Effects of Two Natural Bisbenzylisoquinolines, Curine and Guattegaumerine, Extracted from Isolona hexaloba on Rhodamine Efflux by Abcb1b from Rat Glycocholic-Acid-Resistant Hepatocarcinoma Cells
Abstract
:1. Introduction
2. Results
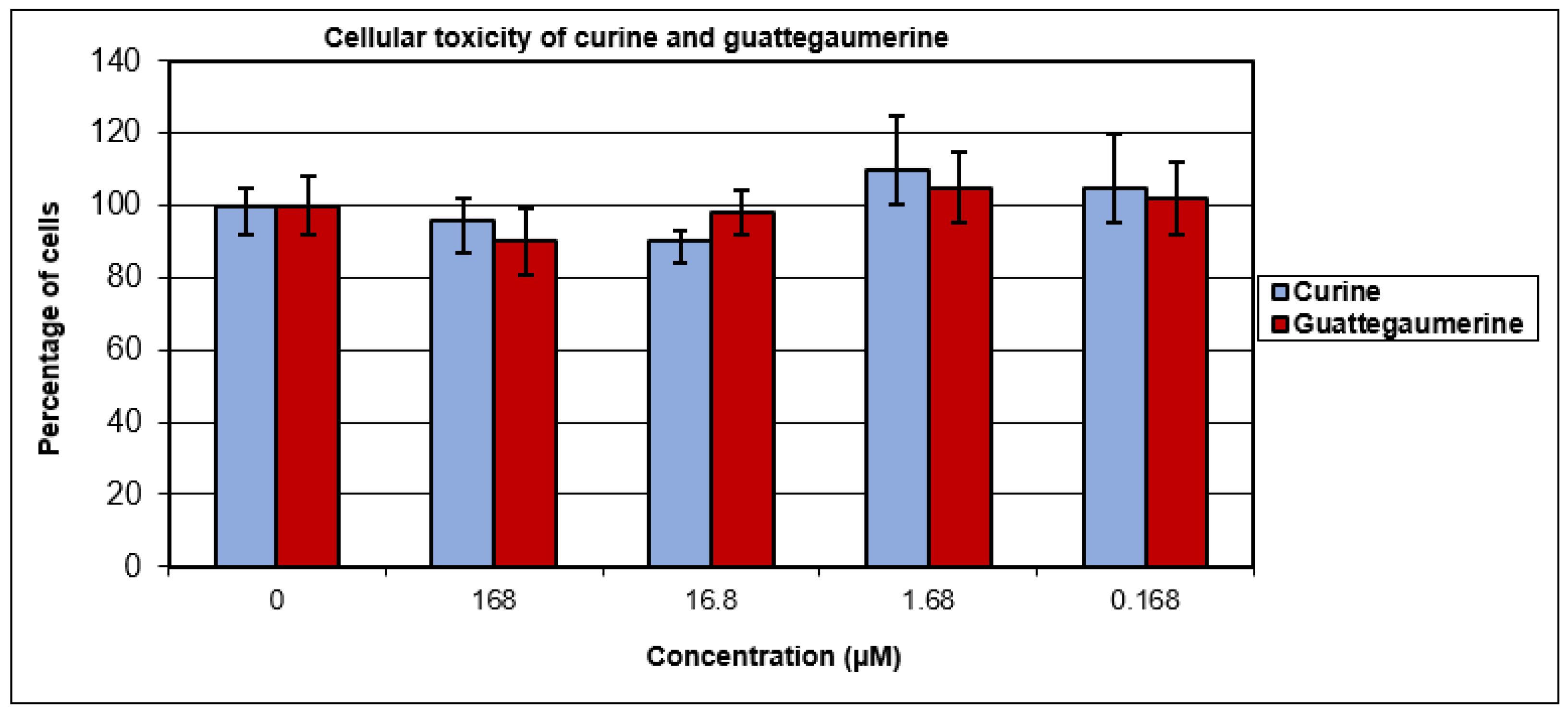
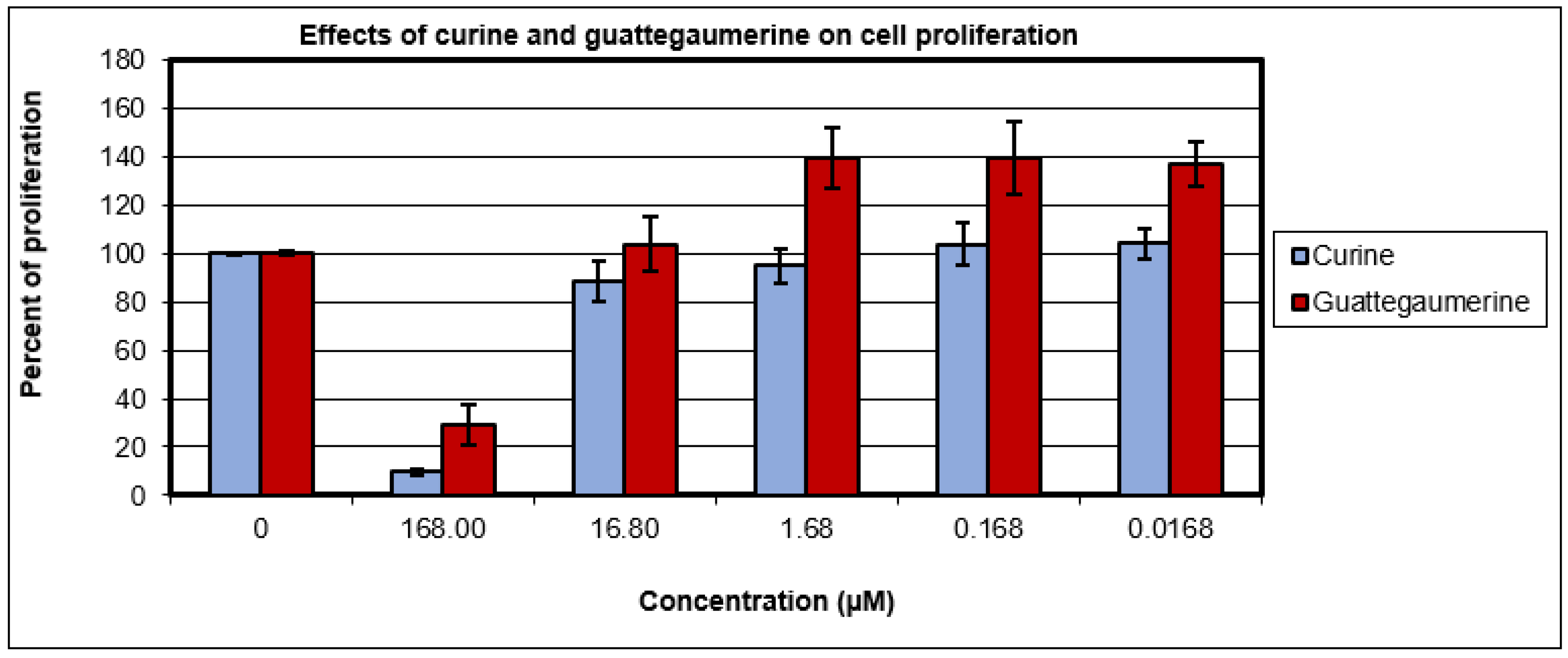
2.1. Evaluation of the Toxicity and Response of HTC-R Cells to Curine and Guattegaumerine Exposure
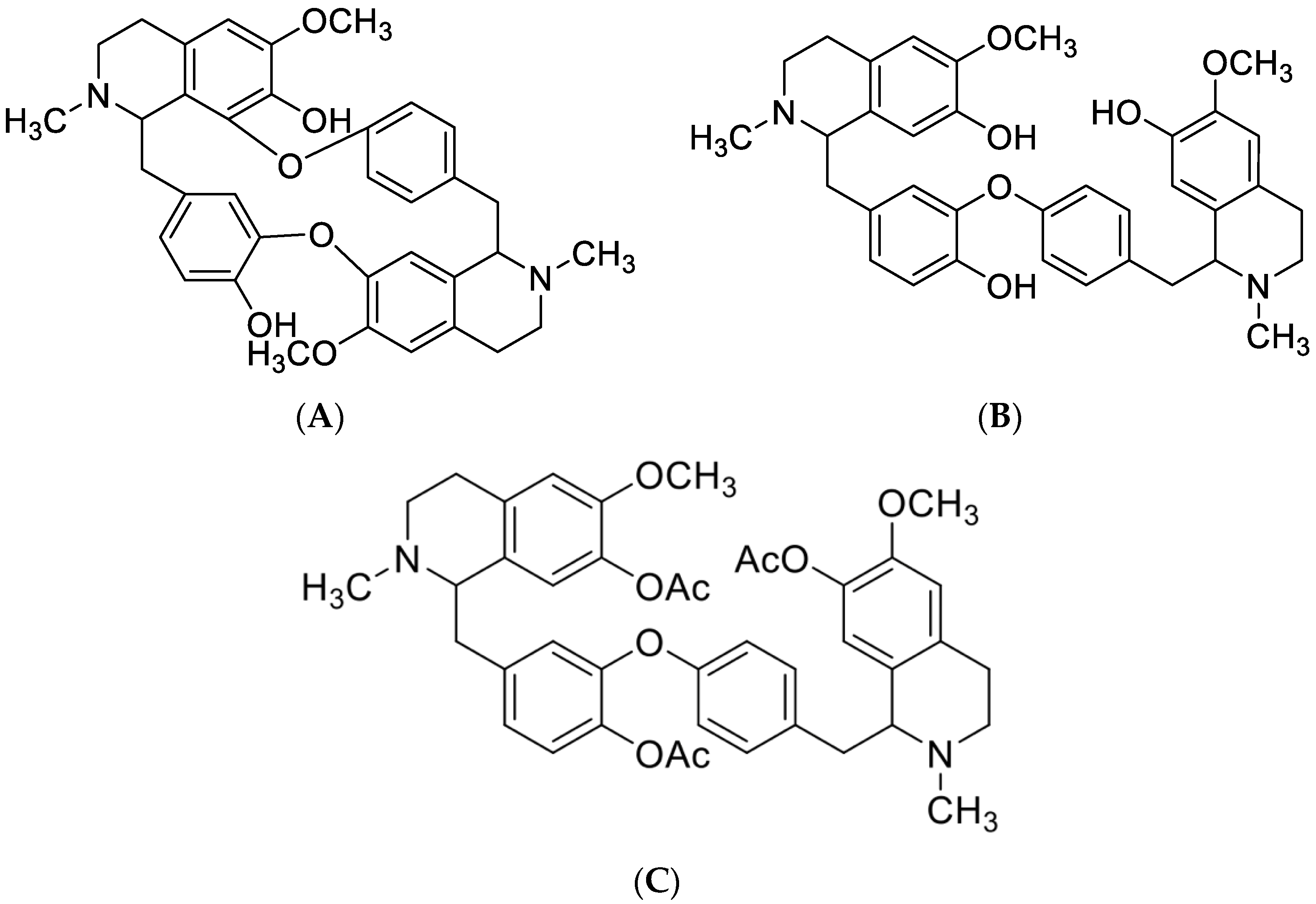
2.1.1. Structures of Compounds Isolated from Isolona hexaloba
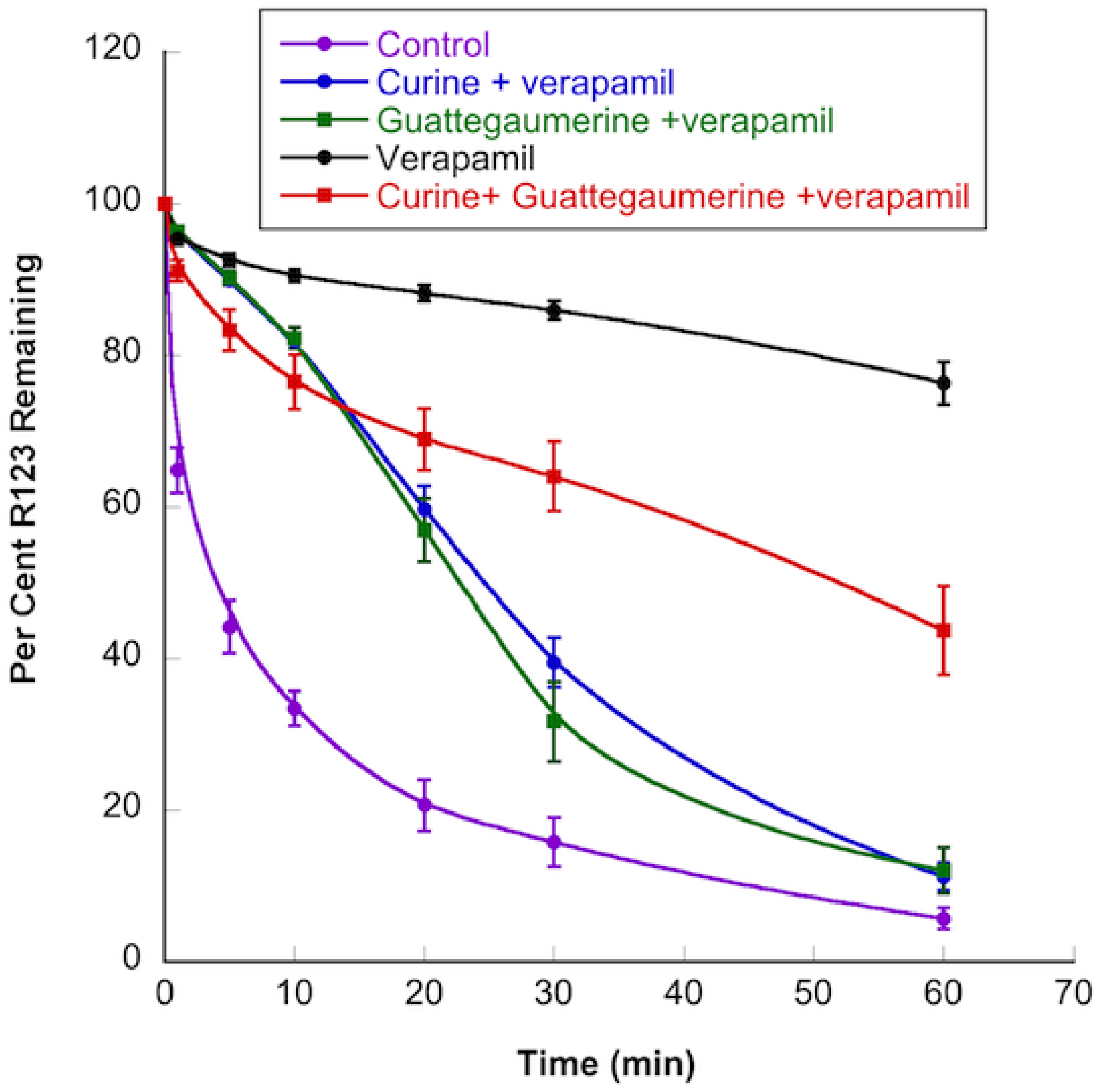
2.1.2. Effects of Curine and Guattegaumerine on Efflux Pumps
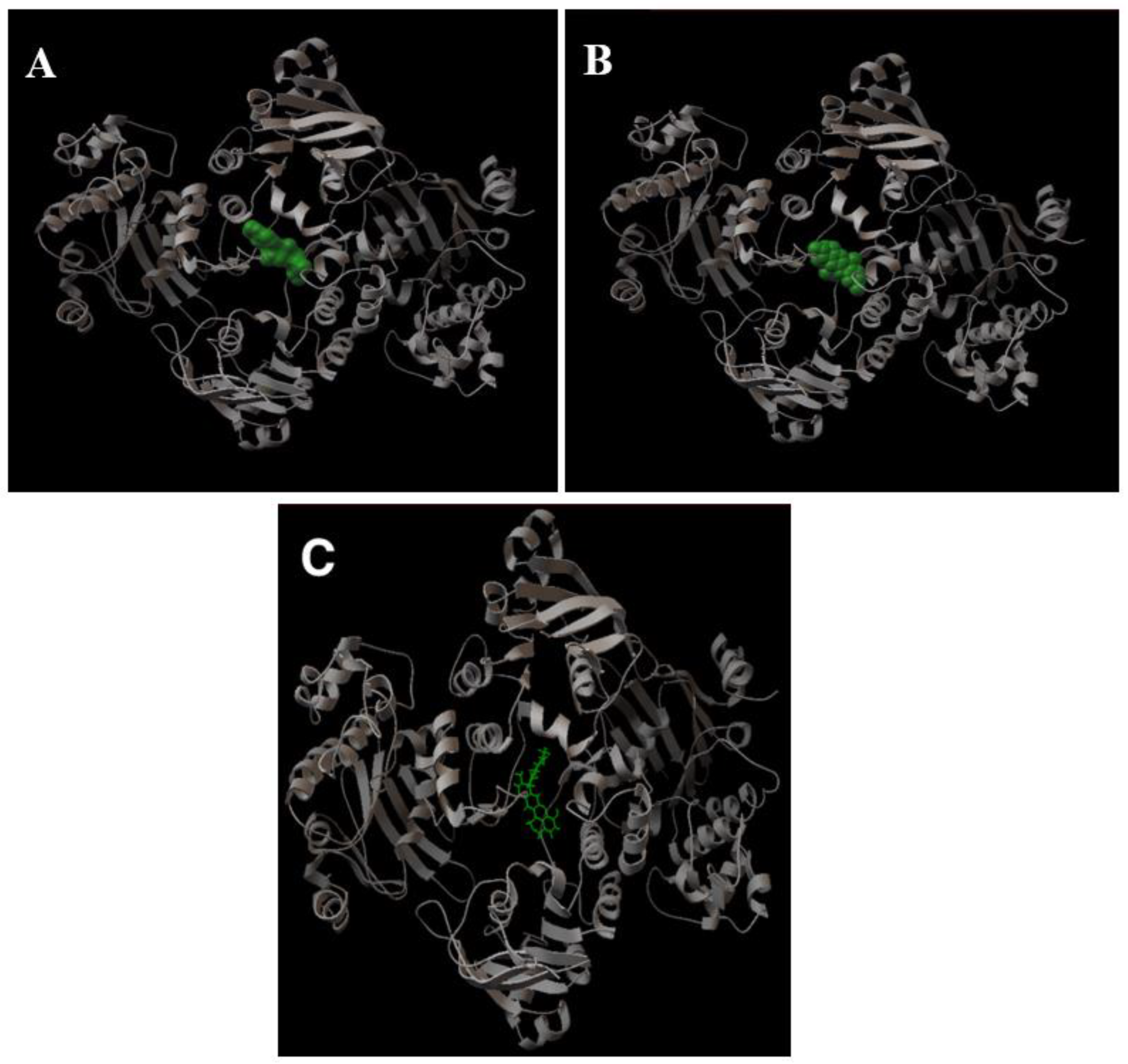
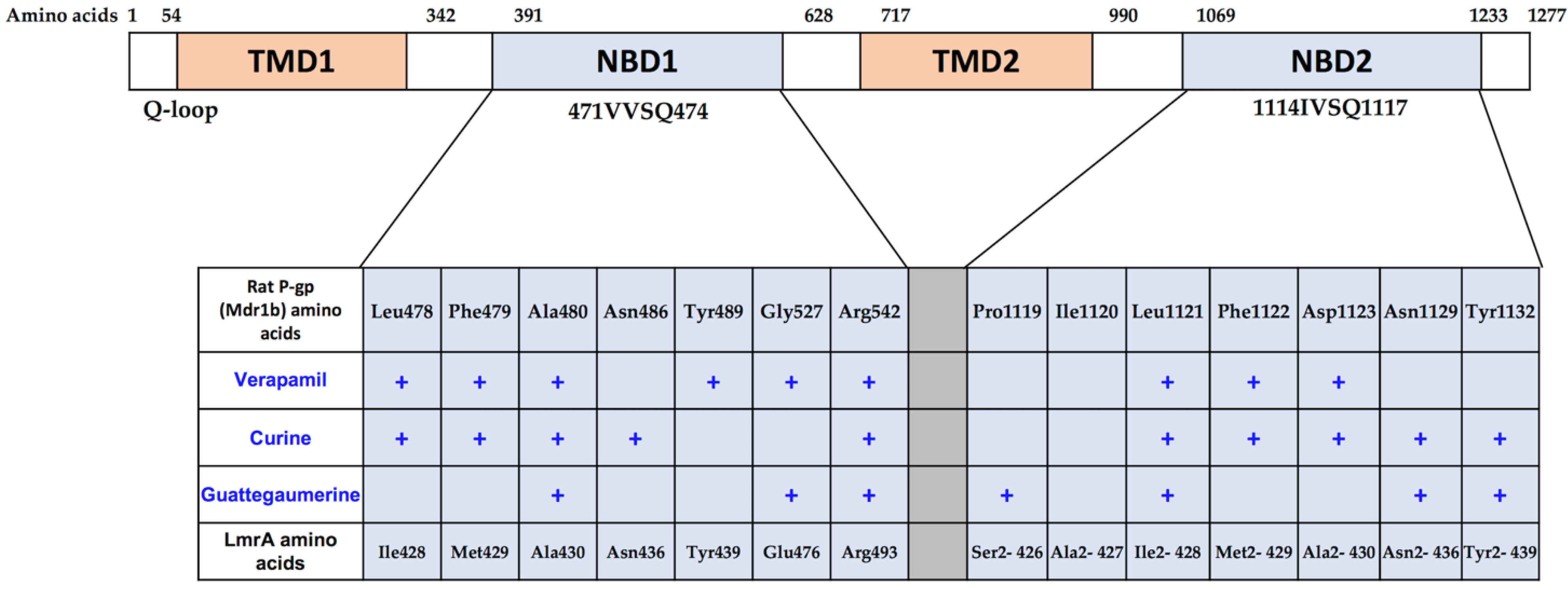
2.1.3. Determination of the Potential Drug Binding Sites through Docking Analysis
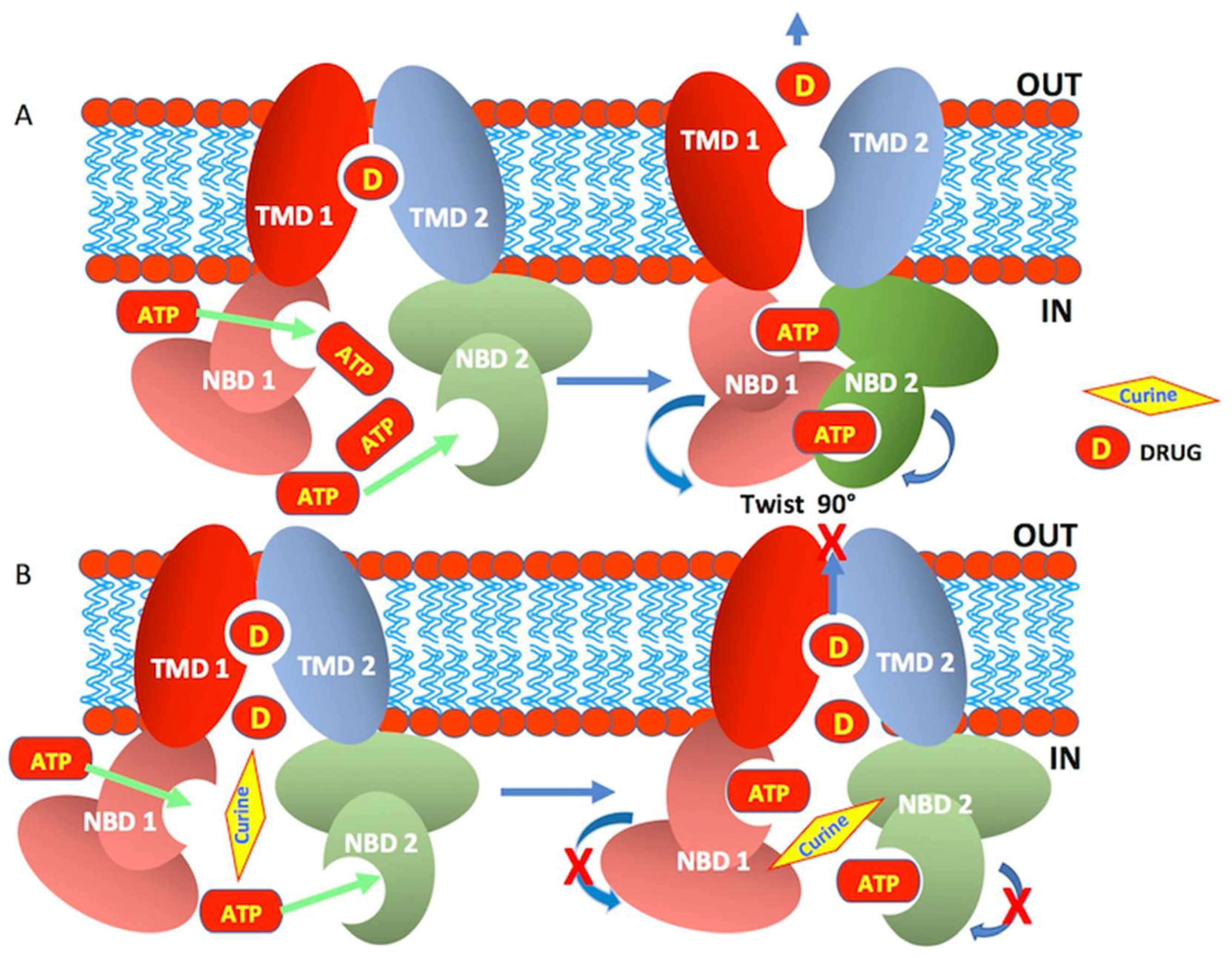
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals
4.3. Alkaloidal Extraction and Isolation of the Bisbenzylisoquinolines
4.4. Triacetylguattegaumerine (C) Preparation
4.5. Molecular Structure Determination
- Curine (A)
- Guattegaumerine (B)
- Triacetylguattegaumerine (C)
4.6. Cell Line and Culture Medium
4.7. Cellular Toxicity of Curine and Guattegaumerine
4.8. Effects of Curine and Guattegaumerine on Cell Proliferation
4.9. Effects of Curine, Guattegaumerine, and Triacetylguattegaumerine on Efflux Mediated by the P-gp Pump
