Abstract
This study aimed to investigate the antioxidant, antimicrobial, and cytotoxic potential of ethanolic extracts obtained from Gentiana asclepiadea L. and Inula helenium L. roots, in relation to their chemical composition. The total polyphenols, flavonoids, and phenolic acids were determined by spectrophotometric methods, while LC-MS analysis was used to evaluate the individual constituents. The antioxidant properties were tested using the FRAP and DPPH methods. The standard well diffusion and broth microdilution assays were carried out to establish in vitro antimicrobial efficacy and minimum inhibitory and bactericidal concentrations. The cytotoxicity was tested on rat intestinal epithelial cells using the MTT assay. The results pointed out important constituents such as secoiridoid glycoside (amarogentin), phenolic acids (caffeic acid, chlorogenic acid, trans-p-coumaric acid, salicylic acid), and flavonoids (apigenin, chrysin, luteolin, luteolin-7-O-glucoside, quercetin, rutoside, and naringenin) and promising antioxidant properties. The in vitro antimicrobial effect was noticed towards several pathogens (Bacillus cereus > Staphylococcus aureus > Enterococcus faecalis > Salmonella typhimurium and Salmonella enteritidis > Escherichia coli), with a pronounced bactericidal activity. Rat intestinal epithelial cell viability was not affected by the selected concentrations of these two extracts. These data support the ethnomedicinal recommendations of these species and highlight them as valuable sources of bioactive compounds.
1. Introduction
For ages, both humans and animals have been instinctively using plants for prevention and treatment of various diseases. In recent decades, the interest in herbal medicines (HMs) and traditional medicine has increased tremendously and led to the start of the “Return to Nature” trend [1]. Medicinal plants exhibit a wide variety of therapeutic effects, including anti-inflammatory, antioxidant, anti-proliferative, antiviral, and antimicrobial [2,3,4]. Currently, 25% of modern medicines are plant-derived, medicinal herbs and their bioactive compounds are regarded as safer and healthier substitutes to the synthetic drugs, especially in long-term use [1]. However, it was estimated that only 15% of species have been studied for their chemical composition, and around 6% for their therapeutic effects [5].
Gentianeae is the most species-rich tribe of the family Gentianaceae, comprising 974 species, from which around 360 belong to the Gentiana L. genus [6]. Gentiana asclepiadea L. (Willow gentian) is a perennial species belonging to the Gentiana genus, found mostly in regions with temperate climate and high altitudes in central, southern, and eastern Europe, Turkey, and Iran [6,7]. Based on its occurrence in nature and risk of extinction, its status varies depending on the region. Therefore, according to International Union for Conservation of Nature and Natural Resources (IUCN), G. asclepiadea is listed as strictly protected in Poland, vulnerable in Hungary and Germany, nearly threatened in Croatia, and of least concern in the rest of the countries [6,7,8]. In traditional medicine, the roots and rhizomes of G. asclepiadea are used for the treatment of digestive system disorders and hepatitis infections [3,8]. Similarly, in Romanian traditional, medicine, G. asclepiadea root tea is used as appetite stimulant, choleretic, anthelmintic and for the treatment of diarrhea [9]. Today, because of its biological effects and chemical composition, this species is commonly used as a substitute for G. lutea (yellow gentian), a species with high therapeutic value that is endangered and under protection in most European countries [7,8]. Scientific studies on the therapeutic effects of G. asclepiadea show that its roots possess antigenotoxic [3], antioxidant [2,3], hepatoprotective [10], antibiofilm [2], antibacterial [2,11], and prebiotic activities [11]. The secondary metabolites found in the chemical composition of G. asclepiadea roots and responsible for its biological activities are bitter secoiridoids glycosides (swertiamarin, gentiopicrin, amarogentin [12,13], sweroside [3]), flavonoids and xanthones (gentioside and gentisin) [13].
Asteraceae is one of the largest plant families that includes 1400–1700 genera and 24,000–35,000 species, representing 10% of all known flowering plant species [14]. The Inula L. genus comprises 78 to 100 species found in Europe, Asia, and Africa, known for the large therapeutic potential of their phytochemical compounds. Inula helenium L. (elecampane) is a widely spread herbaceous perennial species that belongs to the genus Inula [15,16]. Its collection from the spontaneous flora for medicinal purposes has led to a decrease in the populations found in eastern Europe, as indicated in the most recent report provided by IUCN [15]. In traditional medicine, the roots of I. helenium are used for the treatment of respiratory diseases (bronchitis, tuberculosis), gastrointestinal symptoms such as vomiting, diarrhea, abdominal pain, or poor appetite, associated with infectious or parasitic diseases and circulatory diseases [16,17]. I. helenium roots are also used externally in the treatment of wounds, pruritus, and rheumatic pain [16]. Currently, oral administration of I. helenium root tea is recommended in herbal medicine for the alleviation of respiratory symptoms, as a digestive tonic, choleretic, and vermifuge agent; as a topical application, this species is indicated in bacterial and fungal dermatitis and for skin disorders characterized by dry and itchy skin [16,17]. Recent studies have reported that roots of I. helenium exhibit in vitro anti-inflammatory, antioxidant, anti-proliferative, antimicrobial, antibacterial, anticandidal, prebiotic, and anthelmintic effects [4,18,19,20,21]. These proprieties could be attributed to its main secondary metabolites, such as sesquiterpene lactones (alantolactone and isoalantolactone), phenolic acids (gallic acid, caffeic acid, cinnamic acid, coumaric acid), and flavonoids (quercetin, myricetin, kaempferol, catechin) [16,22].
G. asclepiadea and I. helenium species are commonly used in traditional medicine for the treatment of digestive disorders. Their roots are believed to relieve abdominal pain, stimulate the gastrointestinal system and bile secretion, and exhibit anthelmintic activity [10,16]. Additionally, due to the presence of inulin in the roots of I. helenium and gentio-oligosaccharides (gentiobiose and gentianose) in G. asclepiadea roots, both can potentially influence the composition of gut microflora [11,21]. Based on the above-mentioned ethnomedicinal uses, oral administration of herbal medicines containing I. helenium and G. asclepiadea is recommended, their phytochemical constituents being absorbed at intestinal level.
Therefore, taking all the above-mentioned aspects into consideration, the study of G. asclepiadea and I. helenium species and their biological activities appears to be an important subject in order to support their traditional uses. In this context, the present study aimed to perform a comprehensive evaluation of the chemical composition of Inula helenium and Gentiana asclepiadea roots ethanolic extracts by spectrophotometry and LC-MS analysis, and of their in vitro antioxidant and antimicrobial efficacy. Moreover, the in vitro cytotoxicity was investigated using rat intestinal epithelial cell cultures, which to the best of our knowledge is the first report of the cytotoxic effect of G. asclepiadea and I. helenium ethanolic root extracts on primary intestinal cell culture, this being the main element of novelty and originality of the present study. Results obtained hereby may represent important aspects that bring further arguments to sustain the ethnomedicinal uses of these species in digestive disorders.
2. Results and Discussions
2.1. Quantification of Total Polyphenolic (TPC), Flavonoid (TFC), and Phenolic Acids (TPA) Content
Results obtained using spectrophotometrical methods for the quantification of TPC, TFC, and TPA content are presented in Table 1. Values obtained for these assays were significantly higher (p < 0.05) in the I. helenium ethanolic extract compared to the one obtained from G. asclepiadea (Table 1).

Table 1.
Total polyphenolic (TPC), flavonoid (TFC), and phenolic acids (TPA) content of Gentiana asclepiadea and Inula helenium extracts.
