Apple Fibers as Carriers of Blackberry Juice Polyphenols: Development of Natural Functional Food Additives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Polyphenols
2.2. Antioxidant Activity and Inhibition of α-Amylase
2.3. Evaluation of Color Parameters
2.4. Volatile Compounds of Apple Fiber/Blackberry Juice Complexes
2.5. FTIR-ATR Analysis of Apple Fiber/Blackberry Juice Complexes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Apple Fiber/Blackberry Juice Complexes
3.3. Extraction of Complexes
3.4. Evaluation of Total Polyphenols and Proanthocyanidins Contents
3.5. High-Performance Liquid Chromatography (HPLC) Analysis
3.6. Determination of Antioxidant Activity
3.7. Inhibition of α-Amylase
3.8. Color Evaluation
3.9. Evaluation of Volatile Compounds
3.10. Fourier Transform Infrared with Attenuated Total Reflection (FTIR-ATR) Spectroscopy Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary fiber from underutilized plant resources—A positive approach for valorization of fruit and vegetable wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Bonarius, G.A.; Vieira, J.B.; van der Goot, A.J.; Bodnar, I. Rheological behaviour of fibre-rich plant materials in fat-based food systems. Food Hydrocoll. 2014, 40, 254–261. [Google Scholar] [CrossRef]
- Fu, J.-T.; Chang, Y.-H.; Shiau, S.-Y. Rheological, antioxidative and sensory properties of dough and Mantou (steamed bread) enriched with lemon fiber. LWT—Food Sci. Technol. 2015, 61, 56–62. [Google Scholar] [CrossRef]
- Da Silva, L.C.; Viganó, J.; de Souza Mesquita, L.M.; Baião Dias, A.L.; de Souza, M.C.; Sanches, V.L.; Chaves, J.O.; Pizani, R.S.; Contieri, L.S.; Rostagno, M.A. Recent advances and trends in extraction techniques to recover polyphenols compounds from apple by-products. Food Chem. X 2021, 12, 100133. [Google Scholar] [CrossRef]
- Martins, F.C.O.L.; Sentanin, M.A.; de Souza, D. Analytical methods in food additives determination: Compounds with functional applications. Food Chem. 2018, 272, 732–750. [Google Scholar] [CrossRef]
- Liddle, D.M.; Lin, X.; Cox, L.C.; Ward, E.M.; Ansari, R.; Wright, A.J.; Robinson, L.E. Daily apple consumption reduces plasma and peripheral blood mononuclear cell–secreted inflammatory biomarkers in adults with overweight and obesity: A 6-week randomized, controlled, parallel-arm trial. Am. J. Clin. Nutr. 2021, 114, 752–763. [Google Scholar] [CrossRef]