4.10. Docking Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cronquist, A. The Evolution and Classification of Flowering Plants, 2nd ed.; New York Botanical Garden: Bronx, NY, USA, 1988; p. 555. [Google Scholar]
- Le Thomas, A. Flore du Gabon (Aubréville A.), Annonaceae; Muséum National d’Histoire Naturelle: Paris, France, 1969; p. 16. [Google Scholar]
- Makangara, J.J.; Henry, L.; Jonker, S.A.; Nunya, M.H.H. The caulindoles: Dimeric prenylindoles from Isolona cauliflora. Phytochemistry 2004, 65, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Boutique, R. Annonacées. In Flore du Congo Belge et du Ruanda-Urundi, II.; Botanic Garden Meise: Meise, Belgium, 1951; p. 260. [Google Scholar]
- Hocquemiller, R.; Cabalion, P.; Fournet, A. Annonaceae alkaloids XXIII: Bark alkaloids from Isolona campanulata Engler & Diels. Plant. Med. Phytother. 1978, 12, 230–234. [Google Scholar]
- Fournet, A.; Ferreira, M.E.; de Arias, A.R.; Schinini, A.; Nakayama, H.; Torres, S.; Sanabria, R.; Guinaudeau, H.; Bruneton, J. The effect of bisbenzylisoquinoline alkaloids on Trypanosoma cruzi infections in mice. Int. J. Antimicrob. Agents 1997, 8, 163–170. [Google Scholar] [CrossRef]
- Mambu, L.; Martin, M.-T.; Razafimahefa, D.; Ramanitrahasimbola, D.; Rasoanaivo, P.; Frappier, F. Spectral characterisation and antiplasmodial activity of bisbenzylisoquinolines from Isolona ghesquierei Cavaco and Kerauden. Planta Med. 2000, 66, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Gbogbo, M.; Zébré, C.A.; Connil, N.; Koné, M.; Kporou, E.K.; Yapo, P.A.; Feuilloley, M. Evaluation of the cytotoxicity of an Ivorian aphrodisiac of natural origin. World J. Adv. Res. Rev. 2021, 12, 261–267. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Litman, T.; Skovsgoard, T.; Stein, W.D. Pumping of drugs by P-glycoprotein: A two-step process. J. Pharmacol. Exp. Ther. 2003, 307, 846–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathouet, H.; Cazoulat, I.; Oulyadi, H.; Seguin, E.; Daïch, A.; Elomri, A. Cazolobine a new sesquiterpene from Isolona hexaloba (Annonaceae). Z. Nat. B 2004, 59, 1118–1120. [Google Scholar] [CrossRef]
- Mathouet, H.; Elomri, A.; Lameiras, P.; Daïch, A.; Vérité, P. An alkaloid, two conjugate sesquiterpenes and phenylpropanoid from Pachypodanthium confine Engl. and Diels. Phytochemistry 2007, 68, 1813–1818. [Google Scholar] [CrossRef]
- Marshall, S.J.; Russell, P.F.; Wright, C.W.; Anderson, M.M.; Phillipson, J.D.; Kirby, G.C.; Warhust, D.C.; Schiff, P.L., Jr. In vitro antiplasmodial, antiamoebic, and cytotoxic activities of a series of bisbenzylisoquinoline alkaloids. Antimicrob. Agents Chemother. 1994, 38, 96–103. [Google Scholar] [CrossRef] [Green Version]
- González-Coloma, A.; Reina, M.; Sáenz, C.; Lacret, R.; Ruiz-Mesia, L.; Arán, V.J.; Sanz, J.; Martínez-Díaz, R.A. Antileishmanial, antitrypanosomal, and cytotoxic screening of ethnopharmacologically selected Peruvian plants. Parasitol. Res. 2012, 110, 1381–1392. [Google Scholar] [CrossRef] [Green Version]
