Beta-Carotene Affects the Effects of Heme Oxygenase-1 in Isolated, Ischemic/Reperfused Rat Hearts: Potential Role of the Iron
Abstract
1. Introduction
2. Results
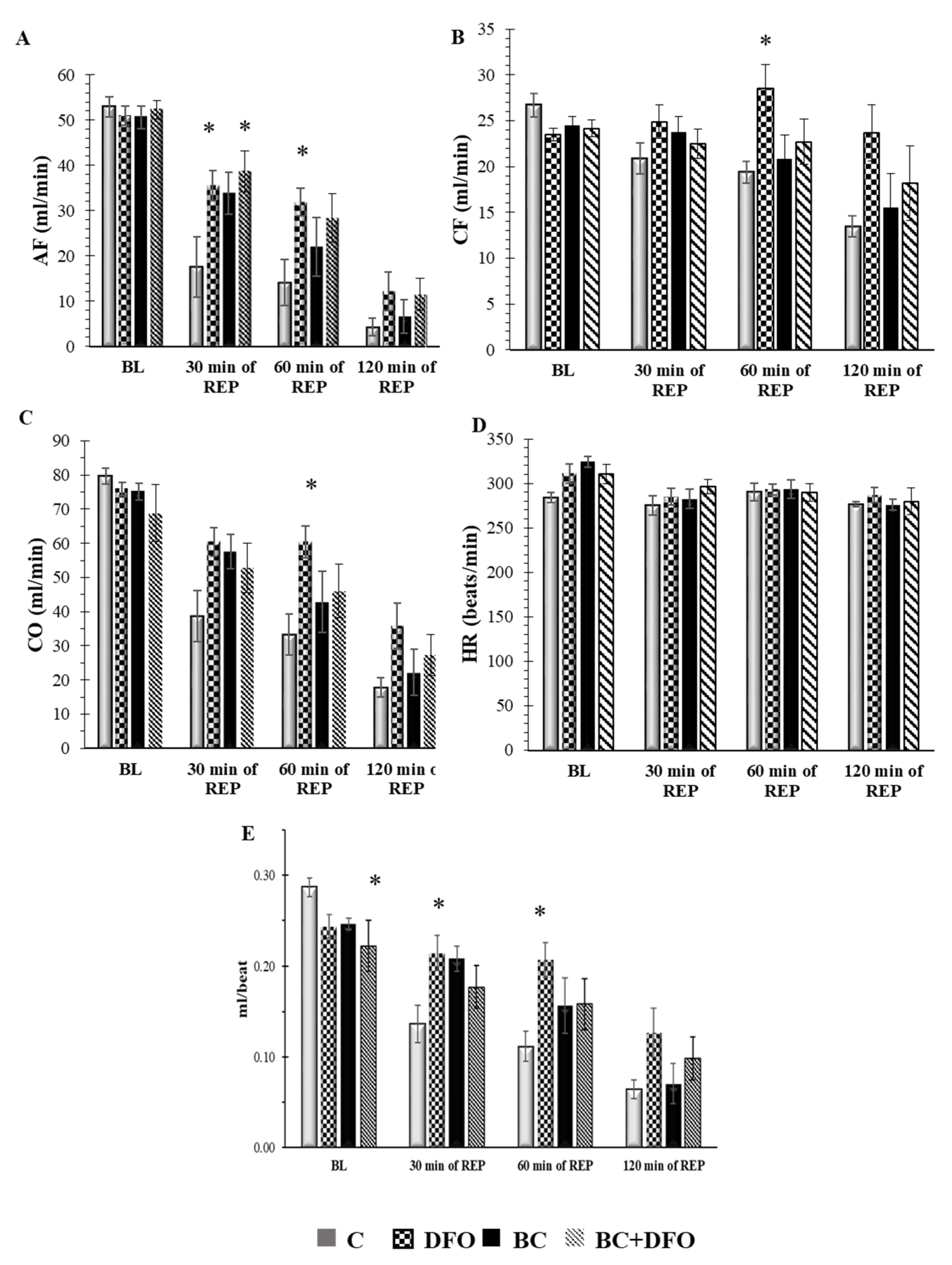
2.1. Cardiac Function in ISA/REP-Injured Hearts Isolated from Vehicle- or BC-Treated Rats with or without DFO Treatment
2.2. The Effect of BC Treatment and DFO Administration on ISA/REP-Induced IS
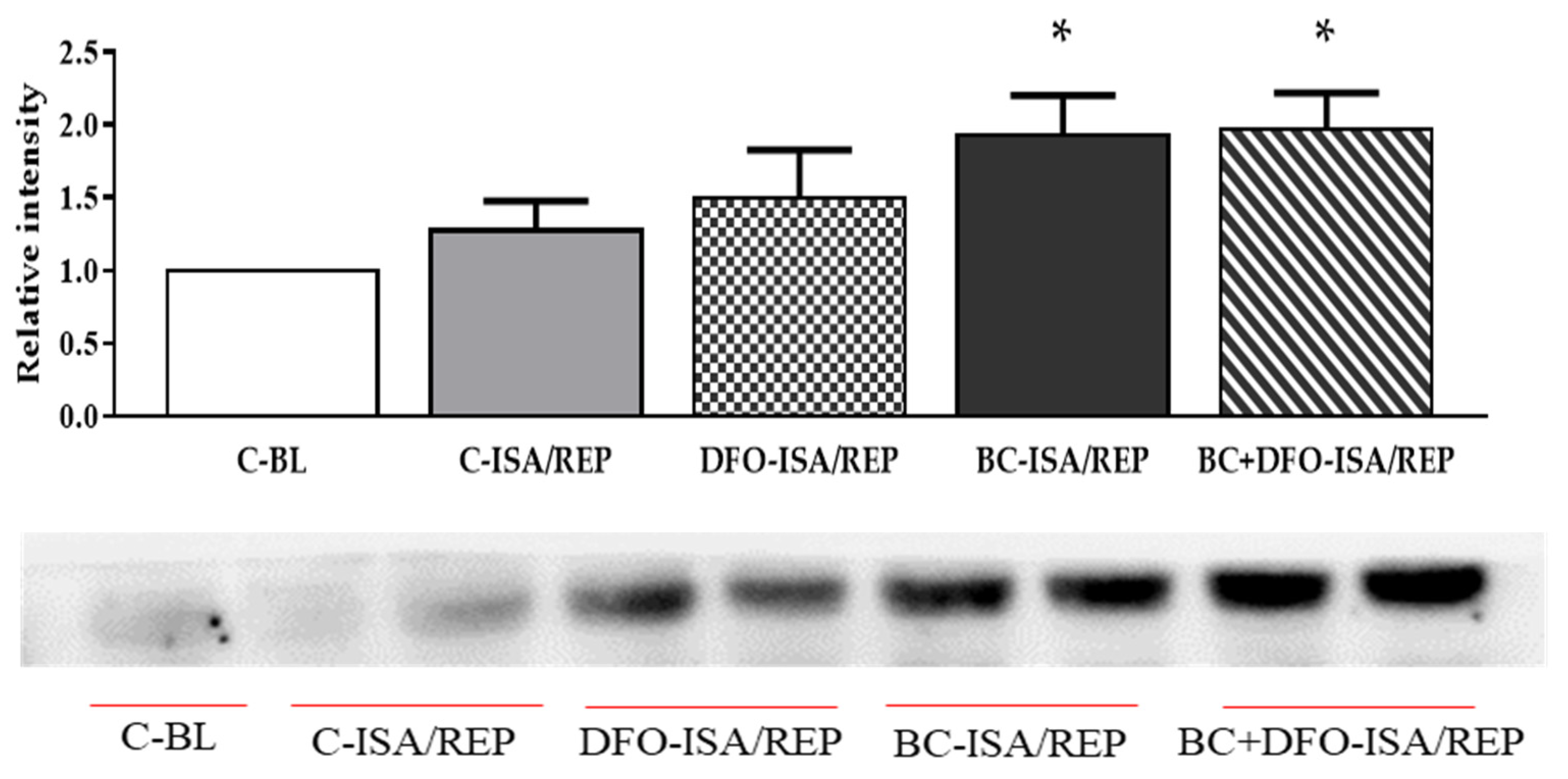
2.3. HO-1 Protein Expression in Hearts Isolated from Vehicle- or BC-Treated Rats with or without the Administration of DFO
3. Discussion
4. Materials and Methods
4.1. Treatment Protocol and Isolated Heart Preparation
4.2. Induction of ISA/REP and Cardiac Function Assessments
4.3. Determination of Infarct Size
4.4. Protein Isolation and Western Blot Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Duarte-Jurado, A.P.; Gopar-Cuevas, Y.; Saucedo-Cardenas, O.; Loera-Arias, M.J.; Montes-de-Oca-Luna, R.; Garcia-Garcia, A.; Rodriguez-Rocha, H. Antioxidant Therapeutics in Parkinson’s Disease: Current Challenges and Opportunities. Antioxidants 2021, 10, 453. [Google Scholar] [CrossRef]
- Alonso-Pineiro, J.A.; Gonzalez-Rovira, A.; Sanchez-Gomar, I.; Moreno, J.A.; Duran-Ruiz, M.C. Nrf2 and Heme Oxygenase-1 Involvement in Atherosclerosis Related Oxidative Stress. Antioxidants 2021, 10, 1463. [Google Scholar] [CrossRef]
- Shah, A.K.; Bhullar, S.K.; Elimban, V.; Dhalla, N.S. Oxidative Stress as A Mechanism for Functional Alterations in Cardiac Hypertrophy and Heart Failure. Antioxidants 2021, 10, 931. [Google Scholar] [CrossRef]
- Daiber, A.; Andreadou, I.; Oelze, M.; Davidson, S.M.; Hausenloy, D.J. Discovery of new therapeutic redox targets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Radic. Biol. Med. 2021, 163, 325–343. [Google Scholar] [CrossRef]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Miller, J.K.; Brzezinska-Slebodzinska, E.; Madsen, F.C. Oxidative stress, antioxidants, and animal function. J. Dairy Sci. 1993, 76, 2812–2823. [Google Scholar] [CrossRef]