Results for the quantification of TPC in I. helenium roots (3.066 g GAE/100 g dry plant) were within the wide range of 1.5–71.24 mg GAE/g reported in other studies [22,23,24]. Based on existing research, the lowest value of TPC (1.5 mg GAE/g dry weight) was obtained by reflux extraction with 95% ethanol [23], and the highest value was obtained by Soxhlet extraction (71.24 mg GAE/g) [24]. Similarly, the TPC of G. asclepiadea roots (2.144 g GAE/100 g dry plant) was in agreement with the previously published data (5.64–146.64 mg GAE/g) [2,3,13].
However, in both plants, the TFC values were lower compared to other studies, the reported range for I. helenium roots being 9.32–50.0 mg RE/g [22,23] and for G. asclepiadea of 3.61–17.54 mg RE/g [2,3]. As per TPA content, the value for the ethanolic extract of I. helenium roots was significantly higher when compared to the G. asclepiadea root extract. This was further confirmed by differences in the concentrations of caffeic and chlorogenic acids, identified and quantified by LC-MS analysis in both tested extracts.
Large variations in these compound contents in extracts could be explained by the differences appearing in exogenous and endogenous factors, such as geographical, climatic conditions, exposure to UV-B radiation, harvesting period, plant age, genetic diversity, solvent, and extraction techniques [24].
2.2. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis
The identification and quantification of the chemical constituents of G. asclepiadea and I. helenium ethanolic extracts were achieved by a LC-MS method. The LC-MS method was validated for linearity, repeatability, limits of detection (LOD), and limits of quantification (LOQ). Major compounds identified in G. asclepiadea roots were amarogentin, apigenin, luteolin-7-O-glucoside, naringenin, and rutoside. In addition to the above-mentioned compounds, G. asclepiadea root extract contained trans-p-coumaric acid, caffeic acid, chlorogenic acid, luteolin, and unquantifiable amounts of salicylic acid, chrysin, and quercetin.
The LC/MS analysis of the I. helenium root ethanolic extract revealed that caffeic acid, chlorogenic acid, chrysin, luteolin, and hesperetin were the major compounds. I. helenium extract was also found to contain luteolin-7-O-glucoside and naringenin (Table 2).

Table 2.
The identified and quantified components in the G. asclepiadea and I. helenium root ethanolic extracts (µg/g dry vegetal material) by the LC-MS analysis.
The G. asclepiadea chromatogram of the major identified compounds is shown in Figure 1.
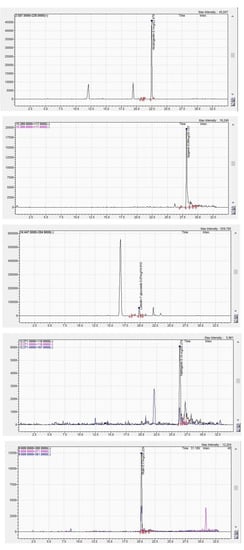
Figure 1.
LC-MS chromatogram peaks of G. asclepiadea ethanolic extract—amarogentin, apigenin, luteolin-7-O-glucoside, naringenin, rutoside (top to bottom).
In total, 12 compounds were identified in the ethanolic extract of G. asclepiadea, of which one bitter secoiridoid glycoside (amarogentin), four phenolic acids (caffeic acid, chlorogenic acid, trans-p-coumaric acid, salicylic acid), and seven flavonoids (apigenin, chrysin, luteolin, luteolin-7-O-glucoside, quercetin, rutoside, and naringenin). In the case of I. helenium ethanolic extract, seven compounds were detected, namely two phenolic acids (caffeic acid, chlorogenic acid) and five flavonoid compounds (chrysin, luteolin, luteolin-7-O-glucoside, naringenin, and hesperetin).
Among the identified compounds, amarogentin was detected in G. asclepiadea roots extract in a concentration of 27.8 ± 0.3 µg/g. The presence of amarogentin in G. asclepiadea roots was previously reported by Szucs et al. [12], but only in trace amounts. This bitter secoiridoid glycoside was commonly isolated from various species of the genus Gentiana and Swertia, family Gentianaceae, such as G. lutea, G. gelida, G. dinarica, S. chirayita, S. alternifolia, S. bimaculata, S. alata, S. nervosa, or S. ciliata [13,25,26,27,28]. Recent studies pointed out a variety of biological effects for amarogenin, including antileishmanial, antioxidant, anti-diabetic, anticancerous, and antithrombotic activity [25,26,27,28].
Phenolic acids were identified in important amounts in the chemical profile of G. asclepiadea roots ethanolic extract. The maximum amount was found for the trans-p-coumaric acid (192.8 ± 1.0 µg/g dry vegetal material), followed by caffeic acid (169 ± 1.2 µg/g dry vegetal material), chlorogenic acid (33.4 ± 0.5 µg/g dry vegetal material), and salicylic acid (<LOQ). Two of these phenolic acids, namely chlorogenic acid and caffeic acid were also detected in high concentrations in I. helenium roots (2284.1 ± 11 and 234.0 ± 2.1 µg/g dry vegetal material, respectively). Both compounds have antioxidant and anti-inflammatory potential [29,30,31]. Furthermore, the chlorogenic acid was proven to modulate lipid metabolism [31].
From the flavonoids group, luteolin, luteolin-7-O-glucoside, chrysin, and naringenin were detected in both G. asclepiadea and I. helenium roots. The presence of luteolin-7-O-glucoside was previously reported in the chemical composition of I. britannica, G. asclepiadea, and G. gelida [13,32]. Similarly, luteolin was isolated from several species of genus Gentiana and Inula, including G. arisanensis, G. veitchiorum, I. britannica, and I. viscosa [32,33,34,35] and has been reported to possess antioxidant, anti-inflammatory, cardio-protective, neuroprotective, and antimicrobial effects [36]. The presence of naringenin was previously reported in G. veitchiorum flowers (0.12 mg/L), but in lower concentrations compared to G. asclepiadea roots [34]. According to recent studies, both naringenin and chrysin exhibit anticancer, anti-inflammatory, antioxidant, and antimicrobial activities [37,38].
Two flavonoids, namely rutoside and apigenin were among the major compounds identified only for the ethanolic extract derived from G. asclepiadea roots (30.8 ± 0.6 and 18.0 ± 0.7 µg/g dry vegetal material, respectively). Apigenin was earlier described as part of the chemical composition in aerial parts of Gentiana species, and roots of G. asclepiadea, G. gelida, and G. paradoxa [13,34]. Similar to other members of the flavonoid group, rutoside and apigenin have been reported to possess antioxidant, anti-inflammatory, and antimicrobial effects [34,39,40,41]. In addition, apigenin was associated with neurovascular protective effect, anti-diabetic activity, and the ability to suppress hepatic lipid accumulation [34,40].
2.3. Antioxidant Activity
The results of DPPH radical scavenging activity and ferric-reducing power (FRAP) of G. asclepiadea and I. helenium roots were expressed using the IC50 value (μg/mL) and μmol TE/100 mL extract, respectively (Table 3).

Table 3.
Antioxidant capacity of G. asclepiadea and I. helenium root ethanolic extracts.
Although the presence of small to moderate quantities of reactive oxygen species (ROS) is regarded as indispensable for maintaining cellular homeostasis, their overproduction plays a key role in the pathogenesis of various inflammatory and neurodegenerative diseases [29]. In search of novel antioxidant agents, plants have been regarded as promising sources of bioactive compounds. Since bioactive compounds found in medicinal plants exert their antioxidant effects through multiple chemical mechanisms, with potential synergistic effects, two methods with different reaction mechanism were used to test the antioxidant activity of I. helenium and G. asclepiadea root extracts. DPPH radical scavenging (DPPH) assay is a method primarily based on single electron donating capacity of hydrophobic antioxidants, and hydrogen atom transfer as secondary mechanism [42]. The Ferric Reducing Antioxidant Power (FRAP) assay was used to detect the redox potential of hydrophilic compounds and is based on hydrogen atom transfer [42].
