Isolation, Structural Elucidation, Antioxidant and Hypoglycemic Activity of Polysaccharides of Brassica rapa L.
Abstract
:1. Introduction
2. Results and Discussion
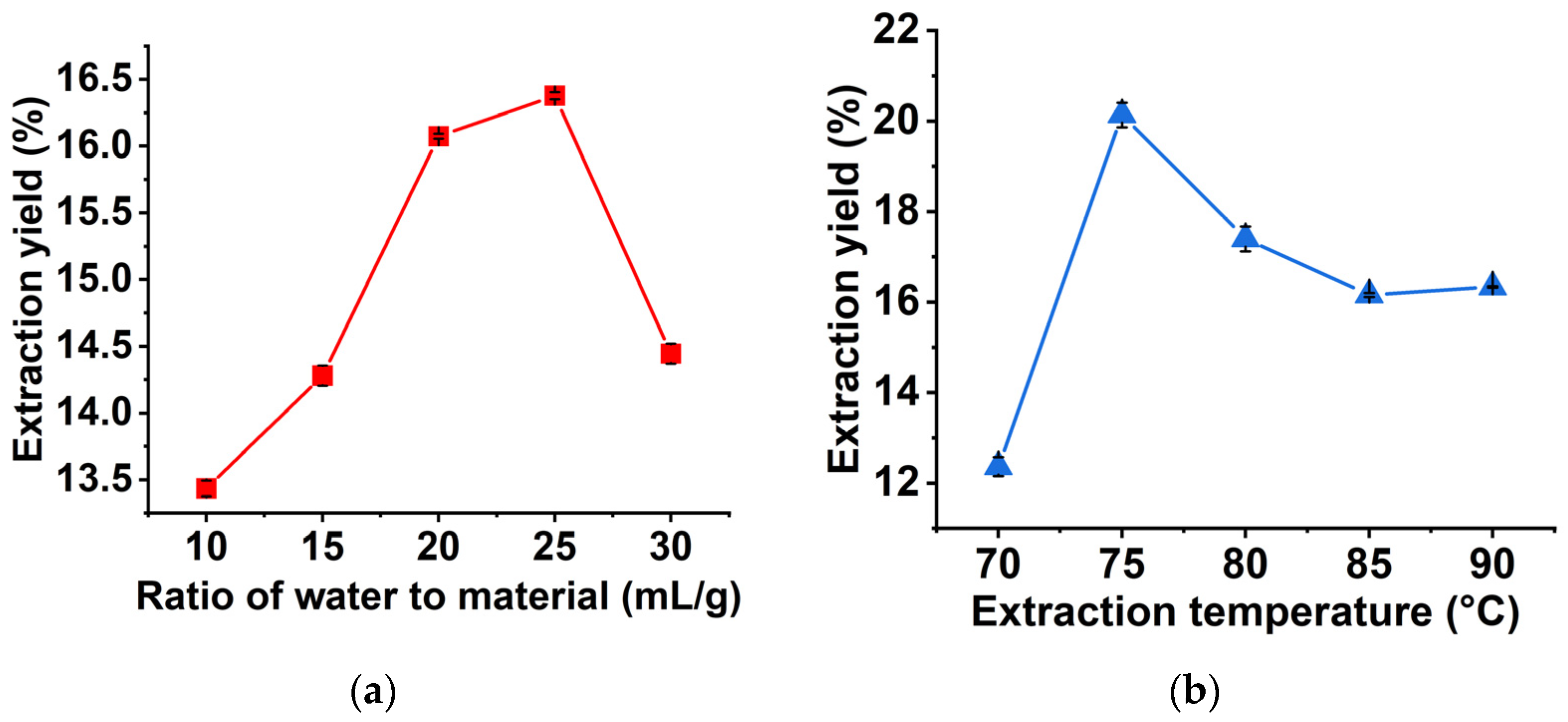
2.1. Single-Factor Experiments Analysis
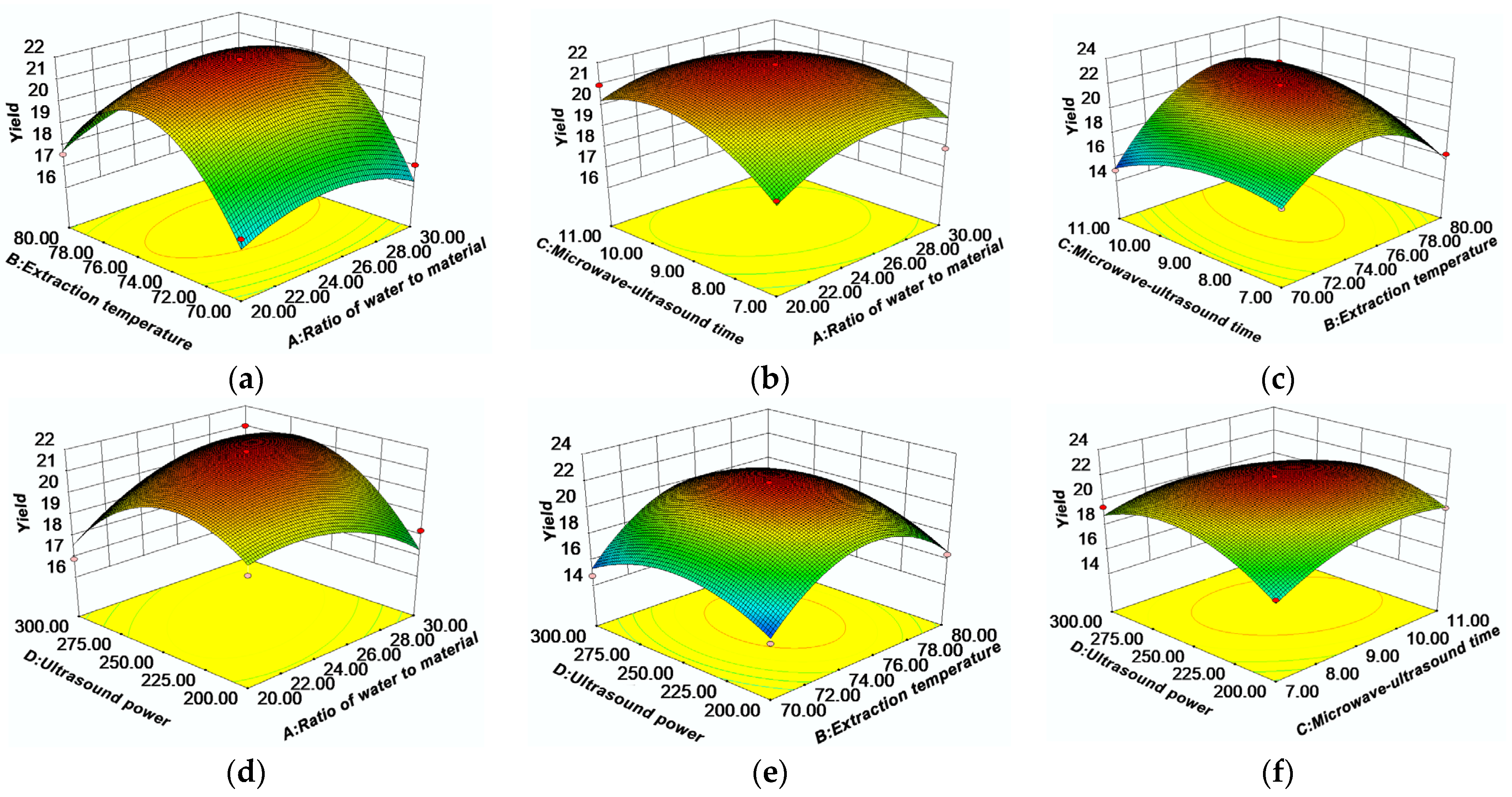
2.2. Response Surface Analysis
2.2.1. Optimization of Extraction Parameters by RSM
1.09A2 − 3.57B2 − 1.39C2 − 2.09D2
2.2.2. Analysis of Response Surface
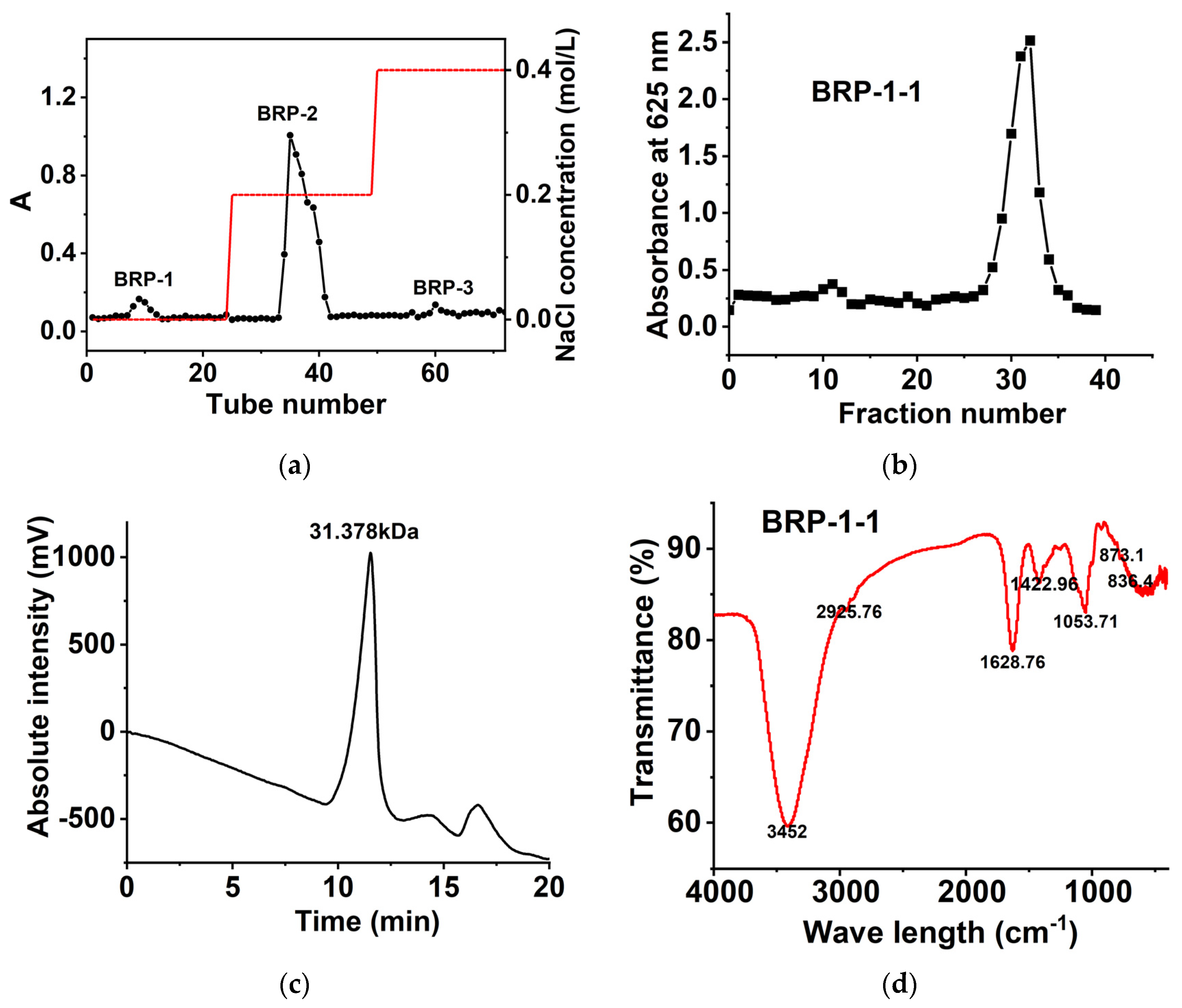
2.3. Polysaccharide Composition
2.4. FT-IR Spectroscopy Analysis
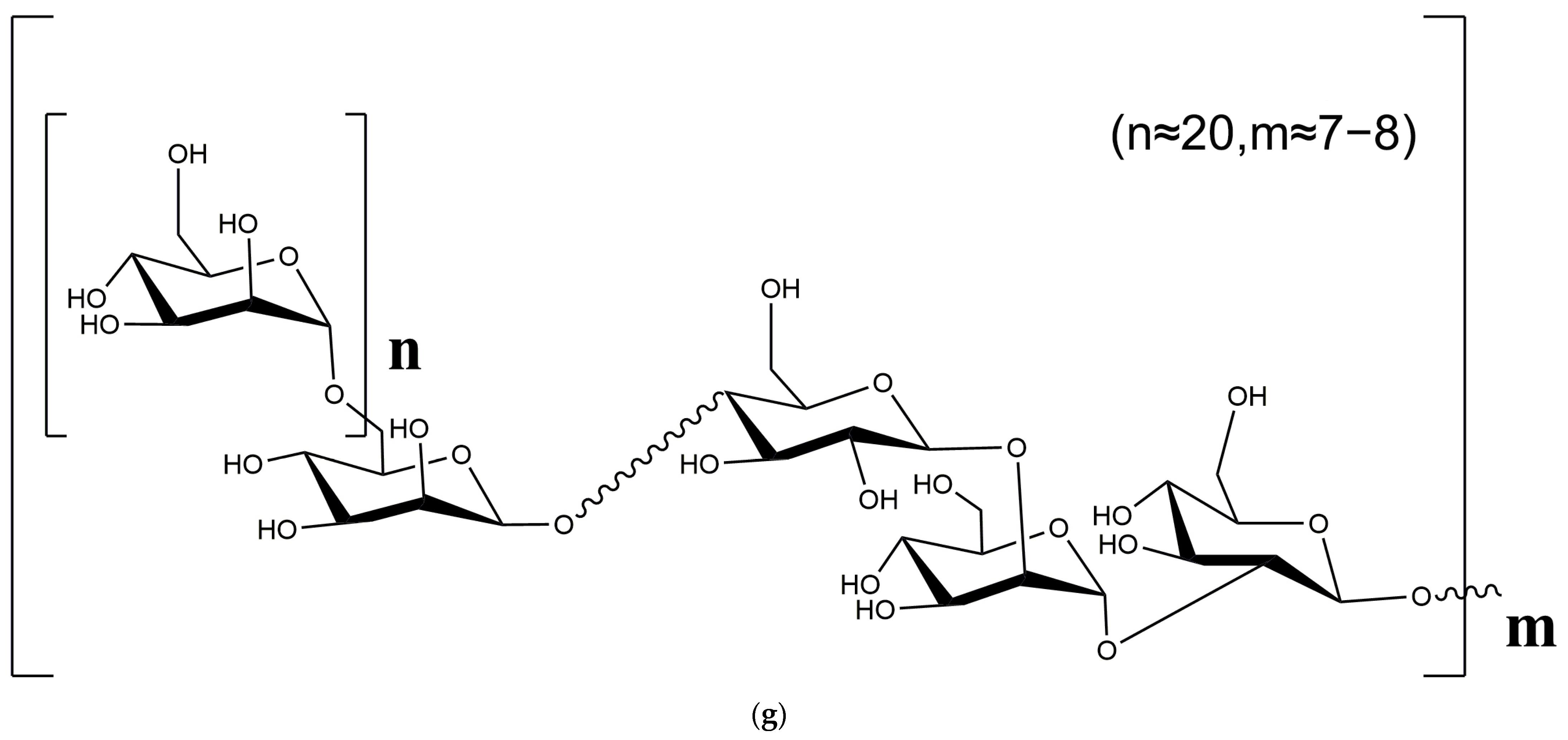
2.5. Methylation Analysis
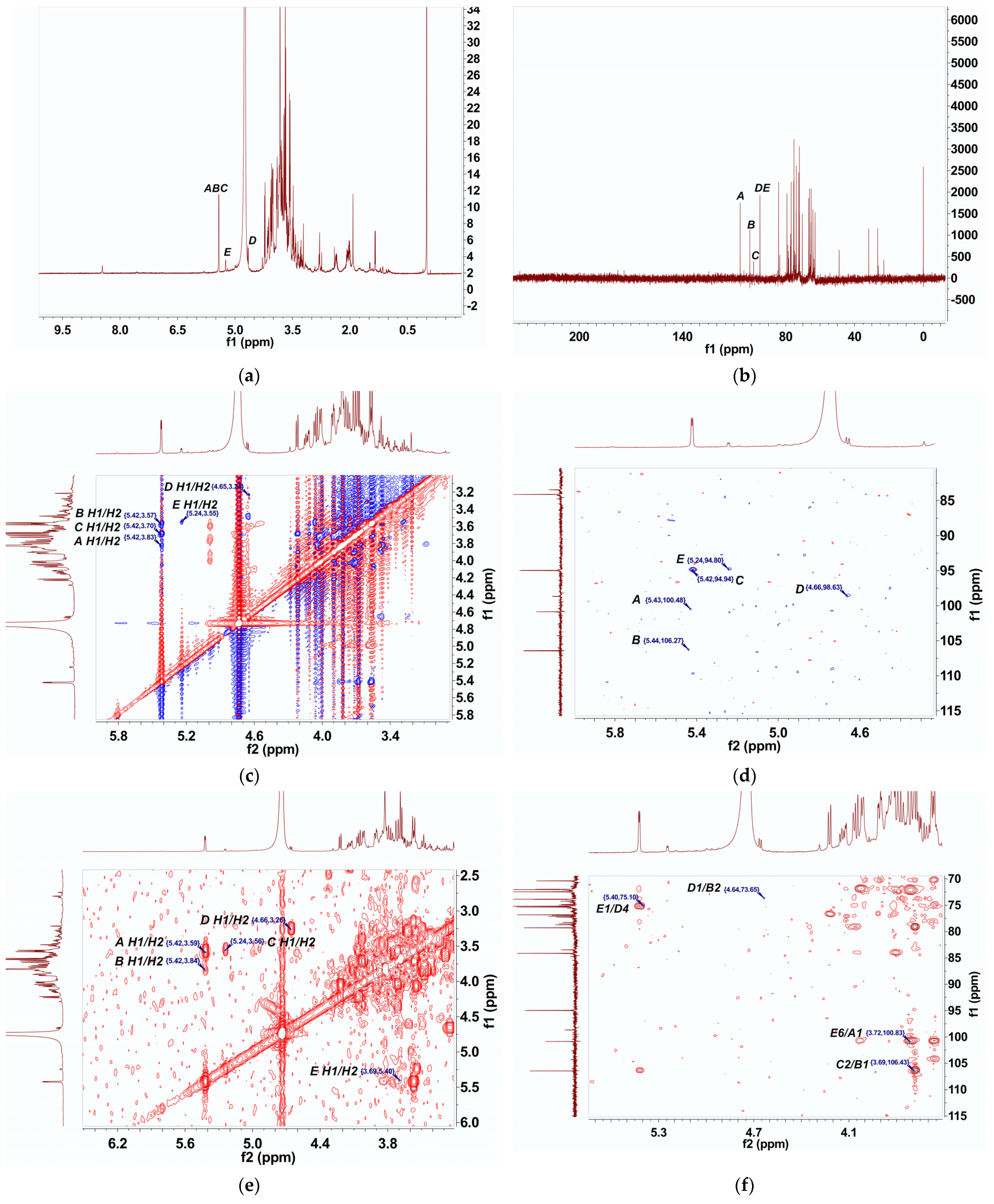
2.6. NMR Analysis
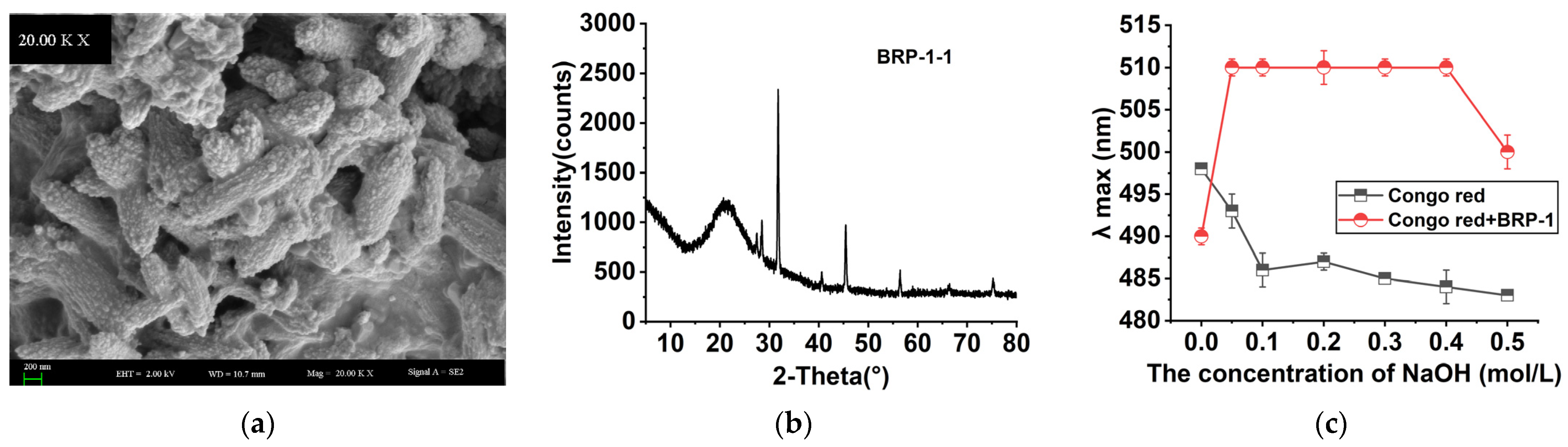
2.7. Morphological Properties
2.8. XRD Analysis
2.9. Congo Red Test Analysis
2.10. Antioxidant Activity
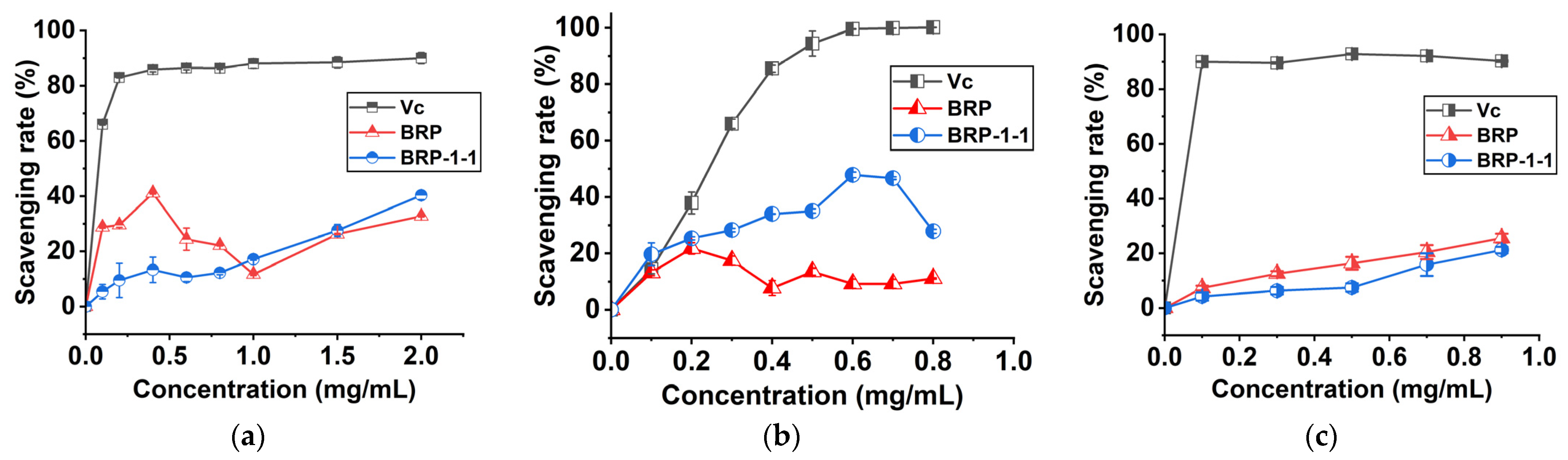
2.10.1. DPPH Scavenging Rate
2.10.2. Hydroxyl Scavenging Rate
2.10.3. ABTS Scavenging Rate
2.11. Hypoglycemic Activity
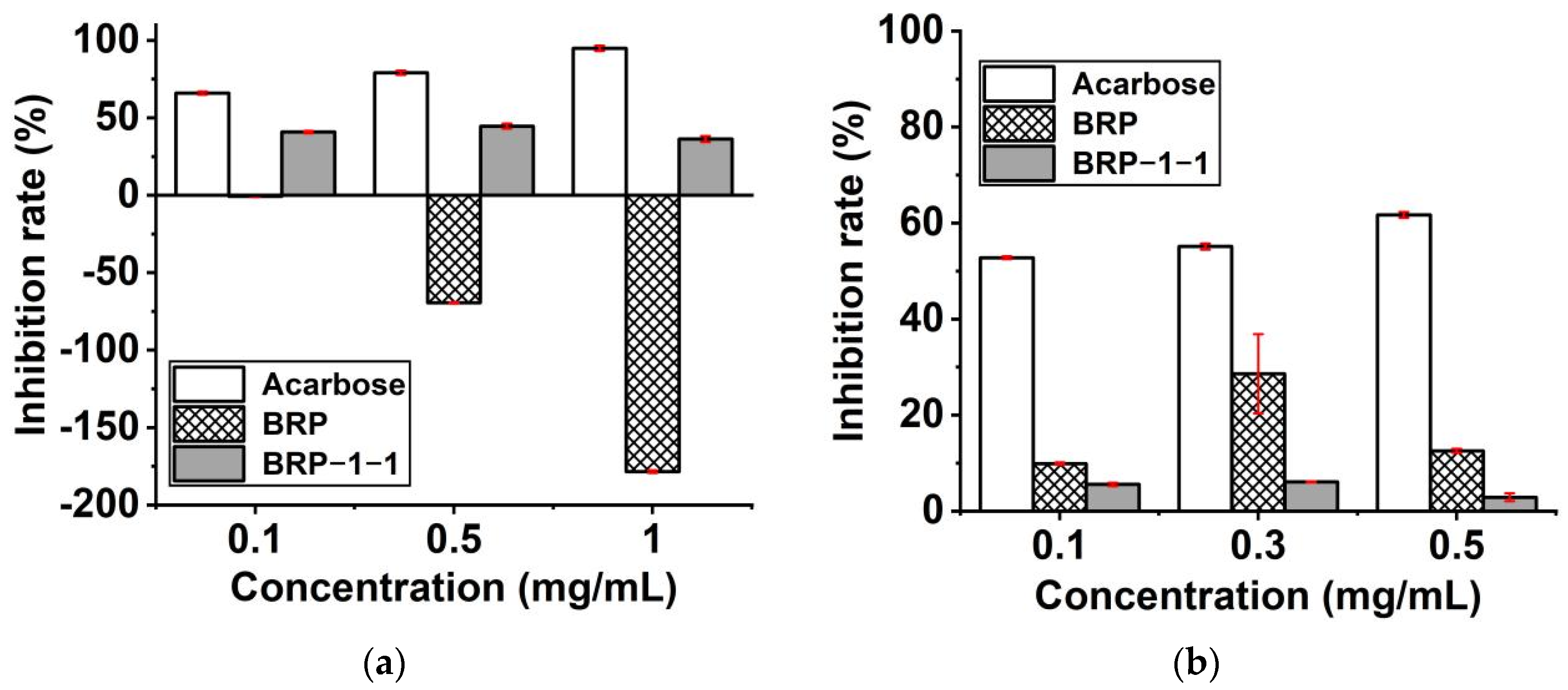
2.11.1. α-Glucosidase Inhibitory Activity
2.11.2. α-Amylase Inhibitory Activity
3. Materials and Methods
3.1. Materials
3.2. Extraction of Polysaccharides
3.2.1. Extraction Process
3.2.2. RSM Experimental Design
3.3. Purification of Polysaccharides
3.4. Composition Analysis
3.5. Molecular Weight
3.6. Monosaccharide Composition
3.7. Infrared Spectral Analysis
3.8. NMR Spectral Analysis
3.9. Methylation Analysis
3.10. XRD Analysis
3.11. Congo Red Test
3.12. Scanning Electron Microscopy
3.13. Antioxidant Activity Assays In Vitro
3.13.1. DPPH Scavenging Rate
3.13.2. Hydroxyl Scavenging Rate
3.13.3. ABTS Scavenging Rate