- Puppel, K.; Kapusta, A.; Kuczynska, B. The etiology of oxidative stress in the various species of animals, a review. J. Sci. Food Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Effects of Carotenoids on Health: Are All the Same? Results from Clinical Trials. Curr. Pharm. Des. 2017, 23, 2422–2427. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, R.; Ricordy, R.; Aglitti, T.; Gatta, V.; Perticone, P.; De Salvia, R. Ascorbic acid and beta-carotene as modulators of oxidative damage. Carcinogenesis 1997, 18, 223–228. [Google Scholar] [CrossRef][Green Version]
- Csepanyi, E.; Czompa, A.; Haines, D.; Lekli, I.; Bakondi, E.; Balla, G.; Tosaki, A.; Bak, I. Cardiovascular effects of low versus high-dose beta-carotene in a rat model. Pharmacol. Res. 2015, 100, 148–156. [Google Scholar] [CrossRef]
- Csepanyi, E.; Czompa, A.; Szabados-Furjesi, P.; Lekli, I.; Balla, J.; Balla, G.; Tosaki, A.; Bak, I. The Effects of Long-Term, Low- and High-Dose Beta-Carotene Treatment in Zucker Diabetic Fatty Rats: The Role of HO-1. Int. J. Mol. Sci. 2018, 19, 1132. [Google Scholar] [CrossRef] [PubMed]
- Alija, A.J.; Bresgen, N.; Sommerburg, O.; Langhans, C.D.; Siems, W.; Eckl, P.M. Cyto- and genotoxic potential of beta-carotene and cleavage products under oxidative stress. Biofactors 2005, 24, 159–163. [Google Scholar] [CrossRef]
- Siems, W.; Salerno, C.; Crifo, C.; Sommerburg, O.; Wiswedel, I. Beta-carotene degradation products—Formation, toxicity and prevention of toxicity. Forum Nutr. 2009, 61, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Siems, W.; Wiswedel, I.; Salerno, C.; Crifo, C.; Augustin, W.; Schild, L.; Langhans, C.D.; Sommerburg, O. Beta-carotene breakdown products may impair mitochondrial functions—Potential side effects of high-dose beta-carotene supplementation. J. Nutr. Biochem. 2005, 16, 385–397. [Google Scholar] [CrossRef]
- Van Helden, Y.G.; Keijer, J.; Knaapen, A.M.; Heil, S.G.; Briede, J.J.; van Schooten, F.J.; Godschalk, R.W. Beta-carotene metabolites enhance inflammation-induced oxidative DNA damage in lung epithelial cells. Free Radic. Biol. Med. 2009, 46, 299–304. [Google Scholar] [CrossRef]