- Faustino, M.; Veiga, M.; Sousa, P.; Costa, E.M.; Silva, S.; Pintado, M. Agro-food byproducts as a new source of natural food additives. Molecules 2019, 24, 1056. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.A.; Bierwirth, J.E.; Greenspan, P.; Pegg, R.B. Blackberry polyphenols: Review of composition, quantity, and health impacts from in vitro and in vivo studies. J. Food Bioact. 2020, 9, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compost. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Costa, T.S.; Rogez, H.; Pena, R.S. Adsorption capacity of phenolic compounds onto cellulose and xylan. Food Sci. Technol. 2015, 35, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Fernández-Prior, Á.; Vioque, B.; Fernández-Bolaños, J. Strawberry dietary fiber functionalized with phenolic antioxidants from olives. Interactions between polysaccharides and phenolic compounds. Food Chem. 2018, 280, 310–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rubio-Senent, F.; Lama-Muñoz, A.; Fernández-Bolañoz, J. Complexation of hydroxytyrosol and 3,4,-dihydroxyphenylglycol with pectin and their potential use for colon targeting. Carbohydr. Polym. 2017, 163, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Oria, A.; Rodríguez-Gutiérrez, G.; Rodríguez-Juan, E.; González-Benjumea, A.; Fernández-Bolaños, J. Molecular interactions between 3,4-dihyroxyphenylglycol and pectin and antioxidant capacity of this complex in vitro. Carbohydr. Polym. 2018, 197, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.C.; Baron, A.; Guyot, S.; Drilleau, J.-F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Melton, L.D.; O’Connor, C.J.; Kilmartin, P.A.; Smith, B.G. Effect of apple cell walls and their extracts on the activity of dietary antioxidants. J. Agric. Food Chem. 2008, 56, 289–295. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Smith, B.G.; O’Connor, C.J.; Melton, D.L. Effect of raw and cooked onion dietary fiber on the antioxidant activity of ascorbic acid and quercetin. Food Chem. 2008, 111, 580–585. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M. Binding of polyphenols to plant cell wall analogues—Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M. Binding of polyphenols to plant cell wall analogues—Part 2: Phenolic acids. Food Chem. 2012, 135, 2287–2292. [Google Scholar] [CrossRef]
