Rediscovering the Therapeutic Potential of Agarwood in the Management of Chronic Inflammatory Diseases
Abstract
1. Introduction
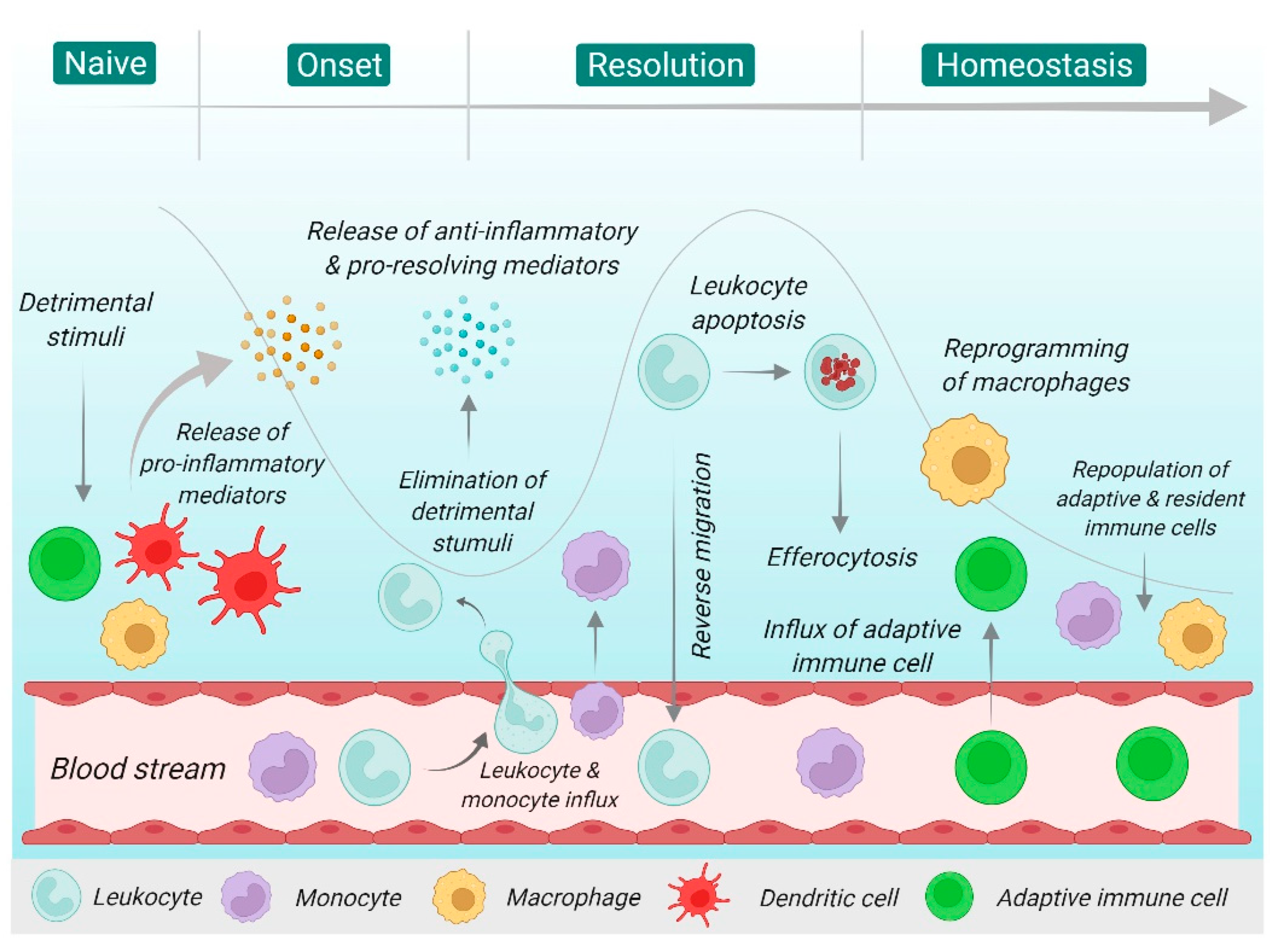
2. Overview of Inflammation
3. Natural Products in Modern Drug Development
3.1. An Overview of Agarwood: Origin, Uses, and Distribution of Agarwood
3.2. Induction of Agarwood
3.3. Distillation of Agarwood Oil
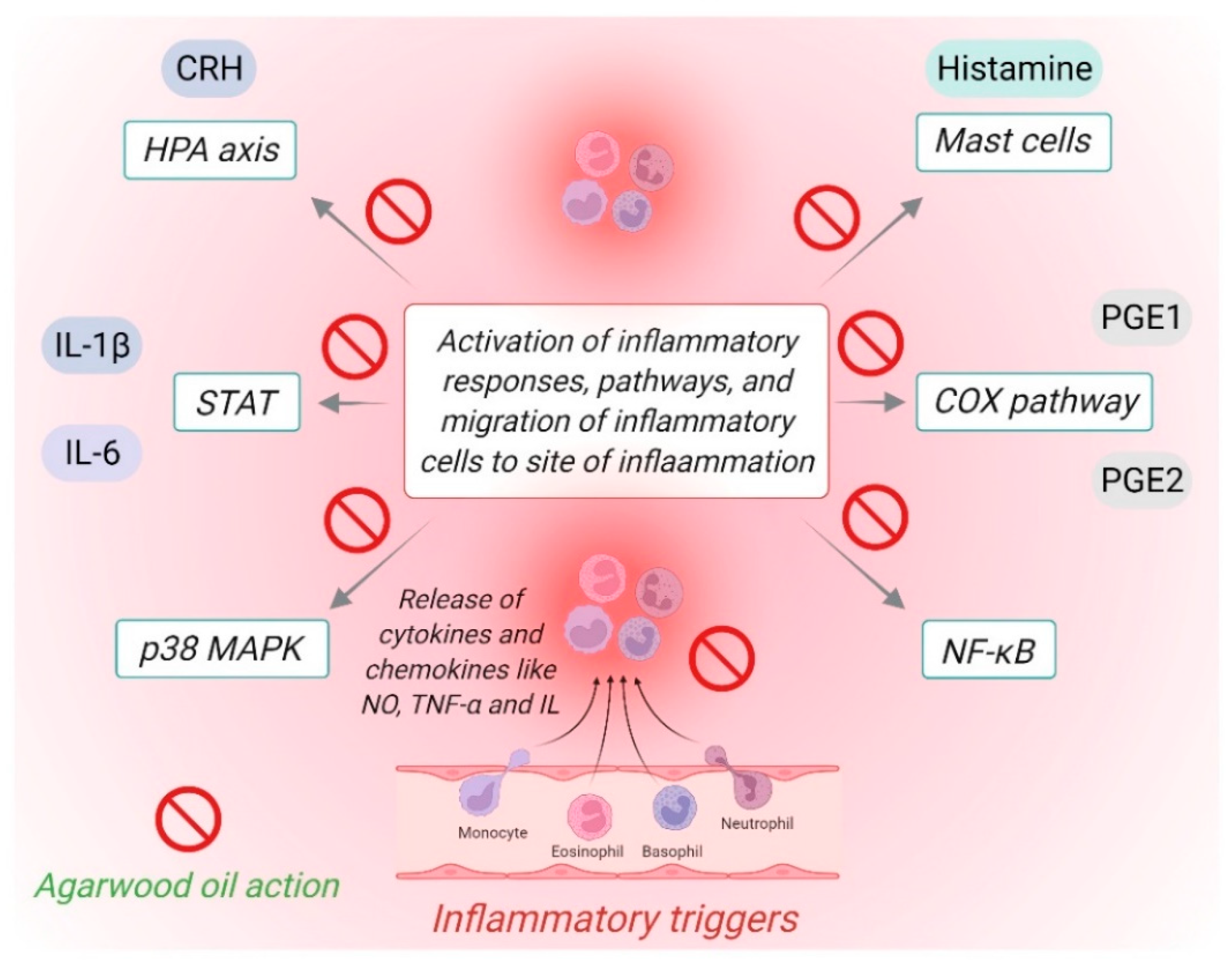
4. Potential of Agarwood Oil against Chronic Inflammatory Diseases
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Punchard, N.A.; Whelan, C.J.; Adcock, I. The Journal of Inflammation. J. Inflamm. 2004, 1, 1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Bansal, P.; Jialal, I. Chronic Inflammation. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2021. [Google Scholar]
- Netea, M.G.; Balkwill, F.; Chonchol, M.; Cominelli, F.; Donath, M.Y.; Giamarellos-Bourboulis, E.J.; Golenbock, D.; Gresnigt, M.S.; Heneka, M.T.; Hoffman, H.M. A guiding map for inflammation. Nat. Immunol. 2017, 18, 826–831. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Hasan, H.; Barkat, M.A.; Hussain, M.S. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharm. Rev. 2011, 5, 120–137. [Google Scholar] [CrossRef]
- Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of anti-inflammatory herbal medicines. Adv. Pharmacol. Sci. 2016, 2016, 9130979. [Google Scholar] [CrossRef]
- Nunes, C.d.R.; Barreto Arantes, M.; Menezes de Faria Pereira, S.; Leandro da Cruz, L.; de Souza Passos, M.; Pereira de Moraes, L.; Vieira, I.J.C.; Barros de Oliveira, D. Plants as sources of anti-inflammatory agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Medicinal plants with anti-inflammatory activities from selected countries and regions of Africa. J. Inflamm. Res. 2018, 11, 307–317. [Google Scholar] [CrossRef]
- Chan, Y.; Ng, S.W.; Liew, H.S.; Pua, L.J.W.; Soon, L.; Lim, J.S.; Dua, K.; Chellappan, D.K. Introduction to chronic respiratory diseases: A pressing need for novel therapeutic approaches. In Medicinal Plants for Lung Diseases; Springer: Berlin/Heidelberg, Germany, 2021; pp. 47–84. [Google Scholar]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Diaz, P.; Jeong, S.C.; Lee, S.; Khoo, C.; Koyyalamudi, S.R. Antioxidant and anti-inflammatory activities of selected medicinal plants and fungi containing phenolic and flavonoid compounds. Chin. Med. 2012, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.B.; Valentão, P. Insights into Natural Products in Inflammation. Int. J. Mol. Sci. 2018, 19, 644. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kismali, G.; Gupta, S.C. Natural Products for the Prevention and Treatment of Chronic Inflammatory Diseases: Integrating Traditional Medicine into Modern Chronic Diseases Care. Evid. Based. Comple. Alt. Med. 2018, 2018, 9837863. [Google Scholar] [CrossRef] [PubMed]
- Yatoo, M.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H. Anti-inflammatory drugs and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders-a review. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.T.; Ly, T.L.; Van Le, T.H.; Tran, T.V.A.; Kwon, S.W.; Park, J.H. Sesquiterpene derivatives from the agarwood of Aquilaria malaccensis and their anti-inflammatory effects on NO production of macrophage RAW 264.7 cells. Phytochemistry 2021, 183, 112630. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Yu, Z.; Wang, C.; Gong, B.; Liu, Y.; Wei, J. Chemical Constituents and Anti-Inflammatory Effect of Incense Smoke from Agarwood Determined by GC-MS. Int. J. Anal. Chem. 2020, 2020, 4575030. [Google Scholar] [CrossRef]
- Afzal, S.; Ahmad, H.I.; Jabbar, A.; Tolba, M.M.; AbouZid, S.; Irm, N.; Zulfiqar, F.; Iqbal, M.Z.; Ahmad, S.; Aslam, Z. Use of Medicinal Plants for Respiratory Diseases in Bahawalpur, Pakistan. Biomed. Res. Int. 2021, 2021, 5578914. [Google Scholar] [CrossRef]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Adam, A.Z.; Lee, S.Y.; Mohamed, R. Pharmacological properties of agarwood tea derived from Aquilaria (Thymelaeaceae) leaves: An emerging contemporary herbal drink. J. Herb. Med. 2017, 10, 37–44. [Google Scholar] [CrossRef]
- Taylor, N.L.; Day, D.A.; Millar, A.H. Targets of stress-induced oxidative damage in plant mitochondria and their impact on cell carbon/nitrogen metabolism. J. Exp. Bot. 2004, 55, 1–10. [Google Scholar] [CrossRef]
- Nonaka, Y.; Izumo, T.; Izumi, F.; Maekawa, T.; Shibata, H.; Nakano, A.; Kishi, A.; Akatani, K.; Kiso, Y. Antiallergic effects of Lactobacillus pentosus strain S-PT84 mediated by modulation of Th1/Th2 immunobalance and induction of IL-10 production. Int. Arch. Allergy Immunol. 2008, 145, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xie, Y.; Song, L.; Wang, Y.; Qiu, H.; Yang, Y.; Li, C.; Wang, Z.; Han, Z.; Yang, L. Seven new 2-(2-phenylethyl) chromone derivatives from agarwood of Aquilaria agallocha with inhibitory effects on nitric oxide production. Fitoterapia 2022, 159, 105177. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Huo, H.; Zhang, H.; Wang, L.; Meng, Y.; Jin, F.; Wang, X.; Zhao, Y.; Zhao, Y.; Tu, P. 2-(2-Phenylethyl) chromone-enriched Extract of the Resinous Heartwood of Chinese Agarwood (Aquilaria sinensis) Protects against Taurocholic Acid-induced Gastric Epithelial Cells Apoptosis through Perk/eIF2α/CHOP Pathway. Phytomedicine 2022, 98, 153935. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Vago, J.P.; Perretti, M.; Teixeira, M.M. Mediators of the resolution of the inflammatory response. Trends Immunol. 2019, 40, 212–227. [Google Scholar] [CrossRef]