3.14. Hypoglycemic Activity Assays In Vitro
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khan, S.; Waqas, M.; Ding, F.; Shamshad, I.; Arp, H.P.H.; Li, G. The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J. Hazard. Mater. 2015, 300, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.; Chen, C.; Li, J.; Chen, X.; Li, Y.; Tang, W.; Jin, L.; Zhang, Y. Effects of Tibetan turnip (Brassica rapa L.) on promoting hypoxia-tolerance in healthy humans. J. Ethnopharmacol. 2017, 195, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Shi, Y.; Yuan, Z.; Huang, Z.; Cai, F.; Zhu, J.; Zhang, W.; Li, J.; Xiong, Q.; Wang, Y.; et al. Isolation, Identification, and Anti-Inflammatory Activity of Polysaccharides of Typha angustifolia. Biomacromolecules 2021, 22, 2451–2459. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, S.; Su, D.; Pi, N.; Ma, C.; Gao, P. Composition analysis and anti-hypoxia activity of polysaccharide from Brassica Rapa L. Int. J. Biol. Macromol. 2010, 47, 528–533. [Google Scholar] [CrossRef]
- Chen, Z.-E.; Wufuer, R.; Ji, J.-H.; Li, J.-F.; Cheng, Y.-F.; Dong, C.-X.; Taoerdahong, H. Structural Characterization and Immunostimulatory Activity of Polysaccharides from Brassica Rapa L. J. Agric. Food Chem. 2017, 65, 9685–9692. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Ye, H.; Hu, B.; Zhou, L.; Jabbar, S.; Zeng, X.; Shen, W. Optimization of extraction, characterization and antioxidant activity of polysaccharides from Brassica rapa L. Int. J. Biol. Macromol. 2016, 82, 979–988. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, W.; Liu, L.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Fractionation, characterization and anti-fatigue activity of polysaccharides from Brassica Rapa L. Process Biochem. 2021, 106, 163–175. [Google Scholar] [CrossRef]