- Vukoja, J.; Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Formulation and stability of cellulose-based delivery systems of raspberry phenolics. Processess 2021, 9, 90. [Google Scholar] [CrossRef]
- Papoutsis, K.; Golding, J.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.; Scarlett, C.; Bowyer, M. Encapsulation of citrus by-product extracts by spray-drying and freeze-drying using combinations of maltodextrin with soybean protein and ι-carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; Hui, X.; Stipkovits, L.; Rachman, A.; Tu, J.; Brennan, M.A.; Brennan, C.S. Whey protein-blackcurrant concentrate particles obtained by spray-drying and freeze-drying for delivering structural and health benefits of cookies. Innov. Food Sci. Emerg. Technol. 2021, 68, 102606. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Watrelot, A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Watrelot, A.A.; Ginies, C.; Imberty, A.; Renard, C.M.G.C. Impact of processing on the non-covalent interactions between procyanidins and cell-walls. J. Agric. Food Chem. 2012, 60, 9484–9494. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M.G.C. Non-covalent interactions between procyanidins and apple cell-wall material. Part II: Quantification and impact of cell-wall drying. Biochim. Biophys. Acta 2005, 1725, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Polyphenols and antioxidant activity of citrus fiber/blackberry juice complexes. Molecules 2021, 26, 4400. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kwon, S.; Jo, Y.; Jin, C.H.; Nam, B.; Lee, S.Y.; Jeong, S.; Im, S.; Oh, S.C.; Cho, L.; et al. Comparison of phytochemicals and antioxidant activity in blackberry (Rubus fruticosus L.) fruits of mutant lines at the different harvest time. Plant Breed. Biotechnol. 