- Abudukelimu, A.; Barberis, M.; Redegeld, F.A.; Sahin, N.; Westerhoff, H.V. Predictable irreversible switching between acute and chronic inflammation. Front. Immunol. 2018, 9, 1596. [Google Scholar] [CrossRef]
- Barnes, P.J. Cellular and molecular mechanisms of asthma and COPD. Clin. Sci. 2017, 131, 1541–1558. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of inflammation: What controls its onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef]
- Ortega-Gómez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef]
- Gradel, K.O.; Nielsen, H.L.; Schønheyder, H.C.; Ejlertsen, T.; Kristensen, B.; Nielsen, H. Increased short-and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology 2009, 137, 495–501. [Google Scholar] [CrossRef]
- Thornton, J.; Emmett, P.; Heaton, K. Diet and Crohn’s disease: Characteristics of the pre-illness diet. Br. Med. J. 1979, 2, 762–764. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rodríguez, L.A.G.; Ruigómez, A.; Panés, J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 2006, 130, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Chacko, A. Influence of environmental factors on the onset and course of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 1088. [Google Scholar] [CrossRef] [PubMed]
- Mansour, L.; El-Kalla, F.; Kobtan, A.; Abd-Elsalam, S.; Yousef, M.; Soliman, S.; Ali, L.A.; Elkhalawany, W.; Amer, I.; Harras, H.; et al. Helicobacter pylori may be an initiating factor in newly diagnosed ulcerative colitis patients: A pilot study. World J. Clin. Cases 2018, 6, 641–649. [Google Scholar] [CrossRef]
- Klein, A.; Eliakim, R. Non steroidal anti-inflammatory drugs and inflammatory bowel disease. Pharmaceuticals 2010, 3, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Levenstein, S.; Rosenstock, S.; Jacobsen, R.K.; Jorgensen, T. Psychological stress increases risk for peptic ulcer, regardless of Helicobacter pylori infection or use of nonsteroidal anti-inflammatory drugs. Clin. Gastroenterol. Hepatol. 2015, 13, 498–506.e1. [Google Scholar] [CrossRef] [PubMed]
- Kemp, W.J.; Bashir, A.; Dababneh, H.; Cohen-Gadol, A.A. Cushing’s ulcer: Further reflections. Asian J. Neurosurg. 2015, 10, 87. [Google Scholar]
- Senthelal, S.; Li, J.; Goyal, A.; Thomas, M.A. Arthritis. In StatPearls [Internet]; Treasure Island (FL): StatPearls Publishing: Mountain View, CA, USA, 2022. [Google Scholar]
- Chauhan, K.; Jandu, J.S.; Goyal, A.; Al-Dhahir, M.A. Continuing Education Activity. In StatPearls [Internet]; Treasure Island (FL): StatPearls Publishing: Mountain View, CA, USA, 2021. [Google Scholar]
- Liao, K.P.; Alfredsson, L.; Karlson, E.W. Environmental influences on risk for rheumatoid arthritis. Curr. Opin. Rheumatol. 2009, 21, 279. [Google Scholar] [CrossRef]
- Kumar, A.; Mendez, M.D. Herpes simplex encephalitis. In StatPearls [Internet]; StatPearls Publishing: Mountain View, CA, USA, 2021. [Google Scholar]
- Daliparty, V.M.; Balasubramanya, R. HIV encephalitis. In StatPearls [Internet]; StatPearls Publishing: Mountain View, CA, USA, 2021. [Google Scholar]
- Gole, S.; Anand, A. Autoimmune Encephalitis. In StatPearls [Internet]; StatPearls Publishing: Mountain View, CA, USA, 2022. [Google Scholar]
- Richie, M.B. Autoimmune Meningitis and Encephalitis. Neurol. Clin. 2022, 40, 93–112. [Google Scholar] [CrossRef]
- Shi, K.; Tian, D.; Li, Z.; Ducruet, A.F.; Lawton, M.T.; Shi, F. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef]
- Mehta, M.; Dhanjal, D.S.; Paudel, K.R.; Singh, B.; Gupta, G.; Rajeshkumar, S.; Thangavelu, L.; Tambuwala, M.M.; Bakshi, H.A.; Chellappan, D.K. Cellular signalling pathways mediating the pathogenesis of chronic inflammatory respiratory diseases: An update. Inflammopharmacology 2020, 28, 795–817. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Paudel, K.R.; Kim, D. Eriobotrya japonica leaf extract attenuates airway inflammation in ovalbumin-induced mice model of asthma. J. Ethnopharmacol. 2020, 253, 112082. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, P.; Choi, S.; An, M.; Jang, A. Effects of Air Pollutants on Airway Diseases. Int. J. Environ. Res. Public Health 2021, 18, 9905. [Google Scholar] [CrossRef] [PubMed]