- Wufuer, R.; Bai, J.; Liu, Z.; Zhou, K.; Taoerdahong, H. Biological activity of Brassica rapa L. polysaccharides on RAW264.7 macrophages and on tumor cells. Bioorg. Med. Chem. 2020, 28, 115330. [Google Scholar] [CrossRef]
- Abuduwaili, A.; Rozi, P.; Mutailifu, P.; Gao, Y.; Nuerxiati, R.; Aisa, H.A.; Yili, A. Effects of different extraction techniques on physicochemical properties and biological activities of polysaccharides from Fritillaria pallidiflora Schrenk. Process Biochem. 2019, 83, 189–197. [Google Scholar] [CrossRef]
- Tang, W.; Liu, D.; Yin, J.-Y.; Nie, S.-P. Consecutive and progressive purification of food-derived natural polysaccharide: Based on material, extraction process and crude polysaccharide. Trends Food Sci. Technol. 2020, 99, 76–87. [Google Scholar] [CrossRef]
- Lin, T.; Liu, Y.; Lai, C.; Yang, T.; Xie, J.; Zhang, Y. The effect of ultrasound assisted extraction on structural composition, antioxidant activity and immunoregulation of polysaccharides from Ziziphus jujuba Mill var. spinosa seeds. Ind. Crops Prod. 2018, 125, 150–159. [Google Scholar] [CrossRef]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations A review of current status. Chem. Eng. Process.-Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Zheng, Y.; Cui, J.; Chen, A.-H.; Zong, Z.-M.; Wei, X.-Y. Optimization of Ultrasonic-Microwave Assisted Extraction and Hepatoprotective Activities of Polysaccharides from Trametes orientalis. Molecules 2019, 24, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sillero, L.; Prado, R.; Labidi, J. Simultaneous microwave-ultrasound assisted extraction of bioactive compounds from bark. Chem. Eng. Process.