- Dias, C.S.; Barbosa-Filho, J.M.; Lemos, V.S.; Côrtes, S.F. Mechanisms involved in vasodilatator effect of curine in rat resistance arteries. Planta Med. 2002, 68, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, M.A.; Pinho, J.F.; De-Lira, D.P.; Barbosa-Filho, J.M.; Araújo, D.A.; Cortes, S.F.; Lemos, V.S.; Cruz, J.S. Curine, a bisbenzylisoquinoline alkaloid, blocks L-type Ca2+ channels and decreases intracellular Ca2+ transients in A7r5 cells. Eur. J. Pharmacol. 2011, 669, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, S.; Xu, D.; Zou, F.; Peng, R. (-)-Curine induces cell cycle arrest and cell death in hepatocellular carcinoma cells in a p53-independent way. Biomed. Pharmacother. 2017, 89, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Filho, J.; Carvalho Leite, F.; Surrage Calheiros, A.; de Brito Carneiro, A.; Alves Azeredo, J.; Fernandes de Assis, E.; da Silva Dias, C.; Piuvezam, M.R.; Bozza, P.T. Curine Inhibits Macrophage activation and neutrophil recruitment in a mouse model of Lipopolysaccharide-induced inflammation. Toxins 2019, 11, 705. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, J.; Quetin, J.; de Pauw-Gillet, M.-C.; Bassleer, R.; Angenot, L. Antimitotic and cytotoxic activities of guattegaumerine, a bisbenzylisoquinoline alkaloid. Planta Med. 1987, 53, 116–117. [Google Scholar] [CrossRef]