- Shastri, M.D.; Allam, V.S.R.R.; Shukla, S.D.; Jha, N.K.; Paudel, K.R.; Peterson, G.M.; Patel, R.P.; Hansbro, P.M.; Chellappan, D.K.; Dua, K. Interleukin-13: A pivotal target against influenza-induced exacerbation of chronic lung diseases. Life Sci. 2021, 283, 119871. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Dharwal, V.; Patel, V.K.; Galvao, I.; Wadhwa, R.; Malyla, V.; Shen, S.S.; Budden, K.F.; Hansbro, N.G.; Vaughan, A. Role of lung microbiome in innate immune response associated with chronic lung diseases. Front. Med. 2020, 7, 554. [Google Scholar] [CrossRef] [PubMed]
- Dharwal, V.; Paudel, K.R.; Hansbro, P.M. Impact of bushfire smoke on respiratory health. Med. J. Aust. 2020, 213, 284–284.e1. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Raju Allam, V.S.R.; Paudel, K.R.; Singh, S.K.; Gulati, M.; Dhanasekaran, M.; Gupta, P.K.; Jha, N.K.; Devkota, H.P.; Gupta, G. Nutraceuticals: Unlocking newer paradigms in the mitigation of inflammatory lung diseases. Crit. Rev. Food Sci. Nutr. 2021, 1–31. [Google Scholar] [CrossRef]
- Chan, Y.; Ng, S.W.; Dua, K.; Chellappan, D.K. Plant-based chemical moieties for targeting chronic respiratory diseases. In Targeting Cellular Signalling Pathways in Lung Diseases; Springer: Berlin/Heidelberg, Germany, 2021; pp. 741–781. [Google Scholar]
- Sack, M.; Hofbauer, A.; Fischer, R.; Stoger, E. The increasing value of plant-made proteins. Curr. Opin. Biotechnol. 2015, 32, 163–170. [Google Scholar] [CrossRef]
- Chan, Y.; Ng, S.W.; Tan, J.Z.X.; Gupta, G.; Negi, P.; Thangavelu, L.; Balusamy, S.R.; Perumalsamy, H.; Yap, W.H.; Singh, S.K. Natural products in the management of obesity: Fundamental mechanisms and pharmacotherapy. S. Afr. J. Bot. 2021, 143, 176–197. [Google Scholar] [CrossRef]
- Patil, R.Y.; Patil, S.A.; Chivate, N.D.; Patil, Y.N. Herbal drug nanoparticles: Advancements in herbal treatment. Res. J. Pharm. Tech. 2018, 11, 421–426. [Google Scholar] [CrossRef]
- Clarke, R.; Lundy, F.T.; McGarvey, L. Herbal treatment in asthma and COPD–current evidence. Clin. Phytosci. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Wang, H.; Mei, W.; Dai, H. Natural products in agarwood and Aquilaria plants: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 528–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, Z.; Wang, C.; Wu, C.; Guo, P.; Wei, J. Chemical constituents and pharmacological activity of agarwood and Aquilaria plants. Molecules 2018, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Ohdo, S.; Koyanagi, S.; Matsunaga, N. Chronopharmacological strategies focused on chrono-drug discovery. Pharmacol Ther. 2019, 202, 72–90. [Google Scholar] [CrossRef]
- Alam, J.; Mujahid, M.; Rahman, M.; Akhtar, J.; Khalid, M.; Jahan, Y.; Basit, A.; Khan, A.; Shawwal, M.; Iqbal, S.S. An insight of pharmacognostic study and phytopharmacology of Aquilaria agallocha. J. Appl. Pharm. Sci. 2015, 5, 173–181. [Google Scholar] [CrossRef]
- Araújo, W.L.; Tohge, T.; Ishizaki, K.; Leaver, C.J.; Fernie, A.R. Protein degradation-an alternative respiratory substrate for stressed plants. Trends Plant Sci. 2011, 16, 489–498. [Google Scholar] [CrossRef]
- Kalita, P.; Roy, P.K.; Sen, S. Agarwood: Medicinal Side of the Fragrant Plant. In Herbal Medicine in India; Springer: Berlin/Heidelberg, Germany, 2020; pp. 223–236. [Google Scholar]
- Liu, Y.; Chen, H.; Yang, Y.; Zhang, Z.; Wei, J.; Meng, H.; Chen, W.; Feng, J.; Gan, B.; Chen, X. Whole-tree agarwood-inducing technique: An efficient novel technique for producing high-quality agarwood in cultivated Aquilaria sinensis trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, S.; Song, J.; Zhou, X.; Zhou, D.; Liu, X.; Hong, Z.; Xu, D. Effects of various artificial agarwood-induction techniques on the metabolome of Aquilaria sinensis. BMC Plant Biol. 2021, 21, 591. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mohamed, R. The origin and domestication of Aquilaria, an important agarwood-producing genus. In Agarwood; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–20. [Google Scholar]
- Comparative Tabulation Report. Available online: https://trade.cites.org/en/cites_trade/download/view_results?filters%5Btime_range_start%5D=2016&filters%5Btime_range_end%5D=2021&filters%5Bexporters_ids%5D%5B%5D=all_exp&filters%5Bimporters_ids%5D%5B%5D=all_imp&filters%5Bsources_ids%5D%5B%5D=all_sou&filters%5Bpurposes_ids%5D%5B%5D=all_pur&filters%5Bterms_ids%5D%5B%5D=all_ter&filters%5Btaxon_concepts_ids%5D%5B%5D=13257&filters%5Breset%5D=&filters%5Bselection_taxon%5D=taxonomic_cascade&web_disabled=&filters%5breport_type%5d=comptab (accessed on 24 March 2022).
- Aquilaria Malaccensis. Available online: https://mycites.frim.gov.my/en/species/aquilaria-malaccensis/overview/habitat/ (accessed on 24 March 2022).
- Wang, Y.; Hussain, M.; Jiang, Z.; Wang, Z.; Gao, J.; Ye, F.; Mao, R.; Li, H. Aquilaria Species (Thymelaeaceae) Distribution, Volatile and Non-Volatile Phytochemicals, Pharmacological Uses, Agarwood Grading System, and Induction Methods. Molecules 2021, 26, 7708. [Google Scholar] [CrossRef]
- Hashim, Y.Z.H.; Kerr, P.G.; Abbas, P.; Mohd Salleh, H. Aquilaria spp. (agarwood) as source of health beneficial compounds: A review of traditional use, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 189, 331–360. [Google Scholar] [CrossRef]