- Kobayashi, M.; Suhara, T.; Baba, Y.; Kawasaki, N.K.; Higa, J.K.; Matsui, T. Pathological Roles of Iron in Cardiovascular Disease. Curr. Drug Targets 2018, 19, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Paterek, A.; Mackiewicz, U.; Maczewski, M. Iron and the heart: A paradigm shift from systemic to cardiomyocyte abnormalities. J. Cell. Physiol. 2019, 234, 21613–21629. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Shen, Z.; Wang, J.; Zhou, B. Role of iron homeostasis in the heart: Heart failure, cardiomyopathy, and ischemia-reperfusion injury. Herz 2022, 47, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zweier, J.L.; Talukder, M.A. The role of oxidants and free radicals in reperfusion injury. Cardiovasc. Res. 2006, 70, 181–190. [Google Scholar] [CrossRef]
- Lillo-Moya, J.; Rojas-Sole, C.; Munoz-Salamanca, D.; Panieri, E.; Saso, L.; Rodrigo, R. Targeting Ferroptosis against Ischemia/Reperfusion Cardiac Injury. Antioxidants 2021, 10, 667. [Google Scholar] [CrossRef]
- Haines, D.D.; Lekli, I.; Teissier, P.; Bak, I.; Tosaki, A. Role of haeme oxygenase-1 in resolution of oxidative stress-related pathologies: Focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol. 2012, 204, 487–501. [Google Scholar] [CrossRef]
- Dong, Z.; Lavrovsky, Y.; Venkatachalam, M.A.; Roy, A.K. Heme oxygenase-1 in tissue pathology: The Yin and Yang. Am. J. Pathol. 2000, 156, 1485–1488. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Sy, C.; Dangles, O.; Borel, P.; Caris-Veyrat, C. Iron-induced oxidation of (all-E)-beta-carotene under model gastric conditions: Kinetics, products, and mechanism. Free Radic. Biol. Med. 2013, 63, 195–206. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.A.; Bilton, R.F.; Young, A.J. Beta-carotene enhances hydrogen peroxide-induced DNA damage in human hepatocellular HepG2 cells. FEBS Lett. 1999, 449, 255–258. [Google Scholar] [CrossRef]
- Lowe, G.M.; Booth, L.A.; Young, A.J.; Bilton, R.F. Lycopene and beta-carotene protect against oxidative damage in HT29 cells at low concentrations but rapidly lose this capacity at higher doses. Free Radic. Res. 1999, 30, 141–151. [Google Scholar] [CrossRef]