-Process Intensif. 2020, 156, 108100. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Zhu, C.-p.; Zhai, X.-c.; Li, L.-q.; Wu, X.-x.; Li, B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef]
- Raza, A.; Li, F.; Xu, X.; Tang, J. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int. J. Biol. Macromol. 2017, 94, 335–344. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Zhang, Z.; Hou, T. Optimization of ultrasound-assisted extraction of polysaccharides from perilla seed meal by response surface methodology: Characterization and in vitro antioxidant activities. J. Food Sci. 2021, 86, 306–318. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Wen, X.; Chen, X.; He, Z.; Ni, Y. Optimization of ultrasound-assisted extraction of okra (Abelmoschus esculentus (L.) Moench) polysaccharides based on response surface methodology and antioxidant activity. Int. J. Biol. Macromol. 2018, 114, 1056–1063. [Google Scholar] [CrossRef]
- Yang, X.; Chen, F.; Huang, G. Extraction and analysis of polysaccharide from Momordica charantia. Ind. Crops Prod. 2020, 153, 112588. [Google Scholar] [CrossRef]
- Ye, G.; Li, J.; Zhang, J.; Liu, H.; Ye, Q.; Wang, Z. Structural characterization and antitumor activity of a polysaccharide from Dendrobium wardianum. Carbohydr. Polym. 2021, 269. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K. NMR-Spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Zhang, X.; Wan, L.; Zheng, Q.; Xu, D.; Li, Y.; Liang, Y.; Chen, M.; Li, B.; et al. Structural characterization of polysaccharide from Centipeda minima and its hypoglycemic activity through alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2021, 82, 104478. [Google Scholar] [CrossRef]