- Naziz, P.S.; Das, R.; Sen, S. The scent of stress: Evidence from the unique fragrance of agarwood. Front. Plant Sci. 2019, 10, 840. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cites.org/eng/app/appendices.php (accessed on 24 March 2022).
- Available online: https://www.iucnredlist.org/ (accessed on 24 March 2022).
- Azren, P.D.; Lee, S.Y.; Emang, D.; Mohamed, R. History and perspectives of induction technology for agarwood production from cultivated Aquilaria in Asia: A review. J. For. Res. 2019, 30, 1–11. [Google Scholar] [CrossRef]
- Yan, T.; Yang, S.; Chen, Y.; Wang, Q.; Li, G. Chemical profiles of cultivated Agarwood induced by different techniques. Molecules 2019, 24, 1990. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Mohamed, R. Understanding agarwood formation and its challenges. In Agarwood; Springer: Berlin/Heidelberg, Germany, 2016; pp. 39–56. [Google Scholar]
- Mohamed, R.; Jong, P.; Zali, M. Fungal diversity in wounded stems of Aquilaria malaccensis. Fungal Divers. 2010, 43, 67–74. [Google Scholar] [CrossRef]
- Tibpromma, S.; Zhang, L.; Karunarathna, S.C.; Du, T.; Phukhamsakda, C.; Rachakunta, M.; Suwannarach, N.; Xu, J.; Mortimer, P.E.; Wang, Y. Volatile constituents of endophytic Fungi isolated from Aquilaria sinensis with descriptions of two new species of Nemania. Life 2021, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Jong, P.L.; Kamziah, A.K. Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery. J. For. Res. 2014, 25, 201–204. [Google Scholar] [CrossRef]
- Chhipa, H.; Kaushik, N. Fungal and bacterial diversity isolated from Aquilaria malaccensis tree and soil, induces agarospirol formation within 3 months after artificial infection. Front. Microbiol. 2017, 8, 1286. [Google Scholar] [CrossRef]
- Sangareswari, M.; Parthiban, K.T.; Kanna, S.U.; Karthiba, L.; Saravanakumar, D. Fungal microbes associated with agarwood formation. Am. J. Plant Sci. 2016, 7, 1445. [Google Scholar] [CrossRef]
- Martin, D.; Tholl, D.; Gershenzon, J.; Bohlmann, J. Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol. 2002, 129, 1003–1018. [Google Scholar] [CrossRef]
- Ye, W.; Wu, H.; He, X.; Wang, L.; Zhang, W.; Li, H.; Fan, Y.; Tan, G.; Liu, T.; Gao, X. Transcriptome sequencing of chemically induced Aquilaria sinensis to identify genes related to agarwood formation. PLoS ONE 2016, 11, e0155505. [Google Scholar] [CrossRef]
- Van Thanh, L.; Van Do, T.; Son, N.H.; Sato, T.; Kozan, O. Impacts of biological, chemical and mechanical treatments on sesquiterpene content in stems of planted Aquilaria crassna trees. Agrofor. Syst. 2015, 89, 973–981. [Google Scholar] [CrossRef]
- Okudera, Y.; Ito, M. Production of agarwood fragrant constituents in Aquilaria calli and cell suspension cultures. Plant Biotechnol. 2009, 26, 307–315. [Google Scholar] [CrossRef]
- Xiao, Z.; Jia, S.; Bao, H.; Niu, Y.; Ke, Q.; Kou, X. Protection of agarwood essential oil aroma by nanocellulose-graft-polylactic acid. Int. J. Biol. Macromol. 2021, 183, 743–752. [Google Scholar] [CrossRef]
- Ahmad, Z.; Ahmad, B.; Yusoff, Z.B.; Fikri, A.; Awang, B.; Azizi, M.; Bin, F.; Rudin, M.N.; Saiful, M.; Bin, H.; et al. Hydro-Distillation Process in Extracting of Agarwood Essential Oil. In Proceedings of the Technology and Innovation National Conference, Kuching Sarawak, Malayisa, 9–11 June 2015. [Google Scholar] [CrossRef]
- Thuy, D.T.T.; Tuyen, T.T.; Thuy, T.T.T.; Minh, P.T.H.; Tran, Q.T.; Long, P.Q.; Nguyen, D.C.; Bach, L.G.; Chien, N.Q. Isolation process and compound identification of agarwood essential oils from Aquilaria crassna cultivated at three different locations in vietnam. Processes 2019, 7, 432. [Google Scholar] [CrossRef]
- Pushpangadan, P.; George, V. Basil. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 55–72. [Google Scholar]
- Samadi, M.; Zainal Abidin, Z.; Yoshida, H.; Yunus, R.; Awang Biak, D. Towards higher oil yield and quality of essential oil extracted from Aquilaria Malaccensis wood via the subcritical technique. Molecules 2020, 25, 3872. [Google Scholar] [CrossRef]
- Tam, C.; Yang, F.; Zhang, Q.; Guan, J.; Li, S. Optimization and comparison of three methods for extraction of volatile compounds from Cyperus rotundus evaluated by gas chromatography–mass spectrometry. J. Pharm. BioMed. Anal. 2007, 44, 444–449. [Google Scholar] [CrossRef]
- Ibrahim, A.; Al-Rawi, S.; Majid, A.A.; Rahman, N.A.; Abo-Salah, K.; Ab Kadir, M. Separation and fractionation of Aquilaria malaccensis oil using supercritical fluid extraction and tthe cytotoxic properties of the extracted oil. Procedia Food Sci. 2011, 1, 1953–1959. [Google Scholar] [CrossRef]
- Sulaiman, N.; Idayu, M.I.; Ramlan, A.; Fashya, M.N.; Farahiyah, A.N.; Mailina, J.; Azah, M.N. Effects of extraction methods on yield and chemical compounds of gaharu (Aquilaria malaccensis). J. Trop. Sci. 2015, 413–419. [Google Scholar] [CrossRef]
- Ayala, R.S.; De Castro, M.L. Continuous subcritical water extraction as a useful tool for isolation of edible essential oils. Food Chem. 2001, 75, 109–113. [Google Scholar] [CrossRef]