Based on the obtained results, both ethanolic extracts presented significant antioxidant capacities. This potential was significantly (p < 0.05) higher in case of I. helenium, which manifested greater ability to reduce ferric ions and showed a higher DPPH radical scavenging activity (629.04 μmol TE/100 mL and a IC50 value of 173.2). The G. asclepiadea extract exhibited a result of 145.23 μmol TE/100 mL extract for the FRAP assay and an IC50 value of 363.7 μg/mL for the DPPH assay, respectively. The DPPH assay results for G. asclepiadea and I. helenium root extracts fell within the large range previously reported in other studies, both plants exhibiting low to moderate DPPH radical scavenging activity [2,3,13,24,43]. Furthermore, the variations of FRAP and DPPH results were in accordance with the differences of plants total polyphenols, flavonoids, and phenolic acids content. This observation is supported also by the Pearson coefficients established for FRAP and TPC, TFC, and TPA content (r2 = 0.999, r2 = 0.996, and r2 = 0.999, respectively, p < 0.05). Statistical analysis pointed out significant negative correlation between DPPH values and the above-mentioned compounds (r2 = −0.999, r2 = −0.997, and r2 = −0.999, respectively, p < 0.05). Taking into account the DPPH results interpretation, radical scavenging activity also depends on the TPC, TFC, and TPA content. The direct correlation between the antioxidant capacity of plant extracts and their total phenolic and flavonoid concentrations, as well as other compounds with antioxidant activity, was confirmed in other similar studies [22,29,43,44].
Among the major compounds identified in I. helenium root extract by the LC-MS analysis, phenolic acid compounds chlorogenic acid and caffeic acid, and flavonoid compound luteolin were previously reported to exhibit concentration-dependent antioxidant activity by donating a hydrogen atom or electrons to free radicals [29,30,36]. Between the compounds detected in the ethanolic extract of G. asclepiadea roots, potent in vitro and in vivo antioxidant activity was previously reported for bitter secoiridoid glycoside (amarogentin), present in various species of genus Gentiana and Swertia [13,25,26,27,28,45]. Both in vitro and in vivo studies reported that amarogentin exhibits strong radical scavenging activity and possesses the ability to increase the radical-absorbing capacity of cells [28,45]. Another compound that might be responsible for G. asclepiadea root extract antioxidant activity is the flavonoid compound apigenin, that according to recent study exhibits remarkable ABTS and DPPH radical scavenging activity in vitro and regulates cholesterol metabolism in vivo [34]. Additionally, the antioxidant activity of G. asclepiadea roots can be attributed to the presence of phenolic acids (caffeic acid, chlorogenic acid), flavonoids (luteolin, naringenin, chrysin), and other unidentified compounds [2,3,36,37].
2.4. Antibacterial Activity

Table 4.
Antibacterial activity of G. asclepiadea and I. helenium root ethanolic extracts (agar well-diffusion assay).

Table 5.
Antibacterial activity of G. asclepiadea and I. helenium root ethanolic extracts (broth microdilution assay).
Overall, both ethanolic extracts displayed in vitro antimicrobial activity (Table 4) against all selected bacterial strains. The potency of the antimicrobial efficacy varied depending mostly on the bacterial type, with a more intense inhibitory effect expressed against the Gram-positive species (Bacillus cereus > Staphylococcus aureus > Enterococcus faecalis) compared to the Gram-negative (Salmonella enteritidis = Salmonella typhimurium > Escherichia coli). Values obtained for the inhibition zone diameter ranged from 10.00 to 17.33 mm and 8.67 to 18.00 mm in the case of G. asclepiadea and I. helenium root extracts, respectively, thus significantly (p < 0.05) lower compared to those of gentamicin, the standard antibacterial agent. Two of the Gram-positive species, namely Staphylococcus aureus and Bacillus cereus, showed higher susceptibility when exposed to a combination of the two extracts, with diameters of the inhibition zone of 18.33 ± 0.47 and 21.00 ± 0.00, respectively. These values are similar to those induced by gentamicin (p > 0.05) (Table 4).
Values obtained for MIC, MBC, and the resulting MIC index obtained for the two extracts using the broth microdilution method are presented in Table 5. The bactericidal efficacy was clearly pointed out against all tested bacterial species (MBC/MIC ≤ 4).
The bacterial strains were selected given their antimicrobial resistance pattern and prevalence. Over recent decades, the research interest in antimicrobial resistance (AMR) has increased significantly. Recently, it was estimated that if no actions are taken, by 2050 AMR will be responsible for 10 million deaths each year [46]. Misleadingly referred by many as a “silent pandemic”, AMR has worsened since the outbreak of COVID-19 pandemic due to the widespread use of surface disinfectants, misuse, and overuse of antimicrobials [47]. Therefore, finding a safe therapeutic alternative to conventional drugs has become increasingly important for global health and the economy.
Previous studies documented the in vitro antimicrobial efficacy of G. asclepiadea- [2,48,49] and I. helenium-derived products [18,19,20,43,50], tested alone or in combination with different compounds.
An aqueous extract obtained from I. helenium was found active against Bacillus mycoides for MIC 5 mg/mL, and with synergistic activity combined with sodium nitrite and potassium sorbate against Bacillus subtilis and Pseudomonas fluorescens [19]. The in vitro anti-Staphylococcus aureus efficacy against both antibiotic-resistant and susceptible clinical isolates was documented for a hydroethanolic extract obtained from the rhizome and roots at concentrations between 0.9 and 9.0 mg/mL [50]. Similar in vitro anti-staphylococcal activity was reported for (hydro)ethanolic root extracts of I. helenium L. (elecampane) naturalized in Ireland supporting their traditional usage [20]. Additionally, these products demonstrated efficacy against other Gram-positive bacteria such as Group-A Streptococcus pyogenes, Group-B Streptococcus agalactiae, Listeria monocytogenes, and also Gram-negative Escherichia faecalis ATCC 29212 and Escherichia coli, as well as Mycobacterium tuberculosis H37Ra (ATCC 25177) [20]. In vitro antibacterial (Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa) and antifungal (Candida albicans and Candida tropicalis) properties were demonstrated by the agar dilution method in case of methanolic extracts obtained from three Inula species, I. viscosa, I. helenium ssp. turcoracemosa, and I. montbretiana, collected from different locations of Anatolia [43]. Moreover, a mixture of sesquiterpene lactones and essential oil extracted from I. helenium cultivated in Hungary exhibited considerable inhibitory effects against six species of fungi (Candida albicans, Candida glabrata, Candida cruzei, Candida parapsilosis, Saccharomyces cerevisiae, Aspergilus niger) and seven species of bacteria (Staphylococcus aureus, methicillin-resistant Staphylococcus aureus, Streptococcus pyogenes, Bacillus subtilis, Escherichia coli, E. coli D31, Pseudomonas aeruginosa [51]. For the Romanian cultivar, Diguță et al. [52] reported in vitro antimicrobial potential in the case of an ethanolic extract against veterinary strains of Bacillus subtilis, Bacillus cereus, Enterococcus faecalis, Escherichia coli, Staphylococcus aureus, Candida albicans, C. parapsilosis, C. lipolytica, and Aspergillus niger.