2016, 4, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Conversion of rutin to quercetin by acid treatment in relation to biological activities. Prev. Nutr. Food Sci. 2019, 24, 313–320. [Google Scholar] [CrossRef]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2011, 59, 43–49. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Netzel, G.; Wang, D.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding of dietary polyphenols to cellulose: Structural and nutritional aspects. Food Chem. 2015, 171, 388–396. [Google Scholar] [CrossRef]
- Larsen, L.R.; Buerschaper, J.; Schieber, A.; Weber, F. Interactions of anthocyanins with pectin and pectin fragments in model solutions. J. Agric. Food Chem. 2019, 67, 9344–9353. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.D.T.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding selectivity of dietary polyphenols to different plant cell wall components: Quantification and mechanism. Food Chem. 2017, 233, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Matić, P.; Ištuk, J.; Barron, A.R. Study of interactions between individual phenolics of aronia with barley beta-glucan. Pol. J. Food Nutr. Sci. 2021, 71, 187–196. [Google Scholar] [CrossRef]
- Thyavihalli Girijappa, Y.G.; Mavinkere Rangappa, S.; Parameswaranpillai, J.; Siengchin, S. Natural fibers as sustainable and renewable resource for development of eco-friendly composites: A comprehensive review. Front. Mater. 2019, 6, 226. [Google Scholar] [CrossRef]
- Sobczak, P.; Nadulski, R.; Kobus, Z.; Zawiślak, K. Technology for Apple Pomace Utilization within a Sustainable Development Policy Framework. Sustainability 2022, 14, 5470. [Google Scholar] [CrossRef]
- Da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Rutz, J.K.; da Luz, S.R.; Krumreich, F.D.; Benvenutti, E.V.; Nunes, M.R. Encapsulation of the phenolic compounds of the blackberry (Rubus fruticosus). LWT—Food Sci. Technol. 2014, 58, 527–533. [Google Scholar] [CrossRef]
- Figueiredo-González, M.; Grosso, C.; Valentão, P.; Andrade, P.B. α-Glucosidase and α-amylase inhibitors from Myrcia spp.: A stronger alternative to acarbose? J. Pharm. Biomed. Anal. 2016, 118, 322–327. [Google Scholar] [CrossRef]
- Aleixandre, A.; Gil, J.V.; Sineiro, J.; Rosell, C.M. Understanding phenolic acids inhibition of α-amylase and α-glucosidase and influence of reaction conditions. Food Chem. 2022, 372, 131231. [Google Scholar] [CrossRef]