- Lü, Q.; Xu, X.; He, Z.; Huang, X.; Guo, L.; Wang, H. Guattegaumerine protects primary cultured cortical neurons against oxidative stress injury induced by Hydrogen Peroxide concomitant with serum deprivation. Cell. Mol. Neurobiol. 2009, 29, 355–364. [Google Scholar] [CrossRef]
- Eytan, G.D.; Regew, R.; Oren, G.; Hurwitz, C.D.; Assaraf, Y.G. Efficiency of P-glycoprotein-mediated exclusion of rhodamine dyes from multidrug-resistant cells is determined by their passive transmembrane movement rate. Eur. J. Biochem. 1997, 248, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Loo, T.W.; Clarke, D.M. Location of the rhodamine-binding site in the human multidrug resistance P-glycoprotein. J. Biol. Chem. 2002, 277, 44332–44338. [Google Scholar] [CrossRef] [Green Version]
- Ayesh, S.; Shao, Y.-M.; Stein, W.D. Co-operative, competitive and non-competitive interactions between modulators of P-glycoprotein. Biochim. Biophys. Acta 1996, 1316, 8–18. [Google Scholar] [CrossRef] [Green Version]
- Orlowski, S.; Mir, L.M., Jr.; Belehradek, J.; Garrigos, M. Effects of steroids and verapamil on P-glycoprotein ATPase activity: Progesterone, desoxycorticosterone, corticosterone and verapamil are mutually non-exclusive modulators. Biochem. J. 1996, 317, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, A.B.; Fox, K.; Lam, P.; Ling, V. Stimulation of P-glycoprotein-mediated drug transport by prazosin and progesterone. Evidence for a third drug-binding site. Eur. J. Biochem. 