Certain G. asclepiadea extracts or their fractions prepared by maceration with methanol [49], water, ethanol, ethyl acetate, acetone, and diethyl ether demonstrated a better in vitro inhibitory efficacy against Gram-positive compared to Gram-negative bacteria [2,48]. At a concentration of 2.12 mg/mL, the aqueous extract of roots inhibited 50% of biofilm formation in case of S. aureus ATCC 25923 [2]. As for the active compounds responsible for the antimicrobial properties, the presence of xanthones [49] and of secoiridoid glycosides such as gentiopicroside, swertiamarin, and sweroside [12] appears to be relevant.
2.5. Cytotoxicity Assay
The results of MTT assay showed that G. asclepiadea roots extract (Figure 2a) did not exhibit any cytotoxic activity at the tested concentrations (0.0079–0.4726 μmol GAE/mL), cell viability ranging from 94.83 ± 3.58 to 74.89 ± 0.97%, respectively. The IC50 dose for G. asclepiadea extract was 1.1097 ± 0.028 μmol GAE/mL. At increasing concentrations (C6, C7, and C8), cell viability decreased statistically significantly compared to the untreated cells; however, this was not considered biologically significant. Additionally, a strong linear correlation was observed between the cell viability and the total polyphenolic content of G. asclepiadea extract (r2 = 0.7997).
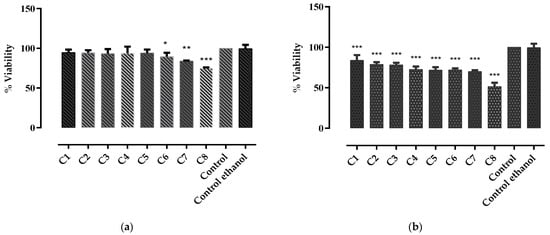
Figure 2.
Inhibitory effects of (a) G. asclepiadea and (b) I. helenium root extracts on rat intestinal epithelial cells, at eight different concentrations C1–C8 calculated according to the TPC (μmol GAE/mL extract): ranging between 0.0079 and 0.4786 for G. asclepiadea extract, and from 0.0135 to 0.8068 for I. helenium extract. Control—untreated cells, Control ethanol—cells treated with ethanol. Values are represented as mean ± SD. Statistically significant differences between treated and untreated cells (control): * p < 0.05; ** p < 0.001; *** p < 0.0001.
Similarly, according to the study conducted by Hudecová et al. [53], the methanolic extract of willow gentian flowers did not exhibit any cytotoxic or genotoxic effect on monkey kidney cell line (COS 1) at concentrations ranging between 0.25 and 2.5 mg/mL. Additionally, previous studies showed that the methanolic extracts of G. lutea and G. rigescens protected the HepG2 (nontumorigenic human hepatoma) and THLE-2 (transformed human liver epithelial) cells from the cytotoxic effect of fatty acids and promoted the growth of HepG2 cells [54].
The results of MTT assay for the extract of I. helenium roots (Figure 2b) showed that at concentrations between 0.0135 and 0.5379 μmol GAE/mL, the ethanolic extract of I. helenium roots did not exhibit any cytotoxic activity, the cell viability ranging from 83.92 ± 6.37 to 70.11 ± 1.55%. However, at a concentration of 0.8068 μmol GAE/mL, a mild cytotoxic activity was observed, cell viability being 51.54 ± 4.68%. The IC50 dose of I. helenium extract was 0.9093 ± 0.016 μmol GAE/mL. Similar to the G. asclepiadea extract, a strong linear correlation was noticed between the cytotoxic effect of I. helenium extract and its TPC (r2 = 0.9485). At the highest tested concentration (C8), both extracts influenced the shape of the intestinal epithelial cells, causing them to become rounded and flat, without affecting their viability.
Similar to our results, alantolactone and isoalantolactone, the major bioactive compounds isolated from I. helenium roots, did not exert any cytotoxic effect on Caco-2 cells, widely used as intestinal epithelial cells model [55]. No cytotoxic effect of I. helenium extract was observed also in PBMC (peripheral blood mononuclear cells), RAW 264.7 macrophage cells, and BV-2 microglial cells [4,56,57]. However, on the human tumor cell lines (HT-29, MCF-7, Capan-2, G1), I. helenium root extract exhibited potent cytotoxic activity [4]. The differences in the cytotoxic activity of the extract on cancer and healthy cells can be explained by variances in their cellular metabolism.
3. Materials and Methods
3.1. Chemicals and Reagents
Methanol, formic acid, salicylic acid, and chrysin used for LC/MS analysis were purchased from Merck (Darmstadt, Germany). All other chemicals used as standards for LC-MS analysis were purchased form Phytolab (Vestenbergsgreuth, Germany). All microorganism strains were distributed by Oxoid Ltd. (Basingstok, Hampshire, UK), while the culture mediums, Mueller Hinton Broth and Mueller Hinton agar, were purchased from Merck (Darmstadt, Germany). Rat intestinal epithelial cells used for the cytotoxic potential were isolated from fetal donors. Collagenase type I, dispase type I, and Hanks’ balanced salt solutions used for the enzymatic digestion of intestine were purchased from Sigma-Aldrich (Darmstadt, Germany). DMEM medium used as isolation and propagation media was purchased from Sigma-Aldrich (Darmstadt, Germany). Fetal bovine serum (FCS) and antibiotic-antimycotic solution used to supplement the isolation and propagation media were purchased from Gibco Life Technologies (Paisley, UK). Non-essential amino acids and epidermal growth factor used to supplement propagation medium were purchased from Sigma-Aldrich (Darmstadt, Germany).
3.2. Plant Material and Extract Preparation
Gentiana asclepiadea and Inula helenium roots were purchased from an authorized herbal online store in Romania (AdServ SRL). The plant materials were identified by Lecturer Irina Ielciu, PhD, and voucher specimens species are deposited at the Department of Pharmaceutical Botany of the “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca (Vouchers number 376–377). For the extract preparation, 5 g of dried roots were powdered at 450 µm particle size using a Gindomix GM 200 mechanical grinder (Retsch GmBH, Eragny, France) and mixed with 100 mL of 70% v/v ethanol. Moisture content was established at 12.5% for G. asclepiadea and at 10.5% for I. helenium using a Kern DLB Thermobalance (Kern&Sohn GmBH, Stuttgart, Germany). Resulting suspensions were vortexed thoroughly for 30 min and left in a dark place at room temperature to macerate for 10 days. The resulting suspensions were centrifuged at 4000 RPM for 10 min. The obtained 70% v/v ethanolic extract was filtered through grade 1 Whatman filter paper and stored in amber glass bottles at 4 °C.
3.3. Quantification of Total Phenolic, Flavonoid, and Phenolic Acids Content
The TPC was determined by a spectrophotometric method using Folin–Ciocâlteu reagent. Gallic acid was used as standard phenolic total, the result being expressed as g GAE per 100 g of dry plant material. Spectrophotometric determination of TFC was performed using aluminum chloride as chromogenic agent, and absorbance was measured at 430 nm. Moreover, rutoside was used as a standard reference solution for the construction of calibration curve, and the results were expressed as g RE per 100 g of dry material. TPA content was determined by spectrophotometric method, using Arnow reagent. The absorbance was determined at 500 nm, and TPA content was expressed as g CAE per 100 g of dry material. All the determinations were performed using a UV–V is spectrophotometer (Specord 200 Plus, Analytik Jena, Germany) [58,59,60,61].
3.4. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis
The LC/MS method was performed on a Shimadzu Nexera I LC/MS—8045 (Kyoto, Japan) UHPLC system equipped with a quaternary pump, autosampler, and an ESI probe and quadrupole rod mass spectrometer. The separation was carried out on a Luna C18 reversed phase column (150 mm × 4.6 mm × 3 μm, 100 Å), from Phenomenex (Torrance, CA, USA). The column was maintained at 40 °C during analysis.