- Da Rocha, R.F.; de Oliveira, M.R.; Schonhofen, P.; Schnorr, C.E.; Dal Pizzol, F.; Moreira, J.C. Long-term vitamin A supplementation at therapeutic doses induces mitochondrial electrons transfer chain (METC) impairment and increased mitochondrial membrane-enriched fraction (MMEF) 3-nitrotyrosine on rat heart. Free Radic. Res. 2010, 44, 505–512. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Leshina, T.V.; Konovalova, T.A.; Kispert, L.D. Carotenoids as scavengers of free radicals in a Fenton reaction: Antioxidants or pro-oxidants? Free Radic. Biol. Med. 2001, 31, 398–404. [Google Scholar] [CrossRef]
- Baba, Y.; Higa, J.K.; Shimada, B.K.; Horiuchi, K.M.; Suhara, T.; Kobayashi, M.; Woo, J.D.; Aoyagi, H.; Marh, K.S.; Kitaoka, H.; et al. Protective effects of the mechanistic target of rapamycin against excess iron and ferroptosis in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H659–H668. [Google Scholar] [CrossRef]
- Tang, L.J.; Luo, X.J.; Tu, H.; Chen, H.; Xiong, X.M.; Li, N.S.; Peng, J. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn Schmiedebergs Arch. Pharmacol. 2021, 394, 401–410. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef]
- Siems, W.; Sommerburg, O.; Schild, L.; Augustin, W.; Langhans, C.D.; Wiswedel, I. Beta-carotene cleavage products induce oxidative stress in vitro by impairing mitochondrial respiration. FASEB J. 2002, 16, 1289–1291. [Google Scholar] [CrossRef]
- Farhangkhoee, H.; Khan, Z.A.; Mukherjee, S.; Cukiernik, M.; Barbin, Y.P.; Karmazyn, M.; Chakrabarti, S. Heme oxygenase in diabetes-induced oxidative stress in the heart. J. Mol. Cell. Cardiol. 2003, 35, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.A.; Barbin, Y.P.; Cukiernik, M.; Adams, P.C.; Chakrabarti, S. Heme-oxygenase-mediated iron accumulation in the liver. Can. J. Physiol. Pharmacol. 2004, 82, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Suttner, D.M.; Dennery, P.A. Reversal of HO-1 related cytoprotection with increased expression is due to reactive iron. FASEB J. 1999, 13, 1800–1809. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Yermakova, V.N.; Sakina, N.L.; Evstigneeva, R.P.; Rozhkova, E.A.; Zheltukhina, G.A. Nα-Acetylcarnosine is a prodrug of L-carnosine in ophthalmic application as antioxidant. Clin. Chim. Acta 1996, 254, 1–21. [Google Scholar] [CrossRef]