- Gorin, P.A.J.; Mazurek, M. Structure of a Phosphonomannan, as Determined by the Effect of Lanthanide Ions on Its Carbon-13 Magnetic Resonance Spectrum. Can. J. Chem. 1974, 52, 3070–3076. [Google Scholar] [CrossRef]
- Wu, J.; Li, C.; Bai, L.; Wu, J.; Bo, R.; Ye, M.; Huang, L.; Chen, H.; Rui, W. Structural differences of polysaccharides from Astragalus before and after honey processing and their effects on colitis mice. Int. J. Biol. Macromol. 2021, 182, 815–824. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, F.; Zhang, R.; Liu, F.; Peng, Q.; Wang, M. An acidic polysaccharide from Ziziphus Jujuba cv. Muzao: Purification and structural characterization. Food Chem. 2019, 274, 494–499. [Google Scholar] [CrossRef]
- Xiong, F.; Li, X.; Zheng, L.; Hu, N.; Cui, M.; Li, H. Characterization and antioxidant activities of polysaccharides from Passiflora edulis Sims peel under different degradation methods. Carbohydr. Polym. 2019, 218, 46–52. [Google Scholar] [CrossRef]
- Gonzalez, P.S.; O’Prey, J.; Cardaci, S.; Barthet, V.J.A.; Sakamaki, J.-i.; Beaumatin, F.; Roseweir, A.; Gay, D.M.; Mackay, G.; Malviya, G.; et al. Mannose impairs tumour growth and enhances chemotherapy. Nature 2018, 563, 719–723. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Song, R.; Cai, J.; Xu, J.; Tang, X.; Li, N. Ginger polysaccharides induced cell cycle arrest and apoptosis in human hepatocellular carcinoma HepG2 cells. Int. J. Biol. Macromol. 2019, 123, 81–90. [Google Scholar] [CrossRef]