- Yoswathana, N.; Eshiaghi, M.; Jaturapornpanich, K. Enhancement of essential oil from agarwood by subcritical water extraction and pretreatments on hydrodistillation. Int. J. Chem. Mol. Eng. 2012, 6, 459–465. [Google Scholar]
- Chen, H.; Wei, J.; Yang, J.; Zhang, Z.; Yang, Y.; Gao, Z.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Hashim, Y.Z.H.; Ismail, N.I.; Abbas, P. Analysis of chemical compounds of agarwood oil from different species by gas chromatography mass spectrometry (GCMS). IIUM Eng. J. 2014, 15. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, W.; Li, J.; Yu, L.; Lin, L. Dynamic analysis of gene expression and determination of chemicals in agarwood in Aquilaria sinensis. J. For. Res. 2020, 31, 1833–1841. [Google Scholar] [CrossRef]
- Chhipa, H.; Chowdhary, K.; Kaushik, N. Artificial production of agarwood oil in Aquilaria sp. by fungi: A review. Phytochem. Rev. 2017, 16, 835–860. [Google Scholar] [CrossRef]
- Faizal, A.; Esyanti, R.R.; Aulianisa, E.N.; Santoso, E.; Turjaman, M. Formation of agarwood from Aquilaria malaccensis in response to inoculation of local strains of Fusarium solani. Trees 2017, 31, 189–197. [Google Scholar] [CrossRef]
- Tan, C.S.; Isa, N.M.; Ismail, I.; Zainal, Z. Agarwood induction: Current developments and future perspectives. Front. Plant Sci. 2019, 10, 122. [Google Scholar] [CrossRef]
- Zhu, Z.; Gu, Y.; Zhao, Y.; Song, Y.; Li, J.; Tu, P. GYF-17, a chloride substituted 2-(2-phenethyl)-chromone, suppresses LPS-induced inflammatory mediator production in RAW264. 7 cells by inhibiting STAT1/3 and ERK1/2 signaling pathways. Int. Immunopharmacol. 2016, 35, 185–192. [Google Scholar] [CrossRef]
- Wang, S.; Tsai, Y.; Fu, S.; Cheng, M.; Chung, M.; Chen, J. 2-(2-Phenylethyl)-4H-chromen-4-one derivatives from the resinous wood of Aquilaria sinensis with anti-inflammatory effects in LPS-induced macrophages. Molecules 2018, 23, 289. [Google Scholar] [CrossRef]
- Huo, H.; Gu, Y.; Sun, H.; Zhang, Y.; Liu, W.; Zhu, Z.; Shi, S.; Song, Y.; Jin, H.; Zhao, Y. Anti-inflammatory 2-(2-phenylethyl) chromone derivatives from Chinese agarwood. Fitoterapia 2017, 118, 49–55. [Google Scholar] [CrossRef]
- Huo, H.; Zhu, Z.; Song, Y.; Shi, S.; Sun, J.; Sun, H.; Zhao, Y.; Zheng, J.; Ferreira, D.; Zjawiony, J.K. Anti-inflammatory dimeric 2-(2-phenylethyl) chromones from the resinous wood of Aquilaria sinensis. J. Nat. Prod. 2017, 81, 543–553. [Google Scholar] [CrossRef]
- Huo, H.; Gu, Y.; Zhu, Z.; Zhang, Y.; Chen, X.; Guan, P.; Shi, S.; Song, Y.; Zhao, Y.; Tu, P. LC-MS-guided isolation of anti-inflammatory 2-(2-phenylethyl) chromone dimers from Chinese agarwood (Aquilaria sinensis). Phytochemistry 2019, 158, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Mohamed, G.A. Natural occurring 2-(2-phenylethyl) chromones, structure elucidation and biological activities. Nat. Prod. Res. 2015, 29, 1489–1520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, D.; Wei, J.; Feng, J.; Zhang, Z.; Yang, Y.; Zheng, W. Four new 2-(2-phenylethyl) chromone derivatives from Chinese agarwood produced via the whole-tree agarwood-inducing technique. Molecules 2016, 21, 1433. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, H.; Li, W.; Yang, L.; Yang, J.; Yuan, J.; Wei, Y.; Jiang, B.; Mei, W.; Dai, H. Filarones A and B, new anti-inflammatory dimeric 2-(2-phenethyl)chromones from agarwood of Aquilaria filaria. Phytochem. Lett. 2021, 46, 11–14. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, C.; Zheng, W.; Chen, D.; Liu, Y.; Yang, Y.; Wei, J. Anti-inflammatory 5, 6, 7, 8-tetrahydro-2-(2-phenylethyl) chromones from agarwood of Aquilaria sinensis. Bioorg. Chem. 2020, 99, 103789. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, Q.; Han, Z.; Yang, L.; Wang, Z. Three new sesquiterpenoids and one new sesquiterpenoid derivative from Chinese eaglewood. Molecules 2016, 21, 281. [Google Scholar] [CrossRef]
- Xie, Y.; Song, L.; Li, C.; Yang, Y.; Zhang, S.; Xu, H.; Wang, Z.; Han, Z.; Yang, L. Eudesmane-type and agarospirane-type sesquiterpenes from agarwood of Aquilaria agallocha. Phytochemistry 2021, 192, 112920. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Ahamed, M.B.K.; Majid, A.M.A. In vivo anti-inflammatory activity of β-caryophyllene, evaluated by molecular imaging. Mol. Med. Chem. 2015, 1. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Andrade, E.L.; Leite, D.F.; Figueiredo, C.P.; Calixto, J.B. Preventive and therapeutic anti-inflammatory properties of the sesquiterpene α-humulene in experimental airways allergic inflammation. Br. J. Pharm. 2009, 158, 1074–1087. [Google Scholar] [CrossRef]
- Rahman, H.; Vakati, K.; Eswaraiah, M.C. In-vivo and in-vitro anti-inflammatory activity of Aquilaria agallocha oil. Available online: https://www.semanticscholar.org/paper/In-Vivo-and-In-Vitro-Anti-Inflammatory-Activity-of-Rahman-Vakati/08c565784b14738cc2e7e830836f086f803bb00c (accessed on 24 March 2022).
