Effects of the Bark Resin Extract of Garcinia nigrolineata on Chronic Stress-Induced Memory Deficit in Mice Model and the In Vitro Monoamine Oxidases and β-Amyloid Aggregation Inhibitory Activities of Its Prenylated Xanthone Constituents
Abstract
1. Introduction
2. Results
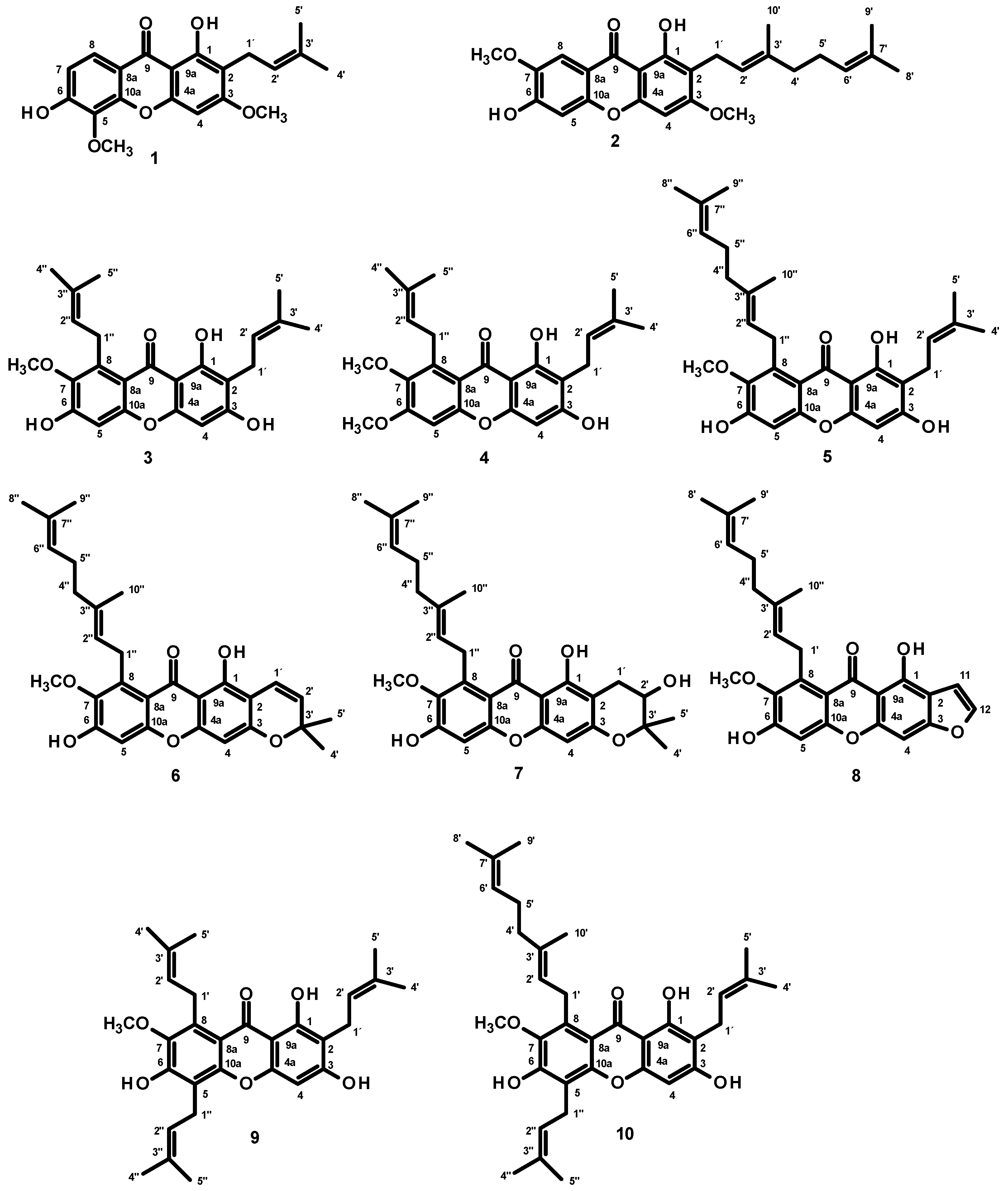
2.1. Isolation and Structure Elucidation of the Constituents of the Extract of the Bark Resin of G. nigroleanata
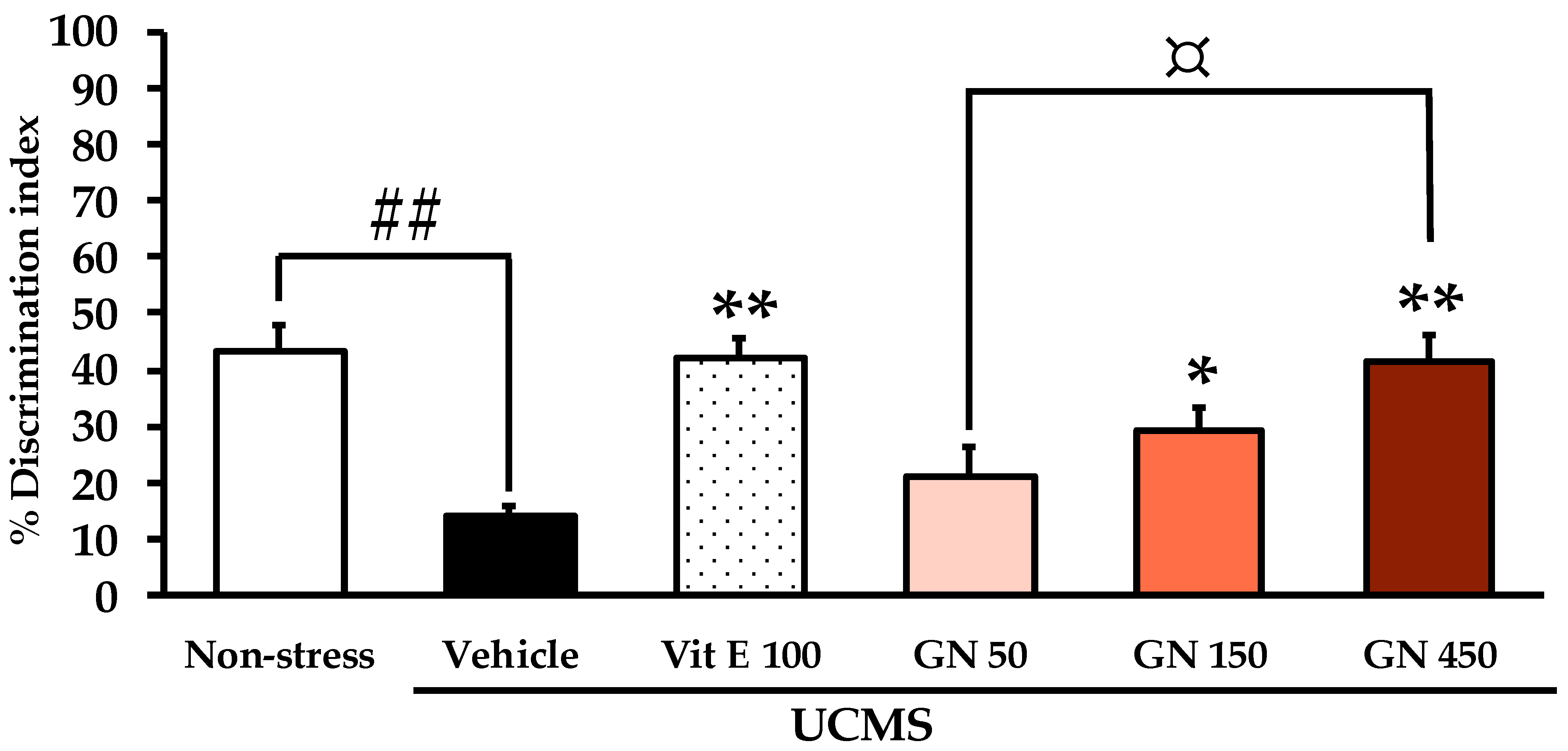
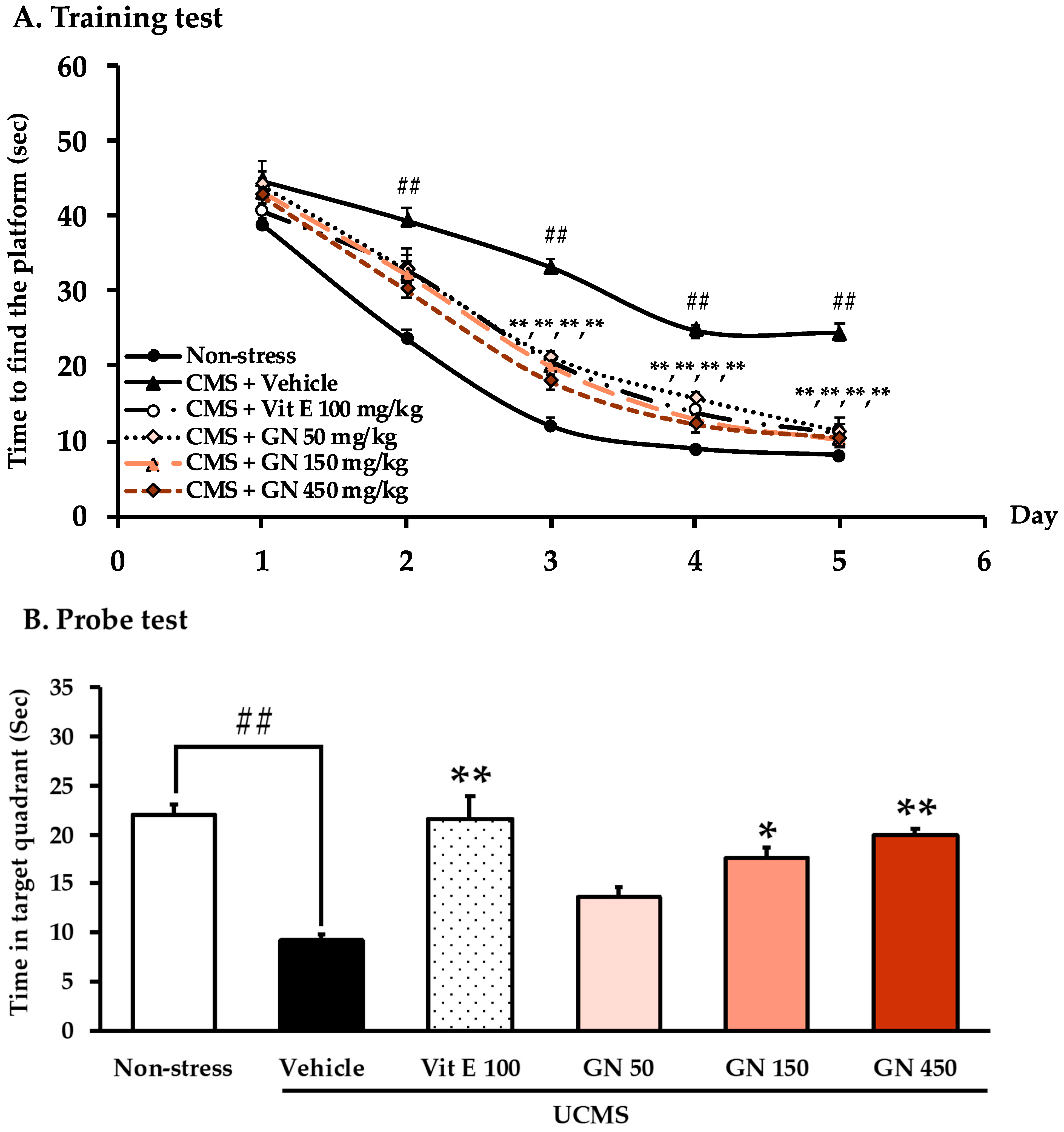
2.2. Effect of the Bark Resin Crude Extract of G. nigrolineata (GN) on UCMS-Induced Cognitive Deficit-like Behavior
2.3. Treatment with the Crude Extract of the Bark Resin of G. nigrolineata (GN) Ameliorated Oxidative Stress in UCMS-Mice
2.4. β-Amyloid (Aβ) Aggregation Inhibitory Activity of the Constituents of G. nigrolineata (GN) Bark Resin
2.5. MAO-A and MAO-B Inhibitory Activities of the Constituents of G. nigrolineata (GN) Bark Resin
3. Discussion
4. Experimental Section
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation of the Constituents
4.4. Animals
4.5. Chronic Unpredictable Stress
4.6. Experimental Design and Drug Treatment
4.7. Behavioral Study
4.7.1. Y-Maze Test
4.7.2. Novel Object Recognition Test (NORT)
4.7.3. Morris Water Maze Test (MWMT)
4.8. Lipid Peroxidation
4.9. Inhibition of Aβ-Self Aggregation
4.10. Inhibitory Activity of Recombinant Human Monoamine Oxidase Enzymes
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bakhtiari-Dovvombaygi, H.; Izadi, S.; Zare, M.; Hassanlouei, E.A.; Dinpanah, H.; Ahmadi-Soleimani, S.M.; Beheshti, F. Vitamin D3 administration prevents memory deficit and alteration of biochemical parameters induced by unpredictable chronic mild stress in rats. Sci. Rep. 2021, 11, 16271. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Ahmad, M.; Sahibzada, M.U.K.; Khusro, A.; Emran, T.B.; Alnasrawi, A.M.; Alkahtani, J.; Elshikh, M.S. Lipid peroxidation reduction and hippocampal and cortical neurons protection against ischemic damage in animal model using Stellaria media. Saudi J. Biol. Sci. 2022, 29, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Shansky, R.M.; Lipps, J. Stress-induced cognitive dysfunction: Hormone-neurotransmitter interactions in the prefrontal cortex. Front. Hum. Neurosci. 