- Gao, J.; Xu, P.; Wang, Y.; Wang, Y.; Hochstetter, D. Combined effects of green tea extracts, green tea polyphenols or epigallocatechin gallate with acarbose on inhibition against α-amylase and α-glucosidase in vitro. Molecules 2013, 18, 11614–11623. [Google Scholar] [CrossRef] [Green Version]
- Dalar, A.; Konczak, I. Phenolic contents, antioxidant capacities and inhibitory activities against key metabolic syndrome relevant enzymes of herbal teas from Eastern Anatolia. Ind. Crops Prod. 2013, 44, 383–390. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Konczak, I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012, 134, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Apostolidis, E.; Shetty, K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglycemia linked to type 2 diabetes. J. Food Biochem. 2008, 32, 15–31. [Google Scholar] [CrossRef]
- Jayaraj, S.; Suresh, S.; Kadeppagari, R.-K. Amylase inhibitors and their biomedical applications. Starke 2013, 65, 535–542. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Ibrahim, A.; Dahiru, N.J.; Mohammed, H.S. Alpha amylase inhibitory potential and mode of inhibition of oils from Allium sativum (Garlic) and Allium cepa (Onion). Clin. Med. Insights Endocrinol. Diabetes 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by-product extract by freeze-drying. LWT 2017, 84, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Estupiñan, D.C.; Schwartz, S.J.; Garzón, G.A. Antioxidant activity, total phenolics content, anthocyanin, and color stability of isotonic model beverages colored with andes berry (Rubus glaucus Benth) anthocyanin powder. J. Food Sci. 2011, 76, S26–S34. [Google Scholar] [CrossRef] [Green Version]
- Vukoja, J.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Cellulose as a delivery system of raspberry juice volatiles and their stability. Molecules 2020, 25, 2624. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Liu, X.; Renard, C.M.G.C.; Bureau, S.; Le Bourvellec, C. Revisiting the contribution of ATR-FTIR spectroscopy to characterize plant cell wall polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the bulk characterization of cell wall residues of fruits and vegetables along a fraction process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, O.; Amin, N.K. Adsorption of phenol from aqueous solutions by Luffa cylindrica fibers: Kinetics, isotherm and thermodynamic studies. Egypt. J. Aquat. Res. 2013, 39, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Prior, R.L.; Fan, E.; Ji, H.; Howell, A.; Nio, C.; Payne, M.J.; Reed, J. Multi-laboratory validation of a standard method for quantifying proanthocyanidins in cranberry powders. J. Sci. Food Agric. 2010, 90, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1994, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, S.M.; Koehnlein, E.A.; Bracht, A.; Castoldi, R.; de Morais, G.R.; Baesso, M.L.; Peralta, R.A.; de Souza, C.G.M.; de Sá-Nakanishi, A.B.; Peralta, R.M. Inhibition of salivary and pancreatic α-amylases by a pinhão coat (Araucaria angustifolia) extract rich in condensed tannin. Food Res. Int. 2014, 56, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kellogg, J.; Grace, M.H.; Lila, M.A. Phlorotannins from alaskan seaweed inhibit carbolytic enzyme activity. Mar. Drugs 2014, 12, 5277–5294. [Google Scholar] [CrossRef]
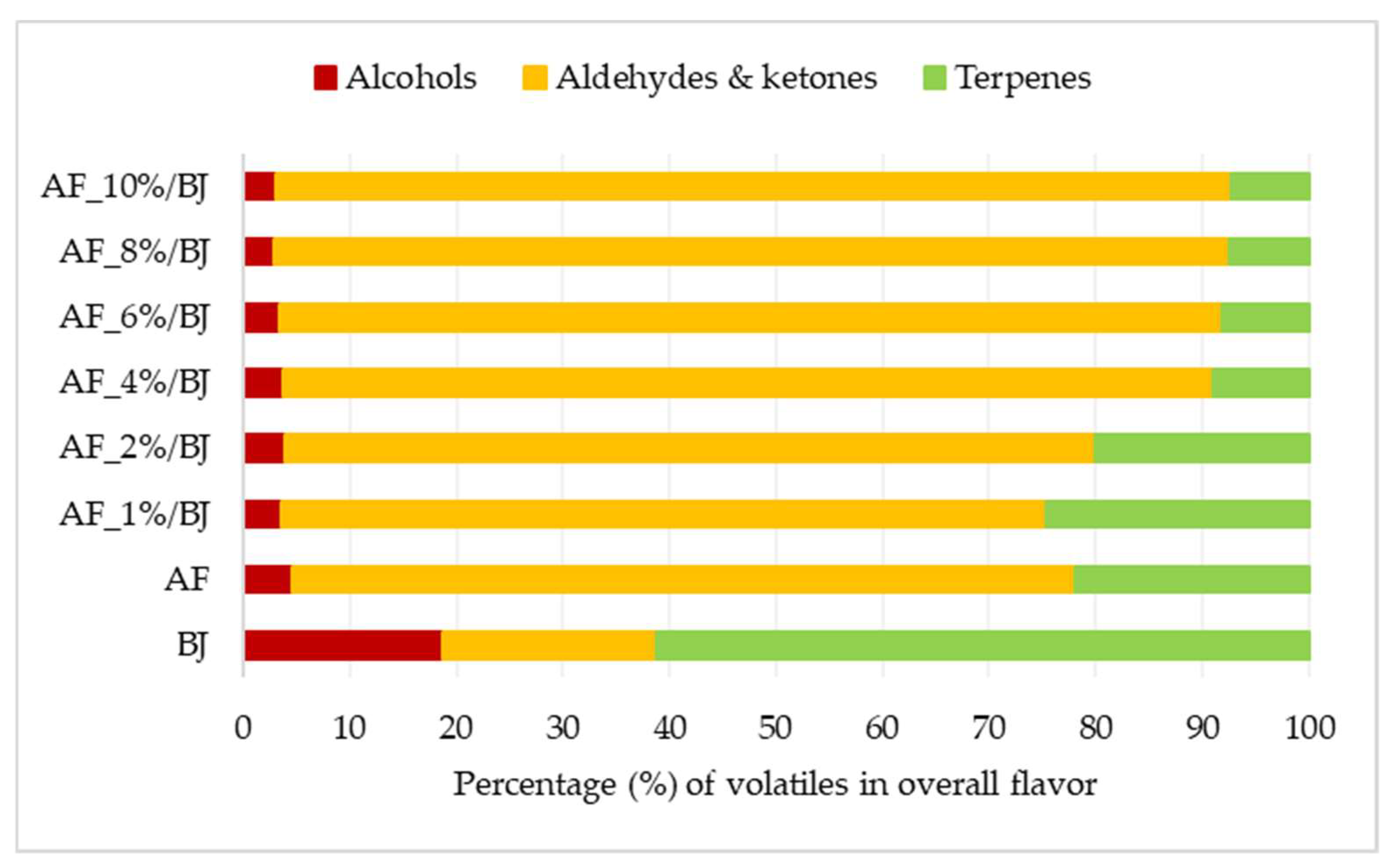
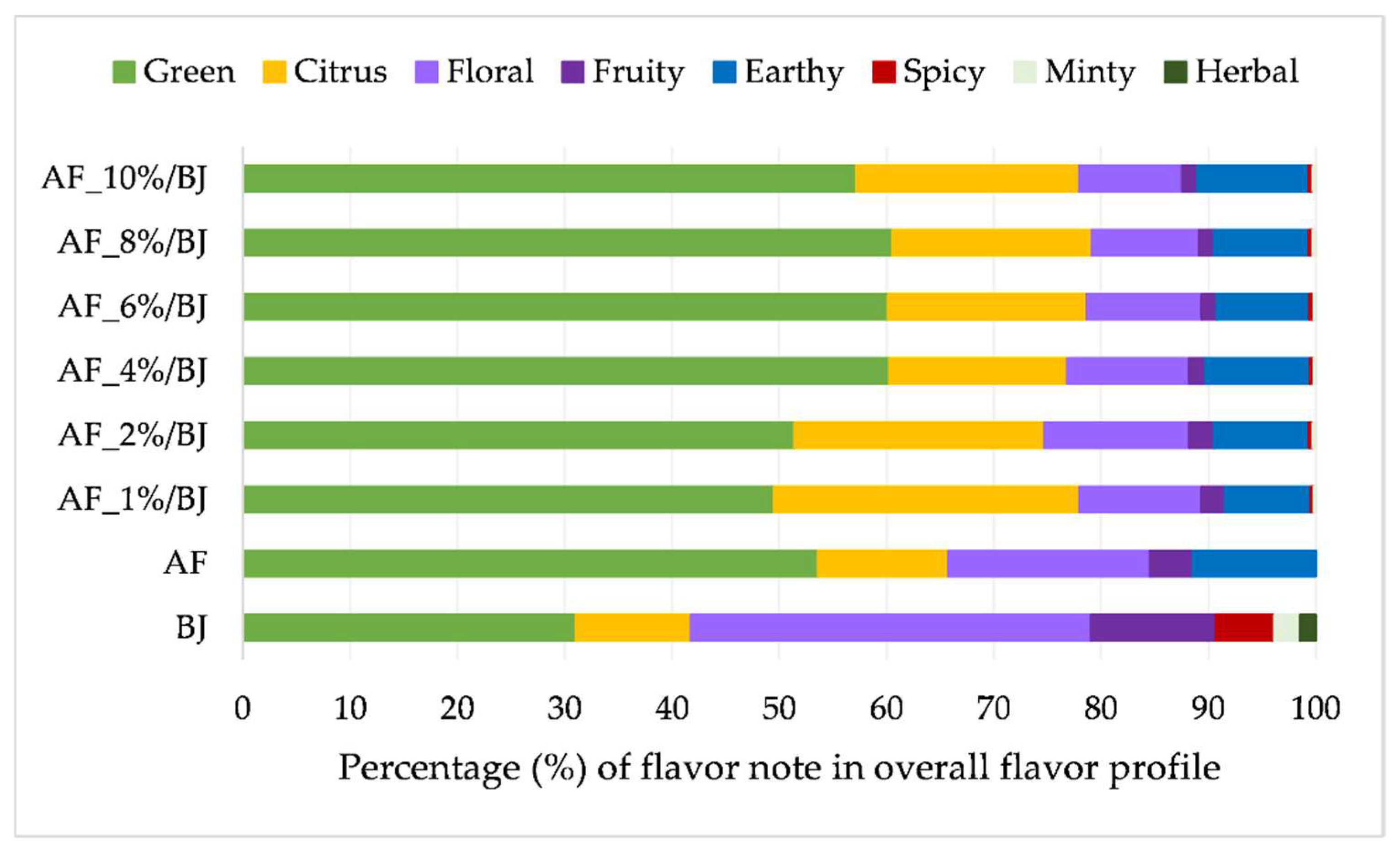
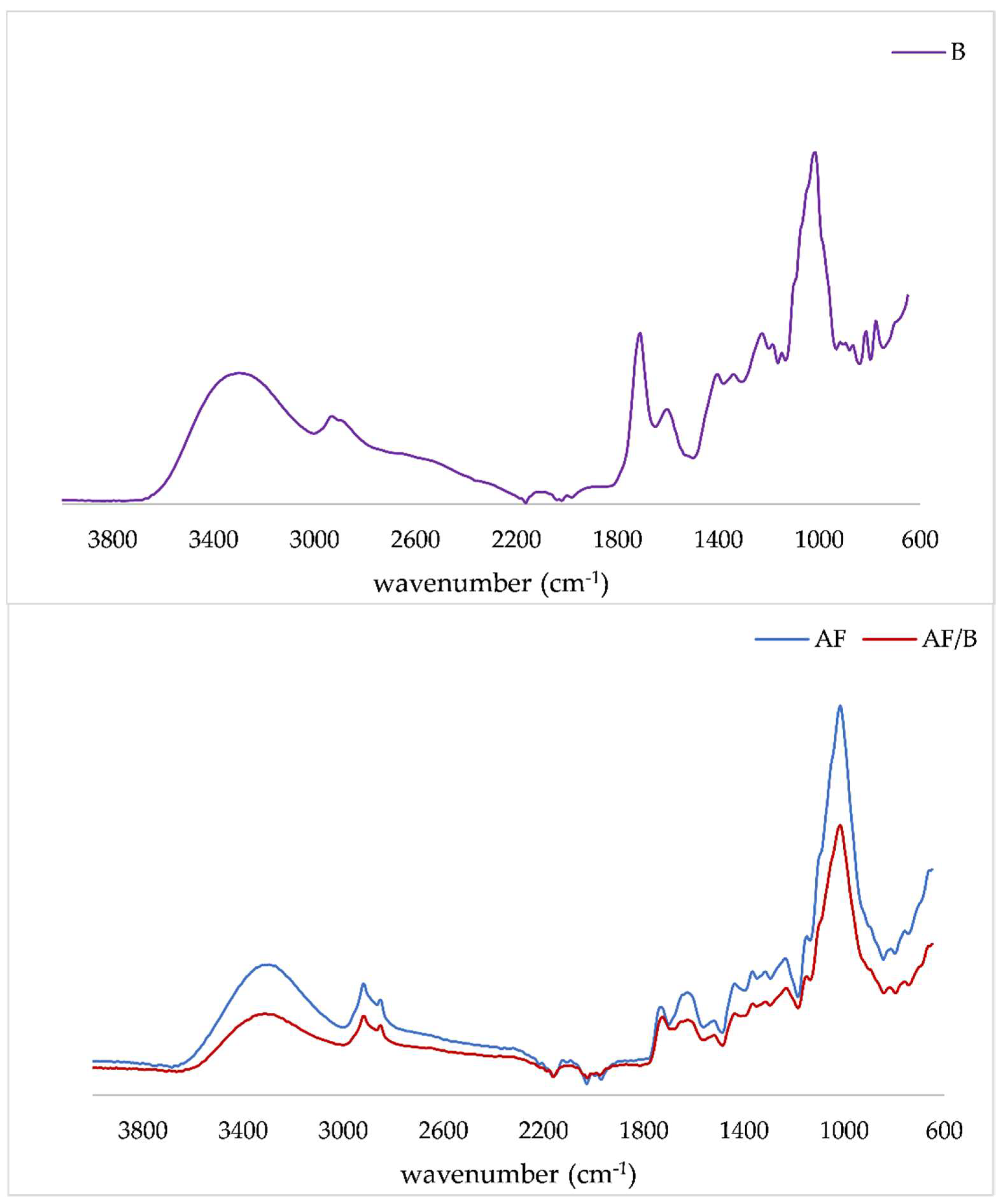
| TPC (g GAE/100 g) | PAC (mg PB2E/100 g) | |
|---|---|---|
| AF_1%/BJ | 1.82 ± 0.00 a | 98.85 ± 1.35 a |
| AF_2%/BJ | 1.79 ± 0.01 b | 93.51 ± 2.93 a |
| AF_4%/BJ | 1.50 ± 0.01 c | 88.56 ± 0.47 b |
| AF_6%/BJ | 1.28 ± 0.02 d | 85.39 ± 2.68 b |
| AF_8%/BJ | 1.25 ± 0.01 e | 73.47 ± 1.96 c |
| AF_10%/BJ | 1.18 ± 0.01 f | 57.43 ± 2.71 d |
| Blackberry Juice | Apple Fiber | ||
|---|---|---|---|
| Individual Polyphenols (mg/100 g) | |||
| C 3-G | 339.8 ± 0.40 | Que | 130.60 ± 5.38 |
| C 3-DG | 118.8 ± 0.03 | Rut | 9.54 ± 1.14 |
| Que | 22.7 ± 0.05 | ChA | 51.64 ± 2.70 |
| Rut | 3.7 ± 0.00 | HC-1 | 17.36 ± 0.13 |
| EA | 27.35 ± 0.00 | HC-2 | 15.72 ± 0.02 |
| CA | 3.8 ± 0.00 | P | 17.64 ± 0.48 |
| ChA | 31.55 ± 0.03 | Ph | 78.21 ± 0.60 |
| p-CA | 41.1 ± 0.00 | ||
| GA | 36.3 ± 0.01 | ||
| TPC (mg GAE/100 g) | 58.33 ± 0.05 | 1136.76 ± 15.50 | |
| PAC (mg PB2E/100 g) | 2.07 ± 0.05 | 153.69 ± 1.65 | |
