary Materials Table S2.
Compound
6: White amorphous powders;
+ 79 (c 0.2, CH
2Cl
2:MeOH
= 1:1); UV (MeCN/H
2O) λmax (%) 220 (100%), 310 (33%) nm;
(+)-HRESIMS:
m/
z 1308.2770 [M+H]+ (calcd for C
56H
58N
15O
11S
6,
1308.2764);
1H and
13C NMR data, see
Supplementary Materials Table S2.
Compound
7: White amorphous powders;
+ 72 (c 0.2, CH
2Cl
2:MeOH
= 1:1); UV (MeCN/H
2O) λmax (%) 222 (100%), 308 (33%) nm;
(+)-HRESIMS:
m/
z 1095.1655 [M+H]+ (calcd for C
47H
43N
12O
8S
6,
1095.1651);
1H and
13C NMR data, see
Supplementary Materials Table S1.
Compound
8: White amorphous powders;
+ 36 (c 0.2, CH
2Cl
2:MeOH
= 1:1); UV (MeCN/H
2O) λmax (%) 221 (100%), 307 (33%) nm; (+)-HRESIMS:
m/
z 1139.1898 [M+H]+ (calcd for C
49H
47N
12O
9S
6,
1139.1913);
1H and
13C NMR data, see
Supplementary Materials Table S1.
Compound
9: White amorphous powders;
+ 24 (c 0.2, CH
2Cl
2:MeOH
= 1:1); UV (MeCN/H
2O) λmax (%) 221 (100%), 308 (33%) nm;
(+)-HRESIMS:
m/
z 1125.1740 [M+H]+ (calcd for C
48H
45N
12O
9S
6,
1125.1757);
1H and
13C NMR data, see
Supplementary Materials Table S1.
3.6. Biological Assays
Isolated compounds of interest were tested against five microbial strains for antimicrobial testing, which were Acinetobacter baumannii (ATCC® 19606™), Klebsiella aerogenes (ATCC® 13048™), Pseudomonas aeruginosa (ATCC® 9027™) Staphylococcus aureus Rosenbach (ATCC® 25923™) and Aspergillus fumigatus (ATCC® 46645™). The minimum inhibition concentration (MIC) and the minimum bactericidal/fungicidal concentration (MBC/MFC) were carried out using the microbroth dilution method according to the Clinical Laboratory Standards Institute (CLSI) guidelines, with some modifications. To establish the MIC values, the bacterial cells were seeded at a concentration of 5.5 × 105 cells/mL and fungal spores at a concentration of 2.5 × 104 spores/mL. The tested compounds were then incubated together with bacterial cells at 37 °C for 24 h and with fungal spores at 25 °C for 72 h, respectively. The OD600 measurements were subsequentially carried out to evaluate the inhibitory effect of the compounds. To further determine the bactericidal and fungicidal effects of the compounds, 5 µL of the treated culture was transferred onto new media microplates. The plates were incubated under the same condition, followed by the OD600 measurement. The cytotoxicity effects of the isolated compounds were also tested on A549 human lung carcinoma cells (ATCC® CCL-185™), where cells were seeded at 3.3 × 104 cells/mL. The cells were then treated with the compounds for 72 h at 37 °C in the presence of 5% CO2. Any Cytotoxic effect was detected with PrestoBlue™ cell viability reagent (ThermoFisher Scientific, Waltham, MA, USA). The cells were read with a fluorescence reading at an excitation of 560 nm and an emission 590 nm. Standard inhibitors, gentamicin (Gibco, Waltham, MA, USA), amphotericin (Sigma-Aldrich, St. Louis, MO, USA) and puromycin (Sigma-Aldrich, St. Louis, MO, USA) were used as the assay controls for the antibacterial, antifungal and cytotoxicity assay. All compounds were tested in triplicates to the ensure reproducibility of the results. GraphPad Prism 8 software (GraphPad, San Diego, CA, USA) was used for the analysis of the bioactivity to determine the respective IC90 and IC50 values.
3.7. GNPS Molecular Networking
An LC-MS/MS data file (.d) created from the Agilent QTOF mass spectrometer were converted to .mgf file formats with an Agilent Qualitative 10.0 and uploaded to the GNPS Web platform (
http://gnps.ucsd.edu., accessed on 20 December 2021) for the classical molecular networking generation. MS-Cluster (0.1 Da tolerance) and a 0.02 Da tolerance for fragment ions were applied to create the consensus parent mass spectra. A network was generated where there were more than six matched fragment ions and the edges were filtered to have a minimal cosine score of 0.7. A maximum size of a molecular family was also set to 100. The output molecular networking was visualized and analyzed using Cytoscape 3.9.0. The GNPS data can be found at
https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=e9f7b2ac30bd4ddabcc847f44b089a4b, accessed on 20 December 2021.