2013, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.U.; Kim, S.; Sim, J.; Yang, S.; An, H.; Nam, M.H.; Jang, D.P.; Lee, C.J. Redefining differential roles of MAO-A in dopamine degradation and MAO-B in tonic GABA synthesis. Exp. Mol. Med. 2021, 53, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ming, Q.; Zhong, X.; Dong, D.; Li, C.; Xiong, G.; Cheng, C.; Cao, W.; He, J.; Wang, X.; et al. The MAOA gene influences the neural response to psychosocial stress in the human brain. Front. Behav. Neurosci. 2020, 14, 65. [Google Scholar] [CrossRef]
- Ahmad, A.; Rasheed, N.; Banu, N.; Palit, G. Alterations in monoamine levels and oxidative systems in frontal cortex, striatum, and hippocampus of the rat brain during chronic unpredictable stress. Stress 2010, 13, 355–364. [Google Scholar] [CrossRef]
- Tamagno, E.; Guglielmotto, M.; Vasciaveo, V.; Tabaton, M. Oxidative stress and beta amyloid in Alzheimer’s disease. Which comes first: The chicken or the egg? Antioxidants 2021, 10, 1479. [Google Scholar] [CrossRef]
- Hemshekhar, M.; Sunitha, K.; Santhosh, M.S.; Devaraja, S.; Kemparaju, K.; Vishwanath, B.S.; Niranjana, S.R.; Girish, K.S. An overview on genus Garcinia: Phytochemical and therapeutical aspects. Phytochem. Rev. 2011, 10, 325–351. [Google Scholar] [CrossRef]
- Espirito Santo, B.L.S.; Santana, L.F.; Junior, W.H.K.; Araújo, F.O.; Bogo, D.; Freitas, K.C.; Guimarães, R.C.A.V.; Hiane, P.A.; Pott, A.; Filiú, W.F.O.; et al. Medicinal potential of Garcinia species and their compounds. Molecules 2020, 25, 4513. [Google Scholar] [CrossRef]
- Lin, F.; Luo, B.; Cheng, Z.; Li, P.; Long, C. Ethnobotanical study on Garcinia (Clusiaceae) in China. Acta Soc. Bot. Pol. 2021, 90, 9012. [Google Scholar] [CrossRef]