1999, 259, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S., Jr.; Lomri, N.; De Voss, J.; Rahmaoui, C.M.; Xie, M.H.; Hua, T.; Lidofsky, S.D.; Scharschmidt, B.F. Enhanced secretion of glycocholic acid in a specially adapted cell line is associated with overexpression of apparently novel ATP-binding cassette protein. Proc. Natl. Acad. Sci. USA 1995, 92, 5421–5425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Veen, H.W.; Callaghan, R.; Soceneantu, L.; Sardini, A.; Konings, W.N.; Higgins, C.F. A bacterial antibiotic-resistant gene that complement the human multidrug-resistance P-glycoprotein gene. Nature 1998, 391, 291–295. [Google Scholar] [CrossRef]
- van Veen, H.W.; Margolles, A.; Müller, M.; Higgins, C.F.; Konings, W.N. The homodimeric ATP-binding cassette transporter LmrA mediates multidrug transport by an alternating two-site (two-cylinder engine) mechanism. EMBO J. 2000, 19, 2503–2514. [Google Scholar] [CrossRef] [Green Version]
- Hocquemiller, R.; Cabalion, P.; Fournet, A.; Cave, A. Alcaloïdes des Annonacées XIIX: Alcaloïdes d’Isolona hexaloba Engler & Diels, I. zenkeri Engler and Diels et I. pilosa Diels. Planta Med. 1984, 50, 23–25. [Google Scholar]
- Becker, J.-P.; Depret, G.; van Bambeke, F.; Tulkens, P.M.; Prévost, M. Molecular models of human P-glycoprotein in two different catalytic states. BMC Struct. Biol. 2009, 9, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Orelle, C.; Gubellini, F.; Durand, A.; Marco, S.; Levy, D.; Di Pietro, A.; Jault, J.M. Conformational change induced by ATP binding in the multidrug ATP-binding cassette transporteur BmrA. Biochemistry 2008, 47, 2404–2412. [Google Scholar] [CrossRef]