- Li, X.; Cui, S. DPPH Radical Scavenging Mechanism of Ascorbic Acid. Food Sci. 2011, 32, 86–90. [Google Scholar]
- Lu, J.; Gu, G.; Hao, L.; Jin, Z.; Wang, X. Characterization and In vitro Antioxidant Activity of a Polysaccharide from Cordyceps sobolifera. J. Food Process. Preserv. 2016, 40, 447–452. [Google Scholar] [CrossRef]
- Sakai, T.; Imai, J.; Ito, T.; Takagaki, H.; Ui, M.; Hatta, S. The novel antioxidant TA293 reveals the role of cytoplasmic hydroxyl radicals in oxidative stress-induced senescence and inflammation. Biochem. Biophys. Res. Commun. 2017, 482, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.-X.; Yang, X.-H.; Zhang, H.-F.; Feng, J.; Zhang, M.-Y. Chemical Structure, Hypoglycemic Activity, and Mechanism of Action of Selenium Polysaccharides. Biol. Trace Elem. Res. 2021. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Li, C. Structural characterization of a novel acidic polysaccharide from Rosa roxburghii Tratt fruit and its α-glucosidase inhibitory activity. Food Funct. 2018, 9, 3974–3985. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Tan, Y.; Wang, S.; Chen, H.; Zhou, A. Progress in Understanding the Structure-Activity Relationship and Hypoglycemic Mechanism of Polysaccharides. Food Sci. 2021, 42, 355–363. [Google Scholar] [CrossRef]
- Fan, M.; Zhu, J.; Qian, Y.; Yue, W.; Xu, Y.; Zhang, D.; Yang, Y.; Gao, X.; He, H.; Wang, D. Effect of purity of tea polysaccharides on its antioxidant and hypoglycemic activities. J. Food Biochem. 2020, 44, e13277. [Google Scholar] [CrossRef]