- Raksat, A.; Maneerat, W.; Andersen, R.J.; Stephen, G.; Pyne, S.G.; Laphookhieo, S. A tocotrienol quinone dimer and xanthones from the leaf extract of Garcinia nigrolineata. Fitoterapia 2019, 36, 104175. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Ritthiwigrom, T.; Pinsa, A.; Sawangchote, P.; Taylor, W.C. Xanthones from the stem bark of Garcinia nigrolineata. Phytochemistry 2003, 64, 1149–1156. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Kamkaew, M.; Sukavisit, D.; Phongpaichit, S.; Sawangchote, P.; Taylor, W.C. Antibacterial xanthones from the leaves of Garcinia nigrolineata. J. Nat. Prod. 2003, 66, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Tadpetch, K.; Watthanaphanit, A.; Saengsanae, N.; Phongpaichit, S. Benzopyran, biphenyl, and tetraoxygenated xanthone derivatives from the twigs of Garcinia nigrolineata. J. Nat. Prod. 2005, 68, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Tiang, N.; Ahad, M.A.; Murugaiyah, V.; Hassan, Z. Xanthone-enriched fraction of Garcinia mangostana and α-mangostin improve the spatial learning and memory of chronic cerebral hypoperfusion rats. J. Pharm. Pharmacol. 2020, 72, 1629–1644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, Z.; Xu, J.R.; Wang, Y.X.; Hou, L.N.; Qiu, Y.; Chen, H.Z. α-Mangostin, a polyphenolic xanthone derivative from mangosteen, attenuates β-amyloid oligomers-induced neurotoxicity by inhibiting amyloid aggregation. Neuropharmacology 2012, 62, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Mahabusarakam, W.; Chairerk, P.; Taylor, W.C. Xanthones from Garcinia cowa Roxb. latex. Phytochemistry 2005, 66, 1148–1153. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Takakura, T.; Ruangrungsi, N.; Enjo, F.; Tokuda, H.; Nishino, H.; Furukawa, H. Chemical constituents of Garcinia fusca: Structure elucidation of eight new xanthones and their cancer chemopreventive activity. J. Nat. Prod. 2003, 66, 200–205. [Google Scholar] [CrossRef]
- Pattanaprateeb, P.; Ruangrungsi, N.; Cordell, G.A. Cytotoxic constituents from Cratoxylum arborescens. Planta Med. 2005, 71, 181–183. [Google Scholar] [CrossRef]
- Panthong, K.; Pongcharoen, W.; Phongpaichit, S.; Taylor, W.C. Tetraoxygenated xanthones from the fruits of Garcinia cowa. Phytochemistry 2006, 67, 999–1004. [Google Scholar] [CrossRef]