- Zolnerciks, J.K.; Akkaya, B.G.; Snippe, M.; Chiba, P.; Seelig, A.; Linton, K.J. The Q loops of the human multidrug resistance transporter ABCB1 are necessary to couple drug binding to the ATP catalytic cycle. FASEB J. 2014, 28, 4335–4346. [Google Scholar] [CrossRef] [Green Version]
- Dalmas, O.; Orelle, C.; Foucher, A.-E.; Geourjon, C.; Crouzy, S.; di Pietro, A.; Jault, J.-M. The Q-loop disengages from the first intracellular loop during the catalytic cycle of the multidrug ABC transporter BmrA. J. Biol. Chem. 2005, 280, 36857–36864. [Google Scholar] [CrossRef] [Green Version]
- Baldas, J.; Porter, Q.N.; Bick, I.R.C.; Vernengo, M.J. Some observations on the mass-spectrometry of bisbenzylisoquinoline alkaloids. Tetrahedron Lett. 1966, 19, 2059–2068. [Google Scholar] [CrossRef]
- Dehaussy, H.; Tits, M.; Angenot, L. Guattegaumerine, new bisbenzylisoquinoline alkaloid from Guatteria gaumeri Greenm. Planta Med. 1983, 49, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Hulen, H.; Racine, P.-J.; Feuilloley, M.; Elomri, A.; Lomri, N. Effects of two natural bisbenzylisoquinolines, curine and guattegaumerine, extracted from Isolona hexaloba on the inhibition of ABC transporters from Pseudomonas aeruginosa MDR systems. Antibiotics, 2022; submitted. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergent, J.-A.; Mathouet, H.; Hulen, C.; Lameiras, P.; Feuilloley, M.; Elomri, A.; Lomri, N.