| ABTS (µmol TE/100 g) | 5.82 ± 0.02 | 50.37 ± 0.46 | |
| DPPH (µmol TE/100 g) | 2.33 ± 0.01 | 54.30 ± 0.34 | |
| CUPRAC (µmol TE/100 g) | 24.04 ± 0.68 | 532.80 ± 15.99 | |
| FRAP (µmol TE/100 g) | 0.47 ± 0.00 | 6.94 ± 0.11 | |
| AF_1%/BJ | AF_2%/BJ | AF_4%/BJ | AF_6%/BJ | AF_8%/BJ | AF_10%/BJ | |
|---|---|---|---|---|---|---|
| Anthocyanins | ||||||
| C 3-G | 208.01 ± 1.56 b | 219.63 ± 2.85 a | 152.88 ± 2.24 c | 131.19 ± 0.51 d | 109.49 ± 1.32 e | 103.72 ± 0.05 f |
| C 3-DG | 54.35 ± 0.38 b | 57.81 ± 0.93 a | 43.58 ± 0.70 c | 39.02 ± 0.04 d | 33.60 ± 0.04 e | 31.94 ± 0.04 f |
| Total | 262.36 ± 1.94 b | 277.44 ± 3.78 a | 196.46 ± 2.94 c | 170.21 ± 0.55 d | 143.09 ± 1.36 e | 135.66 ± 0.09 e |
| Flavanols | ||||||
| Que | 55.60 ± 0.41 f | 70.39 ± 0.88 e | 80.73 ± 0.67 d | 89.87 ± 0.87 c | 94.14 ± 0.26 b | 96.82 ± 0.55 a |
| Phenolic acids | ||||||
| EA | 37.97 ± 0.38 a | 35.82 ± 0.29 b | 23.71 ± 0.17 c | 20.51 ± 0.03 d | 15.53 ± 0.32 e | 15.25 ± 0.02 e |
| ChA | 17.42 ± 0.05 f | 19.60 ± 0.02 e | 21.84 ± 0.18 d | 23.99 ± 0.01 c | 24.65 ±0.28 b | 26.07 ± 0.08 a |
| HC-1 | 18.00 ± 0.16 c,d | 18.14 ± 0.16 b,d | 18.26 ± 0.01 b | 18.73 ± 0.10 a | 18.25 ± 0.08 b,c | 18.41 ± 0.02 b |
| HC-2 | 16.46 ± 0.04 a,b | 16.22 ± 0.33 b,c | 16.34 ± 0.04 a,c | 16.60 ± 0.02 a | 16.10 ± 0.03 c | 16.10 ± 0.00 c |
| Total | 89.85 ± 0.63 a | 89.78 ± 0.80 a | 80.15 ± 0.40 b | 79.83 ± 0.16 b | 74.53 ± 0.71 c | 75.83 ± 0.12 c |
| Dihydrochalcones | ||||||
| P | 4.06 ± 0.06 f | 5.49 ± 0.02 e | 7.57 ± 0.22 d | 9.03 ± 0.26 c | 9.86 ± 0.32 b | 11.26 ± 0.39 a |
| Ph | 15.23 ± 0.03 f | 22.47 ± 0.18 e | 30.99 ± 0.64 a | 36.27 ± 0.11 c | 38.80 ± 0.42 b | 41.86 ± 0.30 a |
| Total | 19.29 ± 0.09 f | 27.96 ± 0.20 e | 38.56 ± 0.86 d | 45.30 ± 0.37 c | 48.66 ± 0.74 b | 53.12 ± 0.69 a |
| TOTAL | 427.10 ± 3.07 b | 465.57 ± 5.66 a | 395.90 ± 4.87 c | 385.21 ± 1.95 c | 360.42 ± 3.07 d | 361.43 ± 1.45 d |
| ABTS (µmol TE/100 g) | DPPH (µmol TE/100 g) | CUPRAC (µmol TE/100 g) | FRAP (µmol TE/100 g) | α-Amylase Inhibition (%) | |
|---|---|---|---|---|---|
| AF_1%/BJ | 106.86 ± 0.12 a | 109.64 ± 0.12 a | 964.00 ± 3.08 a | 13.19 ± 0.11 a | 33.05 ± 0.67 c |