- GAO, X. Anti-inflammatory effect of Chinese agarwood essential oil via inhibiting p-STAT3 and IL-1β/IL-6. Chin. Pharm. J. 2019, 24, 1951–1957. [Google Scholar]
- Yadav, D.K.; Mudgal, V.; Agrawal, J.; K Maurya, A.; U Bawankule, D.; S Chanotiya, C.; Khan, F.; T Thul, S. Molecular docking and ADME studies of natural compounds of Agarwood oil for topical anti-inflammatory activity. Curr. Comput.-Aided Drug Des. 2013, 9, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Chitre, T.; Bhutada, P.; Nandakumar, K.; Somani, R.; Miniyar, P.; Mundhada, Y.; Gore, S.; Jain, K. Analgesic and anti-inflammatory activity of heartwood of Aquilaria agallocha in laboratory animals. Pharm. Online 2007, 1, 288–298. [Google Scholar]
- Kumphune, S.; Prompunt, E.; Phaebuaw, K.; Sriudwong, P.; Pankla, R.; Thongyoo, P. Anti-inflammatory effects of the ethyl acetate extract of Aquilaria crassna inhibits LPS-induced tumour necrosis factor-alpha production by attenuating P38 MAPK activation. Int. J. Green Pharm. (IJGP) 2011, 5, 43–48. [Google Scholar] [CrossRef]
- Zheng, H.; Gao, J.; Man, S.; Zhang, J.; Jin, Z.; Gao, W. The protective effects of Aquilariae Lignum Resinatum extract on 5-Fuorouracil-induced intestinal mucositis in mice. Phytomedicine 2019, 54, 308–317. [Google Scholar] [CrossRef]
- Wang, C.; Wang, S.; Peng, D.; Yu, Z.; Guo, P.; Wei, J. Agarwood Extract Mitigates Intestinal Injury in Fluorouracil-Induced Mice. Biol. Pharm. Bull. 2019, 42, 1112–1119. [Google Scholar] [CrossRef]
- Wang, C.; Peng, D.; Liu, Y.; Wu, Y.; Guo, P.; Wei, J. Agarwood Alcohol Extract Protects against Gastric Ulcer by Inhibiting Oxidation and Inflammation. Evid.-Based Complementary Altern. Med. 2021, 2021, 9944685. [Google Scholar] [CrossRef]
- Rahman, H.; Eswaraiah, M.C.; Dutta, A. Anti-arthritic activity of leaves and oil of Aquilaria agallocha. Saudi J. Life Sci. 2016, 1, 34–43. [Google Scholar]
- Hamouda, A.F. A Biochemical Study of Agarwood on Methanol Injection in Rat. J. Drug Alcohol Res. 2019, 8, 1–14. [Google Scholar]
- Wang, S.; Wang, C.; Yu, Z.; Wu, C.; Peng, D.; Liu, X.; Liu, Y.; Yang, Y.; Guo, P.; Wei, J. Agarwood essential oil ameliorates restrain stress-induced anxiety and depression by inhibiting HPA axis hyperactivity. Int. J. Mol. Sci. 2018, 19, 3468. [Google Scholar] [CrossRef]
- Hamouda, A.F. A Pilot Study of Antistress Effects of Vitamin B Complex and Agarwood Extract in an Animal Model with Parallel Observations on Depression in Human Subjects. J. Drug Alcohol Res. 2021, 10, 3. [Google Scholar]
- Gądek-Michalska, A.; Tadeusz, J.; Rachwalska, P.; Bugajski, J. Cytokines, prostaglandins and nitric oxide in the regulation of stress-response systems. Pharmacol. Rep. 2013, 65, 1655–1662. [Google Scholar] [CrossRef]
- Alharbi, K.S.; Fuloria, N.K.; Fuloria, S.; Rahman, S.B.; Al-Malki, W.H.; Shaikh, M.A.J.; Thangavelu, L.; Singh, S.K.; Raju, V.S.R.; Jha, N.K. Nuclear factor-kappa B and its role in inflammatory lung disease. Chem. Biol. Interact. 2021, 345, 109568. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.R.; Bartlett, N.W.; Clarke, D.; Birrell, M.; Belvisi, M.; Johnston, S.L. Targeting the NF-κB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol. Ther. 2009, 121, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F. p38 mitogen-activated protein kinase pathways in asthma and COPD. Chest 2011, 139, 1470–1479. [Google Scholar] [CrossRef]
- Inoue, E.; Shimizu, Y.; Masui, R.; Tsubonoya, T.; Hayakawa, T.; Sudoh, K. Agarwood Inhibits Histamine Release from Rat Mast Cells and Reduces Scratching Behavior in Mice: Effect of Agarwood on Histamine Release and Scratching Behavior. J. Pharmacopunct. 2016, 19, 239–245. [Google Scholar] [CrossRef]
- Mokhtar, A.M.A.; Zain, H.H.M.; TA, M.M. Overview of Medicinal Properties and Toxicities of Agarwood Species. EDUCATUM J. Sci. Math. Technol. 2021, 8, 1–16. [Google Scholar]
| Place of Inflammation | Causes | Mediators | Consequences | Reference |
|---|---|---|---|---|
| Intestine | Infections | Campylobacter and Salmonella | Inflammatory bowel disease (IBD) | [32] |
| Food | Food with fatty acid compounds | Crohn’s disease | [33] | |
| Dysbiosis | Gut microbiome | Acute gastroenteritis, IBD | [34] | |
| Environmental factors | Smoking, nutrition, climate, pollution | IBD | [35] | |
| Stomach lining | Infections | H. pylori | Ulcerative colitis | [36] |
| NSAIDs | Reduced prostaglandin production due to inhibition of COX1 and COX2 | Colitis, IBD | [37] | |
| Psychological stress, | Increased acid load, effects of hypothalamic-pituitary-adrenal axis activation on healing, altered blood flow, or cytokine-mediated impairment of mucosal defenses | Peptic ulcer | [38] | |
| Physical stress like brain injury | Traumatic head injury can cause increased intracranial pressure and lead to overstimulation of the vagus nerve and increased secretion of gastric acid. | Cushing’s ulcer. | [39] | |
| Joint | Hyperuricemia | Increase uric acid deposition in joint | Gout (Joint inflammation) | [40] |
| Genetics | HLA-DRB1 alleles: HLA-DRB1*04, HLA-DRB1*01, and HLA-DRB1*10. | Rheumatoid arthritis | [40,41] | |
| Mutations in genes encoding types II, IV, V, and VI collagens | ||||
| Environmental/Diet factors | Smoking and alcohol intake | Rheumatoid arthritis | [42] | |
| Autoimmune | Anti-citrullinated protein/peptide antibodies | Rheumatoid arthritis | [42] | |
| Brain | Infections | Herpes simplex | Encephalitis | [43,44] |
| Human immune deficiency virus | ||||
| Autoimmune disorder | Anti-N-methyl-D-aspartate receptor (anti-NMDA) encephalitis | Autoimmune encephalitis | [45,46] | |
| Autoimmune Meningitis | ||||
| Ischemia | Blocking or narrowing of artery leading to brain | Vascular brain injury, Stroke | [47] | |
| Lung | Cigarette smoke | Components of cigarette smoke that mediate oxidative stress and inflammatory | Airway inflammation, COPD | [48] |
| Allergen | Increase inflammatory cytokines by allergens such as Ovalbumin | Airway inflammation, allergic asthma | [49] | |
| Air pollution | Particulate matter (PM) from traffic, industries, and ozone | Airway disease | [50] | |