-E. Effects of Two Natural Bisbenzylisoquinolines, Curine and Guattegaumerine, Extracted from Isolona hexaloba on Rhodamine Efflux by Abcb1b from Rat Glycocholic-Acid-Resistant Hepatocarcinoma Cells. Molecules 2022, 27, 3030. https://doi.org/10.3390/molecules27093030
Sergent J-A, Mathouet H, Hulen C, Lameiras P, Feuilloley M, Elomri A, Lomri N-E. Effects of Two Natural Bisbenzylisoquinolines, Curine and Guattegaumerine, Extracted from Isolona hexaloba on Rhodamine Efflux by Abcb1b from Rat Glycocholic-Acid-Resistant Hepatocarcinoma Cells. Molecules. 2022; 27(9):3030. https://doi.org/10.3390/molecules27093030
Chicago/Turabian StyleSergent, Jacques-Aurélien, Hilarion Mathouet, Christian Hulen, Pedro Lameiras, Marc Feuilloley, Abdelhakim Elomri, and Nour-Eddine Lomri. 2022. "Effects of Two Natural Bisbenzylisoquinolines, Curine and Guattegaumerine, Extracted from Isolona hexaloba on Rhodamine Efflux by Abcb1b from Rat Glycocholic-Acid-Resistant Hepatocarcinoma Cells" Molecules 27, no. 9: 3030. https://doi.org/10.3390/molecules27093030
APA StyleSergent, J.-A., Mathouet, H., Hulen, C., Lameiras, P., Feuilloley, M., Elomri, A., & Lomri, N.-E. (2022). Effects of Two Natural Bisbenzylisoquinolines, Curine and Guattegaumerine, Extracted from Isolona hexaloba on Rhodamine Efflux by Abcb1b from Rat Glycocholic-Acid-Resistant Hepatocarcinoma Cells. Molecules, 27(9), 3030. https://doi.org/10.3390/molecules27093030