| AF_2%/BJ | 95.85 ± 0.79 b | 101.01 ± 0.17 b | 888.65 ± 3.76 b | 11.81 ± 0.14 b | 35.90 ± 0.37 b |
| AF_4%/BJ | 82.77 ± 0.42 c | 100.45 ± 0.64 b,c | 848.27 ± 2.93 c | 11.46 ± 0.10 b | 37.53 ± 0.10 a |
| AF_6%/BJ | 82.27 ± 0.47 c | 98.81 ± 0.56 c | 830.95 ± 2.68 d | 10.70 ± 0.18 c | 33.14 ± 0.23 c |
| AF_8%/BJ | 77.62 ± 0.31 d | 95.58 ± 0.83 d | 790.74 ± 2.52 e | 10.25 ± 0.12 c | 33.56 ± 0.29 c |
| AF_10%/BJ | 73.74 ± 0.86 e | 88.90 ± 0.45 e | 687.97 ± 2.15 f | 9.04 ± 0.05 d | 22.10 ± 0.21 d |
| L* | a* | b* | °h | C* | |
|---|---|---|---|---|---|
| AF_1%/BJ | 45.90 ± 0.09 f | 18.03 ± 0.08 d | 8.77 ± 0.01 f | 25.92 ± 0.07 f | 20.01 ± 0.11 e |
| AF_2%/BJ | 46.71 ± 0.03 e | 19.15 ± 0.04 b | 9.76 ± 0.01 e | 26.99 ± 0.08 e | 21.49 ± 0.03 d |
| AF_4%/BJ | 47.89 ± 0.02 d | 19.41 ± 0.01 a | 10.93 ± 0.02 d | 29.38 ± 0.03 d | 22.27 ± 0.02 c |
| AF_6%/BJ | 48.41 ± 0.01 c | 19.33 ± 0.03 a | 11.90 ± 0.01 c | 31.60 ± 0.03 c | 22.70 ± 0.03 a,b |
| AF_8%/BJ | 49.30 ± 0.02 b | 18.99 ± 0.03 b | 12.76 ± 0.03 b | 33.89 ± 0.10 b | 22.88 ± 0.02 a |
| AF_10%/BJ | 49.70 ± 0.01 a | 18.48 ± 0.06 c | 12.93 ± 0.01 a | 34.98 ± 0.08 a | 22.54 ± 0.06 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buljeta, I.; Nosić, M.; Pichler, A.; Ivić, I.; Šimunović, J.; Kopjar, M. Apple Fibers as Carriers of Blackberry Juice Polyphenols: Development of Natural Functional Food Additives. Molecules 2022, 27, 3029. https://doi.org/10.3390/molecules27093029
Buljeta I, Nosić M, Pichler A, Ivić I, Šimunović J, Kopjar M. Apple Fibers as Carriers of Blackberry Juice Polyphenols: Development of Natural Functional Food Additives. Molecules. 2022; 27(9):3029. https://doi.org/10.3390/molecules27093029
Chicago/Turabian StyleBuljeta, Ivana, Mario Nosić, Anita Pichler, Ivana Ivić, Josip Šimunović, and Mirela Kopjar. 2022. "Apple Fibers as Carriers of Blackberry Juice Polyphenols: Development of Natural Functional Food Additives" Molecules 27, no. 9: 3029. https://doi.org/10.3390/molecules27093029
APA StyleBuljeta, I., Nosić, M., Pichler, A., Ivić, I., Šimunović, J., & Kopjar, M. (2022). Apple Fibers as Carriers of Blackberry Juice Polyphenols: Development of Natural Functional Food Additives. Molecules, 27(9), 3029. https://doi.org/10.3390/molecules27093029