| Infections | Influenza-induced exacerbation | Airway inflammation, Chronic lung disease | [51] | |
| Dysbiosis | Lung microbiome | Airway inflammation, Chronic lung disease | [52] | |
| Bushfire/Wildfire smoke | Complex mix of inspirable particles, volatile organics, aldehydes, carbon monoxide, and particulate matter (PM) | Airway inflammation, Chronic lung disease | [53] |
| Species | Agarwood-Producing | Distribution | Reference |
|---|---|---|---|
| Aquilaria acuminata | Yes | Thailand, Indonesia, Papua New Guinea, Philippines | [69] |
| Aquilaria apiculata | Yes | Philippines | [68,69] |
| Aquilaria baillonii | Yes | Cambodia, Laos, Thailand | [68,69] |
| Aquilaria banaensis | Yes | Vietnam | [68,69] |
| Aquilaria beccariana | Yes | Malaysia, Indonesia, Brunei | [68,69] |
| Aquilaria brachyantha | Yes | Malaysia, Philippines | [68,69] |
| Aquilaria citrincarpa | No | Philippines | [68,69] |
| Aquilaria crassna | Yes | Thailand, Vietnam, Laos, India, Cambodia, Malaysia | [68,69] |
| Aquilaria cumingiana | Yes | Indonesia, Philippines | [68,69] |
| Aquilaria filaria | Yes | Indonesia, Singapore, Malaysia, China, Philippines | [68,69] |
| Aquilaria grandiflora | Yes | China | [69] |
| Aquilaria hirta | Yes | Indonesia, Malaysia, Thailand, Singapore | [68,69] |
| Aquilaria khasiana | Yes | Bangladesh, India | [68,69] |
| Aquilaria malaccensis | Yes | Bhutan, Thailand, Malaysia, India, Vietnam, Bangladesh, Indonesia, Iran, Myanmar, Singapore, Philippines | [68,70] |
| Aquilaria microcarpa | Yes | Indonesia, Malaysia, Singapore | [68] |
| Aquilaria ophispermum | No | Indonesia | [68] |
| Aquilaria parvifolia | No | Philippines | [68] |
| Aquilaria pentandra | No | Bhutan, Laos, Thailand, Myanmar | [68] |
| Aquilaria rostrata | Yes | Malaysia | [68] |
| Aquilaria rugosa | No | Thailand, Vietnam | [68] |
| Aquilaria sinensis | Yes | China | [68,71] |
| Aquilaria subintegra | Yes | Malaysia, Thailand | [68] |
| Aquilaria urdanetensis | No | Philippines | [68] |
| Aquilaria yunnanensis | No | China | [68] |
| Type | Examples | Concept | Advantage | Disadvantage | Reference |
|---|---|---|---|---|---|
| Natural | Thunder strike | Wounds are created which then triggers the activation of the tree’s defense system, thereby producing resin | High-quality agarwood Does not require cultivation, plantation, and artificial induction No cost required and eco-friendly | Extremely low agarwood yield Unsustainable and undetermined where agarwood formation is a matter of chance Requires extremely long duration to produce high-quality agarwood Requires extensive and indiscriminate harvesting of wild trees | [73,76] |
| Animal grazing | |||||
| Pest and disease | |||||
| Broken branches | |||||
| Microbial invasion | |||||
| Artificial conventional | Physical wounding | Mimics natural factors by creating physical wounds on the trees which will then trigger the formation of agarwood via tree’s defense mechanism | Cost-effective Does not require personnel with specific knowledge in agarwood | Laborious Localized formation of agarwood only at the wounded area Agarwood formation correlates with the magnitude of induced injury Inferior quality of agarwood with an uncertain yield | [76] |
| Cauterizing | |||||
| Nailing | |||||
| Holing | |||||
| Bark removal | |||||
| Trunk pruning | |||||
| Burning-chisel-drilling | |||||
| Artificial biological | Fungal strains such as Melanotus flavolivens, Penicillium spp., Phytium spp., Lasiodiplodis spp., Botryodyplodis spp., and Fusarium spp. | Introduction of microbial cultures into Aquilaria trees to mimic its pathological infection, thereby triggering the tree’s defense mechanism | Eco-friendly and safe for handling Microbial cultures can be prepared at a low cost and are readily available | Long incubation time is required to produce high-quality agarwood | [73] |
| Time-consuming holing process for inoculating microbial cultures | |||||
| Inconsistency in agarwood quality depending on fungal species and site of inoculation | |||||
| Artificial chemical | Chemicals or signaling molecules such as ferric chloride, ferrous chloride, salicylic acid, sodium methyl bisulfide, hydrogen peroxide, formic acid, cellobiose, and methyl jasmonate | Direct induction of tree’s defense mechanism for the secretion of resin | Easy to apply with rapid action | An appropriate amount must be applied as an excess could kill the tree Skeptical impact on the environment and human health | [73] |
| Minimize the time required for holing processes | |||||
| Suitable for large scale plantations | |||||
| Ease of quality control | |||||
| High-quality agarwood with high and consistent yields | |||||
| Agarwood formation can be induced in the whole tree |
| Compound | Study Model | Anti-Inflammatory Outcomes | Reference | |
|---|---|---|---|---|
| Inflammatory Pathways | Key Findings | |||
| 2-(2-phenylethyl) chromone | In vitro study on RAW 264.7 cells. | Inhibit the activation of MAPK and STAT pathways. | Inhibit the production of NO, TNF-α, IL-6, IL-1β, PGE2. | [104] |
| In vitro study on RAW 264.7 cells. | Inhibit NF-κB activation. | Inhibit the production of NO. | [105] | |
| In vitro study on RAW 264.7 cells. | Not specified. | Inhibit the production of NO. | [106,107,108,109,110,111,112] | |
| Sesquiterpenoids | In vitro study on RAW 264.7 cells. | Not specified. | Inhibit the production of NO. | [17] |
| In vitro study on RAW 264.7 cells. | Not specified. | Inhibit the production of NO. | [113] | |
| In vitro study on RAW 264.7 cells. | Not specified. | Inhibit the production of NO. | [114] | |
| Others: β-caryophyllene | In vivo study on rats with paw edema induced with carrageenan. | Not specified. | Reduced edema in rat paws. | [115] |
| α-humulene | Ovalbumin induced mice model of allergic asthma | inhibition of the activation of p65 NF-kB and c-Jun AP-1 | reduction of eosinophils in the bronchoalveolar lavage fluid as well as inflammatory mediators such as IFN-γ, IL-5, CCL11, and LTB4 levels. Decrease in the production of IL-5 in the mediastinal lymph nodes, mucus secretions in the lungs. | [116] |