- Nguyen, C.N.; Trinh, B.T.D.; Tran, T.B.; Nguyen, L.T.T.; Jäger, A.K.; Nguyen, L.H.D. Anti-diabetic xanthones from the bark of Garcinia xanthochymus. Bioorg. Med. Chem. Lett. 2017, 27, 3301–3304. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K.; Phadungcharoen, T.; Mahidol, C.; Ruchirawat, S. 7-O-Methylgarcinone from Garcinia cowa. Phytochemistry 1997, 45, 1299–1301. [Google Scholar] [CrossRef]
- Boubakri, A.; Leri, M.; Bucciantini, M.; Najjaa, H.; Arfa, A.B.; Stefani, M.; Neffati, M. Allium roseum L. extract inhibits amyloid beta aggregation and toxicity involved in Alzheimer’s disease. PLoS ONE 2020, 15, e0223815. [Google Scholar] [CrossRef] [PubMed]
- Khamphukdee, C.; Monthakantirat, O.; Chulikhit, Y.; Boonyarat, C.; Daodee, S.; Aon-im, P.; Maneenet, J.; Chotritthirong, Y.; Luecha, P.; Sekeroglu, N.; et al. Antidementia effects of Alternanthera philoxeroides in ovariectomized mice supported by NMR-based metabolomic analysis. Molecules 2021, 26, 2789. [Google Scholar] [CrossRef]
- Tonge, P.J. Quantifying the Interactions between biomolecules: Guidelines for assay design and data analysis. ACS Infect. Dis. 2019, 5, 796–808. [Google Scholar] [CrossRef]
- Do, H.T.T.; Cho, J. Mangosteen pericarp and its bioactive xanthones: Potential therapeutic value in Alzheimer’s Disease, Parkinson’s Disease, and depression with pharmacokinetic and safety profiles. Int. J. Mol. Sci. 2020, 21, 6211. [Google Scholar] [CrossRef]
- Richard, S.A.; Zheng, S.; Su, Z.; Gao, J.; Xu, H. The pivotal neuroinflammatory, therapeutic and neuroprotective role of alpha-mangostin. J. Neurol. Res. 2017, 7, 67–79. [Google Scholar] [CrossRef]
- Tiwari, A.; Khera, R.; Rahi, S.; Mehan, S.; Makeen, H.A.; Khormi, Y.H.; Rehman, M.U.; Khan, A. Neuroprotective effect of α-mangostin in ameliorating propionic acid-induced experimental model of autism in Wistar rats. Brain Sci. 2021, 11, 288. [Google Scholar] [CrossRef]
- Nollet, M. Models of depression: Unpredictable chronic mild stress in mice. Curr. Protoc. 2001, 1, e208. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for assessment of spatial working and reference memory in mice. In Pre-Clinical Models. Methods in Molecular Biology; Guest, P.C., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1916, pp. 105–111. [Google Scholar] [CrossRef]