- Cai, L.; Chen, B.; Yi, F.; Zou, S. Optimization of extraction of polysaccharide from dandelion root by response surface methodology: Structural characterization and antioxidant activity. Int. J. Biol. Macromol. 2019, 140, 907–919. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Mutaillifu, P.; Bobakulov, K.; Abuduwaili, A.; Huojiaaihemaiti, H.; Nuerxiati, R.; Aisa, H.A.; Yili, A. Structural characterization and antioxidant activities of a water soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macromol. 2020, 144, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, T.; Guo, F.; Sun, K.; Wang, B.; Wang, J.; Zhang, Z.; Zhang, X.; Zhao, Y.; Chen, Y. Structural characterization and intestinal protection activity of polysaccharides from Sea buckthorn (Hippophae rhamnoides L.) berries. Carbohydr. Polym. 2021, 274, 118648. [Google Scholar] [CrossRef] [PubMed]
- Rozi, P.; Abuduwaili, A.; Mutailifu, P.; Gao, Y.; Rakhmanberdieva, R.; Aisa, H.A.; Yili, A. Sequential extraction, characterization and antioxidant activity of polysaccharides from Fritillaria pallidiflora Schrenk. Int. J. Biol. Macromol. 2019, 131, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sun, Q.; Xu, J.; Li, N.; Lin, J.; Chen, S.; Li, F. Purification, characterization, and bioactivities of a polysaccharide from mycelial fermentation of Bjerkandera fumosa. Carbohydr. Polym. 2017, 167, 115–122. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]

| Retention Time (min) | Deduced Link-Age | PMAA | Mw | Relative Amount (%) | Major Mass Fragments (m/z) |
|---|---|---|---|---|---|
| 8.944 | t-Man(p) | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl mannitol | 323 | 82.611 | 87,102,118,129,145,162,205,239 |