The mobile phase (Table 6) was a gradient made from methanol and ultrapurified water prepared by Simplicity Ultra-Pure Water Purification System (Merck Millipore, Billerica, MA, USA). Formic acid was used as an eluent. The methanol and formic acid were of LC/MS grade. The used flow rate was of 0.5 mL/min. The total time of the analysis was 35 min.

Table 6.
LC-MS mobile phase gradient.
The detection was performed on a quadrupole rod mass spectrometer operated with electrospray ionization (ESI), both in negative and positive MRM (multiple reaction monitoring) ion mode. The interface temperature was set at 300 °C. Gas nitrogen was used for vaporization and as drying at 30 psi, respectively, at 10 L/min. The capillary potential was set at +3000 V.
The references used for quantification can be found in Table 7, 1 μL of each reference at each concentration was injected. The identification was performed by comparison of the retention times, the MS spectra, and its transitions between the separated compounds and standards. The identification and quantification were performed based on the main transition from the MS spectra of the compound.

Table 7.
LC-MS standards identification parameters.
The LC-MS method was validated by evaluating linearity, precision, and accuracy according to International Conference on Harmonization guidelines (ICH). The LOD and LOQ were calculated after injecting a series of different concentrations for each standard. The extracts were assayed for precision under optimized conditions. The method accuracy was determined in duplicate by a recovery experiment. All samples and references were injected in triplicate.
For quantification purposes, the calibration curves were also determined (Figures S1–S13, Supplementary Materials). Calibration curves, equations, their correlation factors, and the determined limit of detection and quantification are presented in Table 8.

Table 8.
LC-MS standards quantification parameters.
3.5. Antioxidant Activity
3.5.1. Ferric-Reducing Antioxidant Power Assay (FRAP)
FRAP reagent was prepared by mixing 10 mM TPTZ solution in 40 mM HCl (2.5 mL), 20 mM FeCl3·6H2O solution (2.5 mL), and acetate buffer (25 mL, pH 3.6). For FRAP assay, 4 mL of plant extract was mixed with 1.8 mL of water, and 6 mL of FRAP reagent. In the negative control, the extract was replaced with 4 mL of water. The absorbance of obtained solutions was read at 450 nm, using Trolox as standard for the calibration curve (R2 = 0.992). The results of FRAP assay were expressed as μmol of Trolox Equivalents (TE) per 100 mL of extract [58,59,60,61]
3.5.2. DPPH Radical Scavenging Activity Assay
DPPH assay was used to determine the antioxidant potential of I. helenium and G. asclepiadea root extracts. For the preparation of DPPH solution, 10 mg of DPPH was weighted and dissolved in 100 mL methanol. For each tested plant extract, a serial dilution was prepared by mixing 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, and 2 mL of extract with methanol, to obtain a final volume of 4 mL. Then, 2.0 mL of DPPH methanolic solution was added to prepare sample dilutions and the final reaction mixtures were incubated at 40 °C for 30 min. For the negative control, plant extract was replaced with 2 mL of DPPH methanolic solution. Absorbance was measured at 517 nm and the extract DPPH radical scavenging activity was calculated using the following formula:
The results of the DPPH assay were expressed as IC50 value (μg/mL), representing the concentration of antioxidant capable of reducing the DPPH radical concentration by half [58,59,60,61].
3.6. Antibacterial Activity
The in vitro antimicrobial potential was screened by agar well-diffusion assay, a modified EUCAST (European Committee on Antimicrobial Susceptibility Testing) [62] disk-diffusion method. Six reference strains were included Staphylococcus aureus ATCC 25923, Bacillus cereus ATCC 14579, Enterococcus faecalis ATCC 29219, Escherichia coli ATCC 25922, Salmonella typhimurium ATCC 14028, and Salmonella enteritidis ATCC 13076. For each organism, an inoculum was made suspending 24 h pure culture in Mueller Hinton (MH) broth to obtain 10E6 colony forming unit (CFU)/mL according to McFarland scale. The MH agar plates surface was “flood-inoculated” with the bacterial inoculum and prepared for the extract’s evaluation; six-millimeter diameter wells (three for each extract) were aseptically made into the MH agar to contain 60 μL of tested product and 70% ethanol, respectively (as the negative control). Gentamicin was also included as standard antibiotic. The growth inhibition zones diameters in millimeters were measured after 24 h of incubation at 37 °C. Furthermore, the extracts minimum inhibitory (MIC) and bactericidal (MBC) concentrations were established using a broth microdilution method. Two-fold serial dilutions were made in 100 µL broth for each of the two extracts; 5.0 µL of a 24 h 1 × 107 CFU/mL bacterial inoculum were added in each well and incubated for 24 h at 37 °C. MICs values were read as the lowest concentrations able to inhibit the visible growth of bacteria (no turbidity in the well), when compared to the negative control (broth). From each well, 10.0 µL were cultured on MH agar plates for 24 h at 37 °C. MBCs values were read as the lowest concentrations associated with no visible bacterial growth on the agar plates. All these tests were performed in triplicate.
Based on the ratio MBC/MIC, the MIC index was also calculated for each extract to evaluate whether the extract exhibits bactericidal (MBC/MIC ≤ 4) or bacteriostatic (MBC/MIC > 4) effect against the tested bacterial strains [63].
3.7. Isolation of Rat Intestinal Epithelial Cells
Intestinal epithelial cell culture was prepared using the method previously described by Evans et al., with some adaptations [64]. Pregnant Wistar female rats, aged 8 to 10 weeks, were sacrificed on the 14th day, following vaginal plug detection, according to the European Union Directive 2010/63/EU [65]. The pregnant uterus was revealed through a transversal abdominal incision, and transferred to a Petri dish with sterile phosphate buffer solution (PBS). Fetuses were collected and immersed in Tyrode solution. After the incision of the abdominal wall, the small intestine was collected from each fetal donor, and washed five times with PBS. Small intestine samples were cut into smaller pieces, and immersed in enzymatic solution, which included 2 mg/mL collagenase type I solution and 0.1 mg/mL dispase type I in HBSS (Hanks’ Balanced Salt Solution), and incubated for 15 min at 25 °C on a magnetic stirrer. After incubation, cells were resuspended by pipetting the cell suspension for 2 min and examined under the inverted microscope to confirm the separation of different tissue components. At this phase, the suspension was composed from muscle fragments, multicellular epithelial aggregates, single cells, and cellular debris. When more than 70% of epithelium of the villosities/crypts were separated, the enzymatic solution was neutralized by adding an equal volume of fetal bovine serum (FCS).
The obtained mixture was allowed to sediment for 1 min, the supernatant containing muscle cells was aspirated, and sediment was resuspended in isolation media composed of DMEM, 10% FCS, and 1% antibiotic-antimycotic. Samples were centrifuged at 3000 RPM for 3 min at 4 °C. Cellular sediment was resuspended in propagation medium DMEM, supplemented with 10% FCS, 1% non-essential amino acids (NEA), 1% antibiotic-antimycotic, 0.25 IU/mL, and 20 ng/mL epidermal growth factor (EGF). Following the evaluation of cell number and viability (0.4% Trypan blue), cells were cultivated on propagation medium and incubated at 37 °C, 5% CO2, and relative humidity of 60–90%.
After 48 h of propagation, nonadherent cells were removed by changing the medium, for the adherent cells the medium was renewed every 2 days. The passage of cells continued until the cell culture reached a 70% confluence. The primary cell culture was passaged 4 times before further use in the toxicity study.