- Bouet, V.; Freret, T.; Schumann-Bard, P.; Boulouard, M. Novel object recognition test in rodents: Which roles for serotonin receptors? In Handbook of Object Novelty Recognition; Ennaceur, A., de Souza Silva, M.A., Eds.; Elsevier Academic Press: Cambridge, MA, USA, 2018; Volume 27, pp. 391–402. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Maneenet, J.; Daodee, S.; Monthakantirat, O.; Boonyarat, C.; Khamphukdee, C.; Kwankhao, P.; Pitiporn, S.; Awale, S.; Chulikhit, Y.; Anake Kijjoa, A. Kleeb Bua Daeng, a Thai traditional herbal formula, ameliorated unpredictable chronic mild stress-induced cognitive impairment in ICR mice. Molecules 2019, 24, 4587. [Google Scholar] [CrossRef] [PubMed]
- Ghadrdoost, B.; Vafaei, A.B.; Rashidy-Pour, A.; Hajisoltani, R.; Bandegi, A.Z.; Motamedi, F.; Haghighi, S.; Sameni, H.R.; Pahlvan, S. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur. J. Pharmacol. 2011, 667, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.A.; Dion-Albert, L.; Kaufmann, F.N.; Tuck, E.; Lebel, M.; Menard, C. Neurobiology of resilience in depression: Immune and vascular insights from human and animal studies. Eur. J. Neurosci. 2021, 53, 183–221. [Google Scholar] [CrossRef] [PubMed]
- Churchwell, J.C.; Morris, A.M.; Musso, N.D.; Kesner, R.P. Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol. Learn. Mem. 2010, 93, 415–421. [Google Scholar] [CrossRef]
- Chulikhit, Y.; Sukhano, W.; Daodee, S.; Putalun, W.; Wongpradit, R.; Khamphukdee, C.; Umehara, K.; Noguchi, H.; Matsumoto, K.; Monthakantirat, O. Effects of Pueraria candollei var mirifica (Airy Shaw and Suvat.) Niyomdham on ovariectomy-induced cognitive impairment and oxidative stress in the mouse brain. Molecules 2021, 26, 3442. [Google Scholar] [CrossRef]
- Rumman, M.; Pandey, S.; Singh, S.; Gupta, M.; Saba Ubaid, S.; Mahdi, A.A. Genistein prevents hypoxia-induced cognitive dysfunctions by ameliorating oxidative stress and inflammation in the hippocampus. Neurotox. Res. 2021, 39, 1123–1133. [Google Scholar] [CrossRef]