| Study Model(s) | Concentration | Study Duration | Anti-Inflammatory Outcomes | Reference | |
|---|---|---|---|---|---|
| Inflammatory Pathways | Key Findings | ||||
| In vivo and in vitro study on carrageenan-induced rat paw edema and HRBC stabilization method | In vivo: 50 and 100 mg/kg | In vivo: 4 h | Inhibition of the cyclooxygenase (COX) inflammatory pathway | Strong inhibition of rat paw edema. Inhibition of the release of prostaglandins. HRBC membrane stabilization. | [117] |
| In vitro: 100, 250, and 500 mcg/mL | Inhibition of cell membrane lysis induced by hypotonicity. | ||||
| In vivo study on carrageenan-induced rat paw edema and xylene-induced ear edema in mice | Mice: 60 to 960 mg/kg | Not specified | Inhibit the expression p-STAT3 gene. | Reduce the production of IL-1β and IL-6. | [118] |
| Rats: 680 mg/kg | |||||
| In vitro study on RAW 264.7 cells | Not specified | Not specified | Not specified. | Inhibit the release of TNF-α and IL-1α. | [18] |
| In vivo study on mice induced with ear inflammation and in silico studies: ADME and QSAR | In vivo: 20 uL/ear for 3 times | 24 h | Not specified. | Reduce inflammation in mice ears. | [119] |
| Inhibit the release of IL-1β, IL-6, and TNF-α. | |||||
| ADME and QSAR results corresponding to anti-inflammatory activity. | |||||
| In vivo study on rats with paw edema induced with carrageenan and with granuloma induced with cotton pellets | 50, 100 and 200 mg/kg | Carrageenan-induced paw edema: 3 h Cotton pellets-induced granuloma: 7 days | Not specified. | Inhibit the activity of prostaglandins (PGE2 and PGI2). | [120] |
| Reduced edema in rat paws. | |||||
| Smaller size granuloma compared to control group. | |||||
| In vitro study on hPBMCs | 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/mL | 24 h | Inhibit the p38 MAPK activation. | Inhibit the production of TNF-α. | [121] |
| In vivo study on mice with intestinal injury induced by 5-flurouracil | 200, 400, and 800 mg/kg | 7 days | Inhibiting the oxidative stress. | Less symptoms of intestinal inflammation. | [122] |
| Inhibiting the expression of inflammatory mediators. | Less tissue inflammation observed on histopathology and improved recovery. | ||||
| Inhibiting the NF-κB pathway. | Decreased levels of COX-2 and TNF-α inflammatory mediators in the intestinal cells. | ||||
| In vivo study on mice with intestinal injury induced by 5-flurouracil | 0.71, 1.42 and 2.84 g/kg | 14 days | Inhibiting oxidative stress. Inhibiting the mRNA expression of inflammatory pathways and mediators. | Improved body weight and intestinal propulsion. | [123] |
| Less mucosal injury. | |||||
| Decreased levels of NO and increased glutathione and superoxide dismutase activity. | |||||
| Decreased the levels of IL-17, IL-33, and increased IL-10. | |||||
| Inhibiting the NF-κB pathway | |||||
| In vivo study on mice with gastric ulcers induced by ethanol | 0.71, 1.42 and 2.84 g/kg | 7 days | Inhibiting oxidative stress. Inhibiting the mRNA expression of inflammatory pathways and mediators. | Protective effect against gastric ulcer and lesser degree of inflammation. | [124] |
| Decreased levels of IL-1β, IL-6, and increased level of IL-10. | |||||
| Inhibition of the NF-κB and p38 MAPK pathways. | |||||
| In vitro bovine serum protein (BSA) denaturation method and in vivo Freund’s-adjuvant-induced arthritic rat model | In vivo: 125 and 250 mg/kg | In vivo: 21 days | Inhibition of protein denaturation. | Reduced paw edema by gross observation and radiography. | [125] |
| In vitro: 100, 250 and 500 mcg/mL | Inhibition of inflammatory mediators. | Improved hematological parameters. | |||
| In vivo study on methanol induced inflammation in livers and brains of rats | 100 mg/kg | 35 days | Inhibit oxidative stress and apoptosis. | Inhibit the release of NO, MDA, ACHE, COX-2, LOX, TNF-α, Caspase-3, MAO, and DNAF neurotransmitters and pro-inflammatory mediators. | [126] |
| In vivo study on stress-induced anxiety and depression in rats | 10, 20 and 40 mg/kg | 10 days | Decreases the levels of IL-1α, IL-1β, and IL-6 in serum. Downregulated the iNOS in the cerebral cortex and hippocampus. | Antidepressant effect. | [127] |
| Anxiolytic effect. | |||||
| Decreased levels of ACTH and CORT serum. | |||||
| In vivo study on rats with stress-induced with epinephrine | 100 mg/kg | 21 days | Inhibition of cortisol production. | Reduced levels of lipid peroxidation, NO, TNF-α, IL-1β, cortisol, COX-2, LOX, AST, ALT, and lipids. | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamil, J.M.R.; Paudel, K.R.; Chan, Y.; Xenaki, D.; Panneerselvam, J.; Singh, S.K.; Gulati, M.; Jha, N.K.; Kumar, D.; Prasher, P.; et al. Rediscovering the Therapeutic Potential of Agarwood in the Management of Chronic Inflammatory Diseases. Molecules 2022, 27, 3038. https://doi.org/10.3390/molecules27093038
Alamil JMR, Paudel KR, Chan Y, Xenaki D, Panneerselvam J, Singh SK, Gulati M, Jha NK, Kumar D, Prasher P, et al. Rediscovering the Therapeutic Potential of Agarwood in the Management of Chronic Inflammatory Diseases. Molecules. 2022; 27(9):3038. https://doi.org/10.3390/molecules27093038
Chicago/Turabian StyleAlamil, Juman Mohammed Rasmi, Keshav Raj Paudel, Yinghan Chan, Dikaia Xenaki, Jithendra Panneerselvam, Sachin Kumar Singh, Monica Gulati, Niraj Kumar Jha, Deepak Kumar, Parteek Prasher, and et al. 2022. "Rediscovering the Therapeutic Potential of Agarwood in the Management of Chronic Inflammatory Diseases" Molecules 27, no. 9: 3038. https://doi.org/10.3390/molecules27093038
APA StyleAlamil, J. M. R., Paudel, K. R., Chan, Y., Xenaki, D., Panneerselvam, J., Singh, S. K., Gulati, M., Jha, N. K., Kumar, D., Prasher, P., Gupta, G., Malik, R., Oliver, B. G., Hansbro, P. M., Dua, K., & Chellappan, D. K. (2022). Rediscovering the Therapeutic Potential of Agarwood in the Management of Chronic Inflammatory Diseases. Molecules, 27(9), 3038. https://doi.org/10.3390/molecules27093038