- Babizhayev, M.A. Biological activities of the natural imidazole-containing peptidomimetics n-acetylcarnosine, carcinine and L-carnosine in ophthalmic and skin care products. Life Sci. 2006, 78, 2343–2357. [Google Scholar] [CrossRef] [PubMed]
- Jukic, I.; Kolobaric, N.; Stupin, A.; Matic, A.; Kozina, N.; Mihaljevic, Z.; Mihalj, M.; Susnjara, P.; Stupin, M.; Curic, Z.B.; et al. Carnosine, Small but Mighty-Prospect of Use as Functional Ingredient for Functional Food Formulation. Antioxidants 2021, 10, 1037. [Google Scholar] [CrossRef]
- Alabovsky, V.V.; Boldyrev, A.A.; Vinokurov, A.A.; Shchavratsky, V. Effect of histidine-containing dipeptides on isolated heart under ischemia/reperfusion. Biochemistry 1997, 62, 77–87. [Google Scholar]
- Gurtler, A.; Kunz, N.; Gomolka, M.; Hornhardt, S.; Friedl, A.A.; McDonald, K.; Kohn, J.E.; Posch, A. Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013, 433, 105–111. [Google Scholar] [CrossRef]
- Ghosh, R.; Gilda, J.E.; Gomes, A.V. The necessity of and strategies for improving confidence in the accuracy of western blots. Expert Rev. Proteom. 2014, 11, 549–560. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csepanyi, E.; Gyongyosi, A.; Lekli, I.; Tosaki, A.; Bak, I. Beta-Carotene Affects the Effects of Heme Oxygenase-1 in Isolated, Ischemic/Reperfused Rat Hearts: Potential Role of the Iron. Molecules 2022, 27, 3039. https://doi.org/10.3390/molecules27093039
Csepanyi E, Gyongyosi A, Lekli I, Tosaki A, Bak I. Beta-Carotene Affects the Effects of Heme Oxygenase-1 in Isolated, Ischemic/Reperfused Rat Hearts: Potential Role of the Iron. Molecules. 2022; 27(9):3039. https://doi.org/10.3390/molecules27093039
Chicago/Turabian StyleCsepanyi, Evelin, Alexandra Gyongyosi, Istvan Lekli, Arpad Tosaki, and Istvan Bak. 2022. "Beta-Carotene Affects the Effects of Heme Oxygenase-1 in Isolated, Ischemic/Reperfused Rat Hearts: Potential Role of the Iron" Molecules 27, no. 9: 3039. https://doi.org/10.3390/molecules27093039
APA StyleCsepanyi, E., Gyongyosi, A., Lekli, I., Tosaki, A., & Bak, I. (2022). Beta-Carotene Affects the Effects of Heme Oxygenase-1 in Isolated, Ischemic/Reperfused Rat Hearts: Potential Role of the Iron. Molecules, 27(9), 3039. https://doi.org/10.3390/molecules27093039