- Naqvi, F.; Saleem, S.; Naqvi, F.; Batool, Z.; Sadir, S.; Tabassum, S.; Ahmed, S.; Liaquat, L.; Haider, S. Curcumin lessens unpredictable chronic mild stress-induced depression and memory deficits by modulating oxidative stress and cholinergic activity. Pak. J. Pharm. Sci. 2019, 32, 1893–1901, PMID: 31680089. [Google Scholar] [PubMed]
- Rei, D.; Mason, X.; Seo, J.; Gräff, J.; Rudenko, A.; Wang, J.; Rueda, R.; Siegert, S.; Cho, S.; Canter, R.G.; et al. Basolateral amygdala bidirectionally modulates stress-induced hippocampal learning and memory deficits through a p25/Cdk5-dependent pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 7291–7296. [Google Scholar] [CrossRef]
- Gunther, E.C.; Strittmatter, S.M. β-amyloid oligomers and cellular prion protein in Alzheimer’s disease. J. Mol. Med. 2010, 88, 331–338. [Google Scholar] [CrossRef][Green Version]
- Wang, S.N.; Li, Q.; Jing, M.H.; Alba, E.; Yang, X.H.; Sabaté, R.; Han, Y.F.; Pi, R.B.; Lan, W.J.; Yang, X.B.; et al. Natural xanthones from Garcinia mangostana with multifunctional activities for the therapy of Alzheimer’s disease. Neurochem. Res. 2016, 41, 1806–1817. [Google Scholar] [CrossRef]
- Durani, L.W.; Hamezah, H.S.; Ibrahim, N.F.; Yanagisawa, D.; Nasaruddin, M.L.; Mori, M.; Azozan, K.A.; Damanhuri, H.A.; Makpol, S.; Ngah, W.Z.; et al. Tocotrienol-rich fraction of palm oil improves behavioral impairments and regulates metabolic pathways in AβPP/PS1 mice. J. Alzheimers Dis. 2018, 64, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Mikalauskaite, K.; Ziaunys, M.; Sneideris, T.; Smirnovas, V. Effect of ionic strength on thioflavin-T affinity to amyloid fibrils and its fluorescence intensity. Inter. J. Mol. Sci. 2020, 21, 8916. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.I.; Cidade, H.; Pinto, M. Dual/multitargeted xanthone derivatives for Alzheimer’s disease: Where do we stand? Future Med. Chem. 2017, 9, 1611–1630. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kaur, D.; Sehgal, A.; Singh, S.; Sharma, N.; Zengin, G.; Andronie-Cioara, F.L.; Toma, M.M.; Bundau, S.; Bumbu, A.G. Role of monoamine oxidase activity in Alzheimer’s disease: An insight into the therapeutic potential of inhibitors. Molecules 2021, 26, 3724. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, N.; Suzuki, T.; Ogasawara, T.; Yagi, K. Xanthone derivatives as inhibitors for monoamine oxidase. J. Mol. Catal. B Enzym. 2000, 10, 291–294. [Google Scholar] [CrossRef]
