Inosine and D-Mannose Secreted by Drug-Resistant Klebsiella pneumoniae Affect Viability of Lung Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. Colony Morphology and Capsular Polysaccharide (CPS) Quantification
2.2. In Vitro Growth Characteristics of the K. pneumoniae Strains
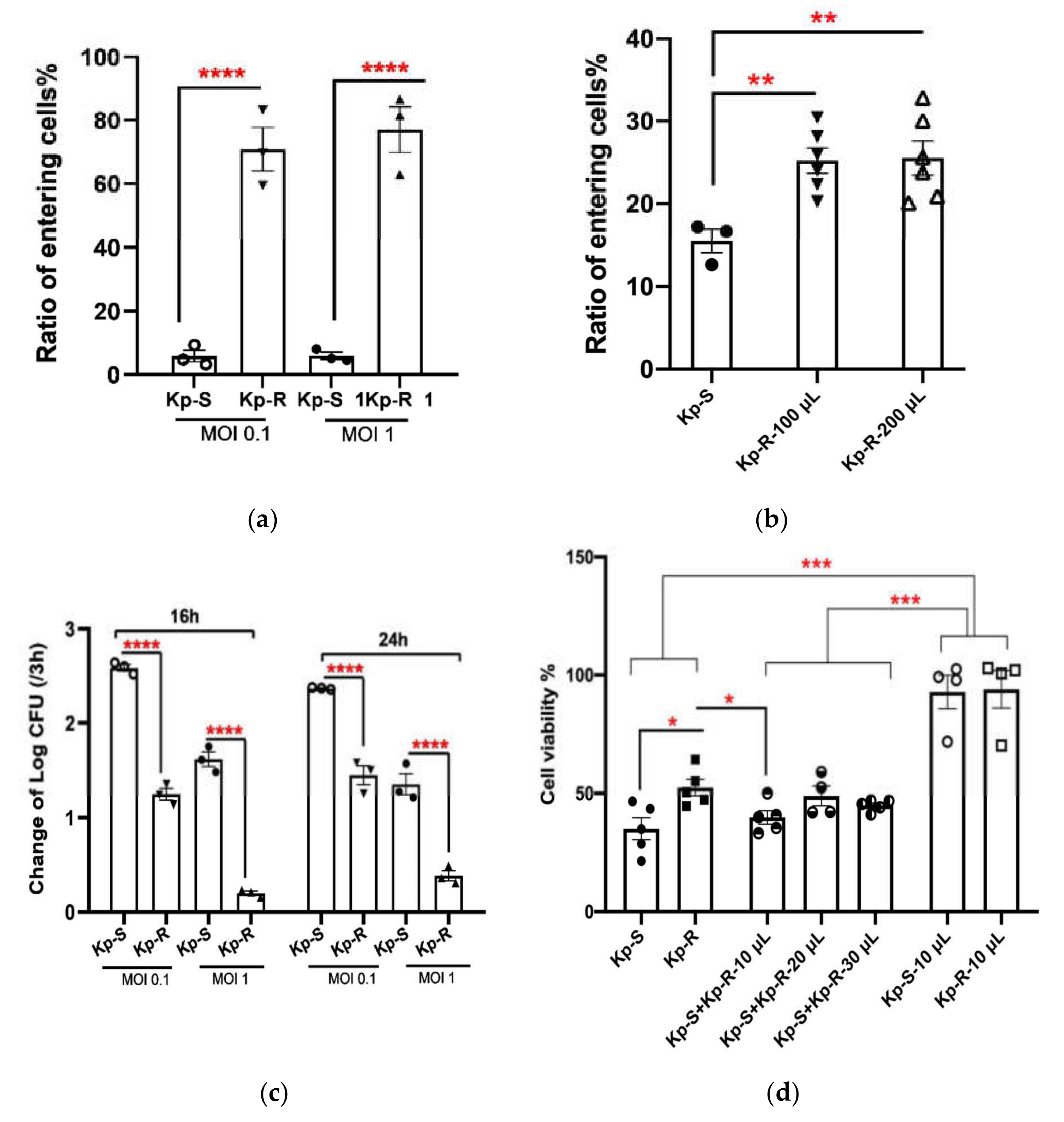