| 13.746 | 2-Man(p) | 1,2,5-tri-O-acetyl-3,4,6-tri-O-methylmannitol | 351 | 3.597 | 87,102,118,129,162,189,203 |
| 24.939 | 2-Glu(p) | 1,2,5-tri-O-acetyl-3,4,6-tri-O-methyl glucitol | 351 | 4.21 | 84,98,116,145,160,226,267 |
| 23.629 | 6-Glu(p) | 1,5,6-tri-O-acetyl-2,3,4-tri-O-methyl glucitol | 351 | 4.869 | 86,102,115,145,157,187,217 |
| 22.29 | 4-Glu(p) | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl glucitol | 351 | 4.712 | 84,98,116,145,160,226,239 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.; Wang, C.; Mayhesumu, X.; Pan, L.; Dang, Y.; Yili, A.; Abuduwaili, A.; Mansur, S. Isolation, Structural Elucidation, Antioxidant and Hypoglycemic Activity of Polysaccharides of Brassica rapa L. Molecules 2022, 27, 3002. https://doi.org/10.3390/molecules27093002
Cao W, Wang C, Mayhesumu X, Pan L, Dang Y, Yili A, Abuduwaili A, Mansur S. Isolation, Structural Elucidation, Antioxidant and Hypoglycemic Activity of Polysaccharides of Brassica rapa L. Molecules. 2022; 27(9):3002. https://doi.org/10.3390/molecules27093002
Chicago/Turabian StyleCao, Wenyang, Chenxi Wang, Xiayidan Mayhesumu, Le Pan, Yan Dang, Abulimiti Yili, Aytursun Abuduwaili, and Sanawar Mansur. 2022. "Isolation, Structural Elucidation, Antioxidant and Hypoglycemic Activity of Polysaccharides of Brassica rapa L." Molecules 27, no. 9: 3002. https://doi.org/10.3390/molecules27093002
APA StyleCao, W., Wang, C., Mayhesumu, X., Pan, L., Dang, Y., Yili, A., Abuduwaili, A., & Mansur, S. (2022). Isolation, Structural Elucidation, Antioxidant and Hypoglycemic Activity of Polysaccharides of Brassica rapa L. Molecules, 27(9), 3002. https://doi.org/10.3390/molecules27093002