- Panda, S.S.; Chand, M.; Sakhuja, R.; Jain, S.C. Xanthones as potent antioxidants. Curr. Med. Chem. 2013, 20, 4481–4507. [Google Scholar] [CrossRef] [PubMed]
- Lueptow, L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017, 126, 55718. [Google Scholar] [CrossRef]
- Zhong, J.Y.; Magnusson, K.R.; Swarts, M.E.; Clendinen, C.A.; Reynolds, N.C.; Moffat, S.D. The application of a rodent-based Morris water maze (MWM) protocol to an investigation of age-related differences in human spatial learning. Behav. Neurosc. 2017, 131, 470–482. [Google Scholar] [CrossRef]
- Svensson, M.; Hallin, T.; Broms, J.; Ekstrand, J.; Tingström, A. Spatial memory impairment in Morris water maze after electroconvulsive seizures. Acta Neuropsychiatr. 2017, 29, 17–26. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford assay for determining protein concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef]
- Daodee, S.; Monthakantirat, O.; Ruengwinitwong, K.; Gatenakorn, K.; Maneenet, J.; Khamphukdee, C.; Sekeroglu, N.; Chulikhit, Y.; Kijjoa, A. Effects of the ethanol extract of Dipterocarpus alatus leaf on the unpredictable chronic mild stress-induced depression in ICR mice and its possible mechanism of action. Molecules 2019, 24, 3396. [Google Scholar] [CrossRef] [PubMed]




| Compounds | % Inhibition of Aβ Aggregation | IC50 (µM) | Si | ||
|---|---|---|---|---|---|
| MAO-A | MAO-B | MAO-A | MAO-B | ||
| 1 | 46.79 ± 5.46 | 100.2 ± 0.35 | 17.99 ± 0.11 | 3.407 | 0.294 |
| 2 | 58.56 ± 3.28 | 119.8 ± 0.75 | 238.50 ± 2.03 | 0.307 | 3.254 |
| 3 | Not detected | 79.81 ± 0.50 | 40.09 ± 0.89 | 1.218 | 0.821 |
| 4 | Not detected | 40.21 ± 0.35 | 15.60 ± 0.26 | 1.579 | 0.6333 |
| 5 | Not detected | 7.10 ± 0.03 | 16.34 ± 1.44 | 0.266 | 3.762 |
| 6 | Not detected | 69.92 ± 0.06 | 0.07 ± 0.01 | 558.273 | 0.00179 |
| 8 | Not detected. | 100.5 ± 0.44 | >400 | <0.153 | 0.6522 |
| 9 | Not detected | 25.41 ± 0.63 | 0.40 ± 0.01 | 38.705 | 0.0256 |