3.8. Cytotoxicity Assay
To evaluate the cytotoxic effect of 70% ethanolic extracts of I. helenium and G. asclepiadea roots, MTT assay (3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) was performed. In the assay, 96-well culture plates, with a density of 1 × 105 cells/well, and 200 µL of culture medium were used. Rat intestinal epithelial cells were treated with 0.25, 0.5, 1.0, 2.5, 5.0, 7.5, 10.0, and 15.0 mL of extracts, and resulting concentrations in the wells (C1, C2, C3, C4, C5, C6, C7, C8) were calculated according to the total phenolic content of each plant extract, and expressed as μmol GAE/mL extract, as follows: G. asclepiadea (C1= 0.0079, C2 = 0.0158, C3 = 0.0315, C4 = 0.0788, C5 = 0.1575, C6 = 0.2363, C7 = 0.3151, and C8 = 0.4726 μmol GAE/mL), and I. helenium (C1 = 0.0135, C2 = 0.0269, C3 = 0.0538, C4 = 0.1345, C5 = 0.2689, C6 = 0.4034, C7 = 0.5379, C8 = 0.8068 μmol GAE/mL). Each concentration was tested in triplicate. The negative control was represented by untreated cells.
After 24 h of exposure to the extract, the culture medium was removed from the wells and 100 μL of MTT solution (0.5 mg MTT/mL HBSS buffer) were added to each well. The culture plates were incubated at 37 °C for 4 h. Subsequently, the MTT reagent was removed and 100 μL of dimethylsulfoxide (DMSO, Sigma) was distributed to each well to solubilize the formazan particles. Absorbance of the chromogenic reaction was measured by spectrophotometry with a BioTek Synergy 2 microplate reader (Winooski, VT, USA), at a wavelength of 450 nm. Results were presented as average % of cell viability (2):
where OD stands for the optical density value. Cell viability and proliferative capacity of treated cells were compared with the negative control. In addition, for each tested plant extract, the half-maximal inhibitory concentration (IC50) value was calculated from the dose response curve obtained using non-linear regression [60].
(%) Viability = (mean sample OD/mean control OD) × 100%,
3.9. Statistical Analysis
The obtained data were statistically analyzed using ANOVA GraphPad Prism software, version 6.0 (San Diego, CA, USA). The samples were analyzed in triplicate and quantitative determinations were given as mean ± standard deviation (SD). The compounds under quantification (<LOQ) limits could be only identified. One-way analysis of variance (ANOVA) was conducted, followed by Tukey’s post hoc test, to determine statistical significance between the chemical profile components and the antioxidant, antimicrobial, and cytotoxic potential of the two extracts, considering statistically significant p < 0.05. In addition, CORREL function was used to calculate Pearson’s correlation coefficients for the analyzed data, namely total phenolic, flavonoid, and phenolic acids content in tested extracts and the antioxidant, antimicrobial, and cytotoxic activity, respectively.
4. Conclusions
To our knowledge, the present study represents the first report in scientific literature regarding the lack of toxicity of G. asclepiadea and I. helenium ethanolic root extracts on rat intestinal epithelial cells.
The study highlighted the fact that these two extracts represent valuable sources of bioactive compounds with therapeutic potential. Particularly, identification and quantification of amarogentin in G. asclepiadea harvested from Romania, is of scientific interest, since the population of other gentian species of high medicinal interest is decreasing worldwide (G. lutea, G. punctata, G. dinarica), positioning it as a promising substitute.
Furthermore, a direct correlation between the biological activities of these plant extracts and their total phenolic and flavonoid concentrations was indicated by the statistical analysis. These data support their ethnomedicinal recommendations in digestive disorders, further studies are intended to develop standardized therapeutic products.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27113560/s1, Figure S1: The calibration curve of caffeic acid; Figure S2: The calibration curve of trans-p-coumaric acid; Figure S3: The calibration curve of salicylic acid; Figure S4: The calibration curve of chlorogenic acid; Figure S5: The calibration curve of amarogentin; Figure S6: The calibration curve of apigenine; Figure S7: The calibration curve of chrysine; Figure S8: The calibration curve of luteolin; Figure S9: The calibration curve of luteolin-7-O-glucoside; Figure S10: The calibration curve of quercetin; Figure S11: The calibration curve of rutoside; Figure S12: The calibration curve of naringenin; Figure S13: The calibration curve of hesperitin.
Author Contributions
Conceptualization, L.C.Ș. and V.B.; methodology, D.H., M.N., E.P., R.F.B., N.-K.O. and I.I. (Irina Ielciu); validation, L.C.Ș., D.H. and M.N.; formal analysis, M.N., D.H., V.B. and I.I. (Irina Ielciu); investigation, V.B., M.N., D.H., E.P., R.F.B., N.-K.O., M.-C.M.-L., I.V., I.I. (Ilinca Iozon), A.R.S., I.I. (Irina Ielciu) and L.C.Ș.; data curation, V.B., D.H., M.N. and L.C.Ș.; writing—original draft preparation, V.B., M.N., D.H., E.P., R.F.B. and N.-K.O.; writing—review and editing, V.B., M.N., D.H., E.P., R.F.B., N.-K.O., M.-C.M.-L., I.V., I.I. (Ilinca Iozon), A.R.S., I.I. (Irina Ielciu) and L.C.Ș.; visualization, L.C.Ș., D.H. and V.B.; supervision, L.C.Ș. and D.H.; project administration, L.C.Ș., V.B. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethics Committee of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca, Romania (No.248/29.03.2021), and by the National Sanitary Veterinary and Food Safety Authority (ANSVSA) of Romania (No.258/13.05.2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Khan, M.S.A.; Ahmad, I. Chapter 1—Herbal Medicine: Current Trends and Future Prospects. In New Look to Phytomedicine; Khan, M.S.A., Ahmad, I., Chattopadhyay, D., Eds.; Academic Press: London, UK, 2019; pp. 3–13. [Google Scholar] [CrossRef]
- Stefanović, O.; Ličina, B.; Vasić, S.; Radojević, I.; Čomić, L. Bioactive extracts of Gentiana asclepiadea: Antioxidant, antimicrobial, and antibiofilm activity. Bot. Serbica 2018, 42, 223–229. [Google Scholar] [CrossRef]
- Mihailovic, V.; Matic, S.; Mišic, D.; Solujic, S.; Stanic, S.; Katanic, J.; Mladenovic, M.; Stankovic, N. Chemical composition, antioxidant and antigenotoxic activities of different fractions of Gentiana asclepiadea L. roots extract. EXCLI J. 2013, 12, 807–823. Available online: https://www.excli.de/index.php/excli/article/view/1194 (accessed on 9 May 2022). [PubMed]
- Dorn, D.C.; Alexenizer, M.; Hengstler, J.G.; Dorn, A. Tumor cell specific toxicity of Inula helenium extracts. Phytother. Res. 2006, 20, 970–980. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Struwe, L. Classification and evolution of the Gentianaceae. In The Gentianaceae: Characterization and Ecology; Rybczyński, J.J., Davey, M.R., Mikula, A., Eds.; Springer: Heidelberg, Germany, 2014; Volume 1, pp. 13–35. [Google Scholar]
- Bilz, M. Gentiana asclepiadea. The IUCN Red List of Threatened Species. 2013, E.T203218A2762391. Available online: https://www.iucnredlist.org/ (accessed on 26 March 2022).
- Zając, A.; Pindel, A. Review of the Willow Gentian, Gentiana asclepiadea L. Biodiversity 2011, 12, 181–185. [Google Scholar] [CrossRef]
- Papp, N.; Tóth, M.; Dénes, T.; Gyergyák, K.; Filep, R.; Bartha, S.G.; Csepregi, R.; Balázs, V.L.; Farkas, Á. Ethnomedicinal treatment of gastrointestinal disorders in Transylvania, Romania. Acta Ethnogr. Hung. 2017, 62, 207–220. [Google Scholar] [CrossRef]
- Mihailović, V.; Mihailović, M.; Uskoković, A.; Arambašić, J.; Mišić, D.; Stanković, V.; Katanić, J.; Mladenović, M.; Solujić, S.; Matić, S. Hepatoprotective effects of Gentiana asclepiadea L. extracts against carbon tetrachloride induced liver injury in rats. Food Chem. Toxicol. 2013, 52, 83–90. [Google Scholar] [CrossRef]
- Milutinović, M.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Plant Extracts Rich in Polyphenols as Potent Modulators in the Growth of Probiotic and Pathogenic Intestinal Microorganisms. Front. Nutr. 2021, 8, 688843. [Google Scholar] [CrossRef]
- Szucs, Z.; Dános, B.; Nyiredy, S.Z. Comparative analysis of the underground parts of Gentiana species by HPLC with diode-array and mass spectrometric detection. Chromatographia 2002, 56, S19–S23. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Gadimli, A.I.; Isaev, J.I.; Kashchenko, N.I.; Prokopyev, A.S.; Kataeva, T.N.; Chirikova, N.K.; Vennos, C. Caucasian Gentiana Species: Untargeted LC-MS Metabolic Profiling, Antioxidant and Digestive Enzyme Inhibiting Activity of Six Plants. Metabolites 2019, 9, 271. [Google Scholar] [CrossRef]
- Mandel, J.R.; Dikow, R.B.; Siniscalchi, C.M.; Thapa, R.; Watson, L.E.; Funk, V.A. A fully resolved backbone phylogeny reveals numerous dispersals and explosive diversifications throughout the history of Asteraceae. Proc. Natl. Acad. Sci. USA. 2019, 116, 14083–14088. [Google Scholar] [CrossRef] [PubMed]
- Khela, S. Inula helenium. The IUCN Red List of Threatened Species. 2013, E.T202982A2758393. Available online: https://www.iucnredlist.org/ (accessed on 26 March 2022).
- Buza, V.; Matei, M.-C.; Ștefănuț, L.C. Inula helenium: A literature review on ethnomedical uses, bioactive compounds and pharmacological activities. Lucr. Ştiinţ. Ser. Med. Vet. 2020, 63, 53–59. [Google Scholar]
- Ghedira, K.; Goetz, P.; Le Jeune, R. Inula helenium L. (Asteraceae): Aunée. Phytothérapie 2011, 9, 176–179. [Google Scholar] [CrossRef]
- Deriu, A.; Zanetti, S.; Sechi, L.A.; Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E. Antimicrobial activity of Inula helenium L. essential oil against Gram-positive and Gram-negative bacteria and Candida spp. Int. J. Antimicrob. Agents 2008, 31, 588–590. [Google Scholar] [CrossRef]
- Stanojević, D.; Ćomić, L.J.; Stefanović, O.; Sukdoloak, S.S. In Vitro synergistic antibacterial activity of Helichrysum arenarium, Inula helenium, Cichorium intybus and some preservatives. Ital. J. Food Sci. 2010, 22, 210–216. [Google Scholar]
- Kenny, C.-R.; Stojakowska, A.; Furey, A.; Lucey, B. From Monographs to Chromatograms: The Antimicrobial Potential of Inula helenium L. (Elecampane) Naturalised in Ireland. Molecules 2022, 27, 1406. [Google Scholar] [CrossRef]
- Abolfathi, M.E.; Tabeidian, S.A.; Foroozandeh Shahraki, A.D.; Tabatabaei, S.N.; Habibian, M. Comparative effects of n-hexane and methanol extracts of elecampane (Inula helenium L.) rhizome on growth performance, carcass traits, feed digestibility, intestinal antioxidant status and ileal microbiota in broiler chickens. Arch. Anim. Nutr. 2019, 73, 88–110. [Google Scholar] [CrossRef]
- Spiridon, I.; Nechita, C.B.; Niculaua, M.; Silion, M.; Armatu, A.; Teacă, C.-A.; Bodîrlău, R. Antioxidant and chemical properties of Inula helenium root extracts. Cent. Eur. J. Chem. 2013, 11, 1699–1709. [Google Scholar] [CrossRef]
- Petkova, N.; Vrancheva, R.; Mihaylova, D.; Ivanov, I.; Pavlov, A.; Denev, P. Antioxidant activity and fructan content in root extracts from elecampane (Inula helenium L.). J. Biosci. Biotechnol. 2015, 4, 101–107. [Google Scholar]
- Zlatić, N.; Jakovljević, D.; Stanković, M. Temporal, Plant Part, and Interpopulation Variability of Secondary Metabolites and Antioxidant Activity of Inula helenium L. Plants 2019, 8, 179. [Google Scholar] [CrossRef]
- Patel, K.; Kumar, V.; Verma, A.; Rahman, M.; Patel, D.K. Amarogentin as Topical Anticancer and Anti-Infective Potential: Scope of Lipid Based Vesicular in its Effective Delivery. Recent Pat. Antiinfect. Drug Discov. 2019, 14, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Phoboo, S.; Pinto, M.; Barbosa, A.C.; Sarkar, D.; Bhowmik, P.C.; Jha, P.K.; Shetty, K. Phenolic-linked biochemical rationale for the anti-diabetic properties of Swertia chirayita (Roxb. ex Flem.) Karst. Phytother Res 2013, 27, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Yen, T.-L.; Lu, W.-J.; Lien, L.-M.; Thomas, P.A.; Lee, T.-Y.; Chiu, H.-C.; Sheu, J.R.; Lin, K.-H. Amarogentin, a Secoiridoid Glycoside, Abrogates Platelet Activation through PLCγ2-PKC and MAPK Pathways. Biomed. Res. Int. 2014, 2014, 728019. [Google Scholar] [CrossRef] [PubMed]
- Disasa, D.; Cheng, L.; Manzoor, M.; Liu, Q.; Wang, Y.; Xiang, L.; Qi, J. Amarogentin from Gentiana rigescens Franch Exhibits Antiaging and Neuroprotective Effects through Antioxidative Stress. Oxid. Med. Cell. Longev. 2020, 2020, 3184019. [Google Scholar] [CrossRef] [PubMed]
- Tavares, W.R.; Seca, A.M.L. Inula L. Secondary Metabolites against Oxidative Stress-Related Human Diseases. Antioxidants 2019, 8, 122. [Google Scholar] [CrossRef]
- Nder, A.E. Efficacy of methanol-water extract of Inula helenium root against oxidative DNA damage. J. Tradit. Chin. Med. 2021, 41, 293–300. [Google Scholar] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Ivanova, V.; Trendafilova, A.; Todorova, M.; Danova, K.; Dimitrov, D. Phytochemical Profile of Inula britannica from Bulgaria. Nat. Prod. Commun. 2017, 12, 153–154. [Google Scholar] [CrossRef]
- Lin, C.N.; Kuo, S.H.; Chung, M.I.; Ko, F.N.; Teng, C.M. A New Flavone C-Glycoside and Antiplatelet and Vasorelaxing Flavones from Gentiana arisanensis. J. Nat. Prod. 1997, 60, 851–853. [Google Scholar] [CrossRef]
- Dou, X.; Zhou, Z.; Ren, R.; Xu, M. Apigenin, flavonoid component isolated from Gentiana veitchiorum flower suppresses the oxidative stress through LDLR-LCAT signaling pathway. Biomed. Pharmacother. 2020, 128, 110298. [Google Scholar] [CrossRef]
- Ozkan, E.; Pehlivan Karakas, F.; Birinci Yildirim, A.B.; Tas, I.; Eker, I.; Zeynep Yavuz, M.; Ucar Turker, A. Promising medicinal plant Inula viscosa L.: Antiproliferative, antioxidant, antibacterial and phenolic profiles. Prog. Nutr. 2019, 21, 652–661. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145 (Suppl. SC), 187–196. [Google Scholar] [CrossRef] [PubMed]
- Arul, D.; Subramanian, P. Naringenin (Citrus Flavonone) Induces Growth Inhibition, Cell Cycle Arrest and Apoptosis in Human Hepatocellular Carcinoma Cells. Pathol. Oncol. Res. 2013, 19, 763–770. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, T.; Yang, H.; Lan, X.; Ying, J.; Du, G. The flavonoid apigenin protects brain neurovascular coupling against amyloid-β₂₅₋₃₅-induced toxicity in mice. J. Alzheimer’s Dis. 2011, 24, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Gökbulut, A.; Ozhan, O.; Satilmiş, B.; Batçioğlu, K.; Günal, S.; Sarer, E. Antioxidant and antimicrobial activities, and phenolic compounds of selected Inula species from Turkey. Nat. Prod. Commun. 2013, 8, 475–478. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.M.; Zhang, B.; Guo, C.Y. Protective Effect of Total Phenolic Compounds from Inula helenium on Hydrogen Peroxide-induced Oxidative Stress in SH-SY5Y Cells. Indian J. Pharm. Sci. 2015, 77, 163–169. [Google Scholar] [CrossRef]
- Kusar, A.; Zupancic, A.; Sentjurc, M.; Baricevic, D. Free radical scavenging activities of yellow gentian (Gentiana lutea L.) measured by electron spin resonance. Hum. Exp. Toxicol. 2006, 25, 599–604. [Google Scholar] [CrossRef]
- O’Neill, J. The review on antimicrobial resistance. In Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; HM Government and Wellcome Trust: London, UK, 2016; Available online: https://amr-review.org (accessed on 10 April 2022).