| 10 | Not detected | 3.58 ± 0.02 | >400 | <0.005 | 183.168 |
| Curcumin 1 | 51.73 ± 2.89 | - | - | - | - |
| Clogyline 2 | - | 0.005 ± 0.00 | 0.015 ± 0.002 | 0.143 | 7 |
| Deprenyl 3 | - | 8.48 ± 0.14 | 0.10 ± 0.02 | 48.565 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamphukdee, C.; Turkmani, I.; Chotritthirong, Y.; Chulikhit, Y.; Boonyarat, C.; Sekeroglu, N.; Silva, A.M.S.; Monthakantirat, O.; Kijjoa, A. Effects of the Bark Resin Extract of Garcinia nigrolineata on Chronic Stress-Induced Memory Deficit in Mice Model and the In Vitro Monoamine Oxidases and β-Amyloid Aggregation Inhibitory Activities of Its Prenylated Xanthone Constituents. Molecules 2022, 27, 3014. https://doi.org/10.3390/molecules27093014
Khamphukdee C, Turkmani I, Chotritthirong Y, Chulikhit Y, Boonyarat C, Sekeroglu N, Silva AMS, Monthakantirat O, Kijjoa A. Effects of the Bark Resin Extract of Garcinia nigrolineata on Chronic Stress-Induced Memory Deficit in Mice Model and the In Vitro Monoamine Oxidases and β-Amyloid Aggregation Inhibitory Activities of Its Prenylated Xanthone Constituents. Molecules. 2022; 27(9):3014. https://doi.org/10.3390/molecules27093014
Chicago/Turabian StyleKhamphukdee, Charinya, Ibrahim Turkmani, Yutthana Chotritthirong, Yaowared Chulikhit, Chantana Boonyarat, Nazim Sekeroglu, Artur M. S. Silva, Orawan Monthakantirat, and Anake Kijjoa. 2022. "Effects of the Bark Resin Extract of Garcinia nigrolineata on Chronic Stress-Induced Memory Deficit in Mice Model and the In Vitro Monoamine Oxidases and β-Amyloid Aggregation Inhibitory Activities of Its Prenylated Xanthone Constituents" Molecules 27, no. 9: 3014. https://doi.org/10.3390/molecules27093014
APA StyleKhamphukdee, C., Turkmani, I., Chotritthirong, Y., Chulikhit, Y., Boonyarat, C., Sekeroglu, N., Silva, A. M. S., Monthakantirat, O., & Kijjoa, A. (2022). Effects of the Bark Resin Extract of Garcinia nigrolineata on Chronic Stress-Induced Memory Deficit in Mice Model and the In Vitro Monoamine Oxidases and β-Amyloid Aggregation Inhibitory Activities of Its Prenylated Xanthone Constituents. Molecules, 27(9), 3014. https://doi.org/10.3390/molecules27093014