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. iScience 2021, 24, 102304. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Matejić, J.; Kitić, D.; Krstev, T.M.; Kitić, N.; Šavikin, K.; Milutinović, M. Antimicrobial Activity of Fractions and The Extract from Gentiana asclepiadea L. Underground Parts with Molecular Docking Analysis. Acta Med. Median. 2022, 61, 14–22. [Google Scholar] [CrossRef]
- Mihailović, V.; Vuković, N.; Nićiforović, N.; Solujić, S.; Mladenović, M.; Mašković, P.; Stanković, M.S. Studies on the antimicrobial activity and chemical composition of the essential oils and alcoholic extracts of Gentiana asclepiadea L. J. Med. Plant Res. 2011, 5, 1164–1174. [Google Scholar] [CrossRef]
- O’Shea, S.; Lucey, B.; Cotter, L. In Vitro activity of Inula helenium against clinical Staphylococcus aureus strains including MRSA. Br. J. Biomed. Sci. 2009, 66, 186–189. [Google Scholar] [CrossRef]
- Budán, F.; Nan, M.; Kocsis, B.; Laczkó-Zöld, E. Antimicrobial activity and potential secondary signal transduction mechanisms of elecampane (Inula helenium L.) root extract. PCBMB 2021, 22, 86–92. [Google Scholar]
- Diguță, C.; Cornea, C.P.; Ioniță, L.; Brîndușe, E.; Farcaș, N.; Bobit, D.; Matei, F. Studies on antimicrobial activity of Inula helenium L. Romanian cultivar. Rom. Biotechnol. Lett. 2014, 19, 9699–9704. [Google Scholar]
- Hudecova, A.; Hasplova, K.; Miadokova, E.; Magdolenova, Z.; Rinna, A.; Galova, E.; Sevcovicova, A.; Vaculcikova, D.; Gregan, F.; Dusinska, M. Cytotoxic and genotoxic effect of methanolic flower extract from Gentiana asclepiadea on COS 1 cells. Neuro Endocrinol. Lett. 2010, 31 (Suppl. S2), 21–25. [Google Scholar]
- Boateng, A. Hepatoprotective Properties of Gentiana SPP: Against Non-Alcoholic Fatty Liver Disease (NAFLD). Ph.D. Thesis, University of Westminster Biomedical Sciences, London, UK, 2018. [Google Scholar]
- Xu, R.; Peng, Y.; Wang, M.; Li, X. Intestinal Absorption of Isoalantolactone and Alantolactone, Two Sesquiterpene Lactones from Radix Inulae, Using Caco-2 Cells. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 295–303. [Google Scholar] [CrossRef]
- Lee, S.-G.; Kang, H. Anti-neuroinflammatory Effects of Ethanol Extract of Inula helenium L. (Compositae). Trop. J. Pharm. Res. 2016, 15, 521–526. [Google Scholar] [CrossRef]
- Chun, J.; Song, K.; Kim, Y.S. Anti-inflammatory Activity of Standardized Fraction from Inula helenium L. via Suppression of NF-κB Pathway in RAW 264.7 Cells. Nat. Prod. Sci. 2019, 25, 16–22. [Google Scholar] [CrossRef][Green Version]
- Ielciu, I.; Sevastre, B.; Olah, N.-K.; Turdean, A.; Chişe, E.; Marica, R.; Oniga, I.; Uifălean, A.; Sevastre-Berghian, A.C.; Niculae, M.; et al. Evaluation of Hepatoprotective Activity and Oxidative Stress Reduction of Rosmarinus officinalis L. Shoots Tincture in Rats with Experimentally Induced Hepatotoxicity. Molecules 2021, 26, 1737. [Google Scholar] [CrossRef] [PubMed]
- Hanganu, D.; Niculae, M.; Ielciu, I.; Olah, N.-K.; Munteanu, M.; Burtescu, R.; Ştefan, R.; Olar, L.; Pall, E.; Andrei, S.; et al. Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts. Molecules 2021, 26, 3104. [Google Scholar] [CrossRef] [PubMed]
- Păltinean, R.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Angenot, L.; Tits, M.; Mocan, A.; Babotă, M.; Frumuzachi, O.; et al. Biological Activities of Some Isoquinoline Alkaloids from Fumaria schleicheri Soy. Will. Plants 2022, 11, 1202. [Google Scholar] [CrossRef]
- Ielciu, I.; Filip, G.A.; Oniga, I.; Olah, N.K.; Bâldea, I.; Olteanu, D.; Burtescu, R.F.; Turcuș, V.; Sevastre-Berghian, A.C.; Benedec, D.; et al. Oxidative Stress and Dna Lesion Reduction of a Polyphenolic Enriched Extract of Thymus marschallianus Willd. In Endothelial Vascular Cells Exposed to Hyperglycemia. Plants 2021, 10, 2810. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrobial Susceptibility Testing EUCAST Disk Diffusion Method; ESCMID: Basel, Switzerland, 2020; Volume 8, pp. 1–21. [Google Scholar]
- Niculae, M.; Hanganu, D.; Oniga, I.; Benedec, D.; Ielciu, I.; Giupana, R.; Sandru, C.D.; Cioc, N. Phytochemical Profile and Antimicrobial Potential of Extracts Obtained from Thymus marschallianus Willd. Molecules 2019, 24, 3101. [Google Scholar] [CrossRef]
- Evans, G.S.; Flint, N.; Somers, A.S.; Eyden, B.; Potten, C.S. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J. Cell Sci. 1992, 101, 219–231. [Google Scholar] [CrossRef]
- National Competent Authorities for the implementation of Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. A Working Document on the Development of a Common Education and Training Framework to Fulfil the Requirements under the Directive; European commission: Brussels, Belgium, 2014; Available online: https://ec.europa.eu/ (accessed on 15 June 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).