2.3. Cell Adhesion and Cell Viability of A549 Cell Line Infected with K. pneumoniae
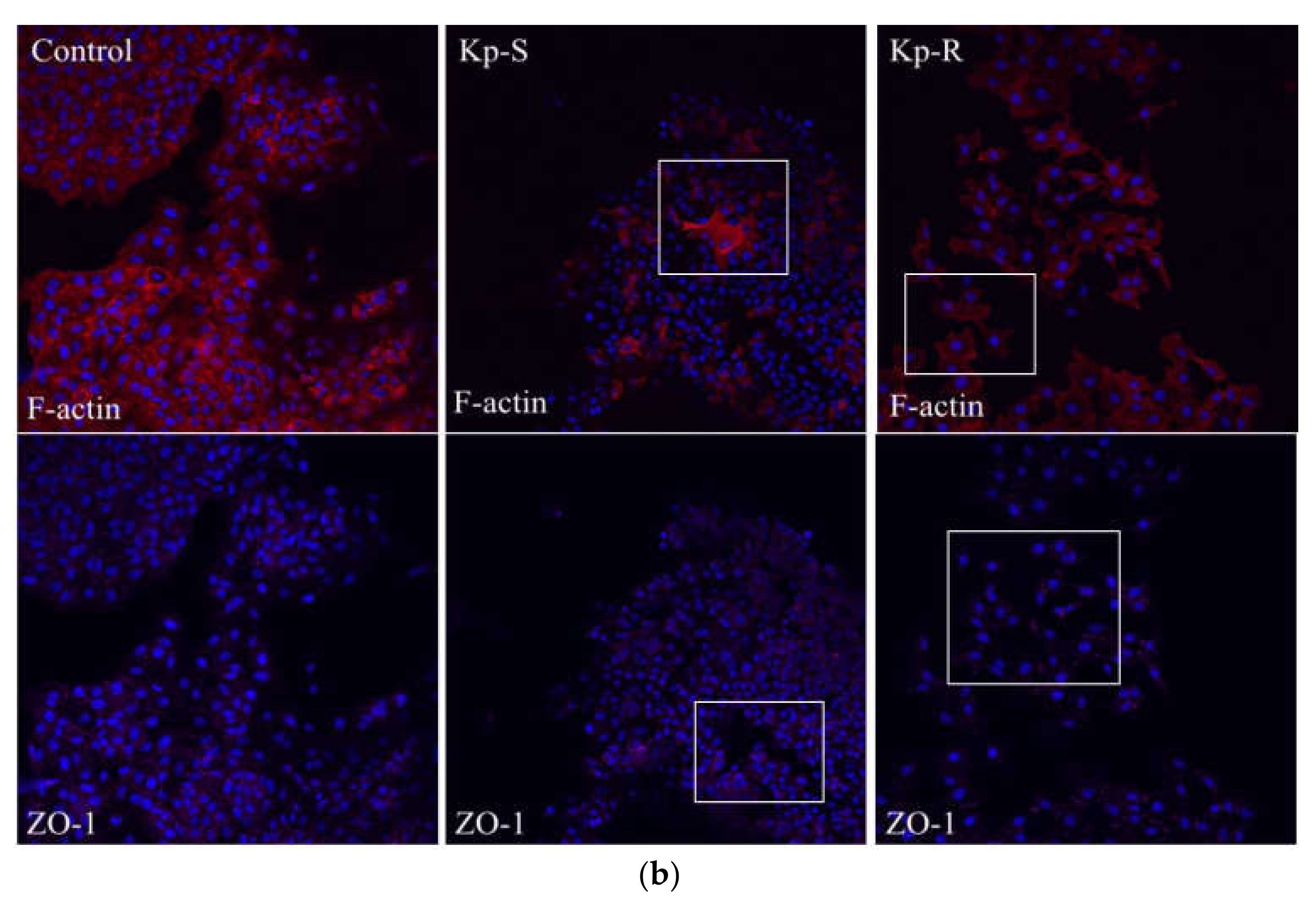
2.4. Immunofluorescence Analysis of the Cytoskeleton (Actin Microfilament F-Actin) and Tight-Junction Protein Zo-1 of the Two K. pneumoniae Strains Infected A549 Cells
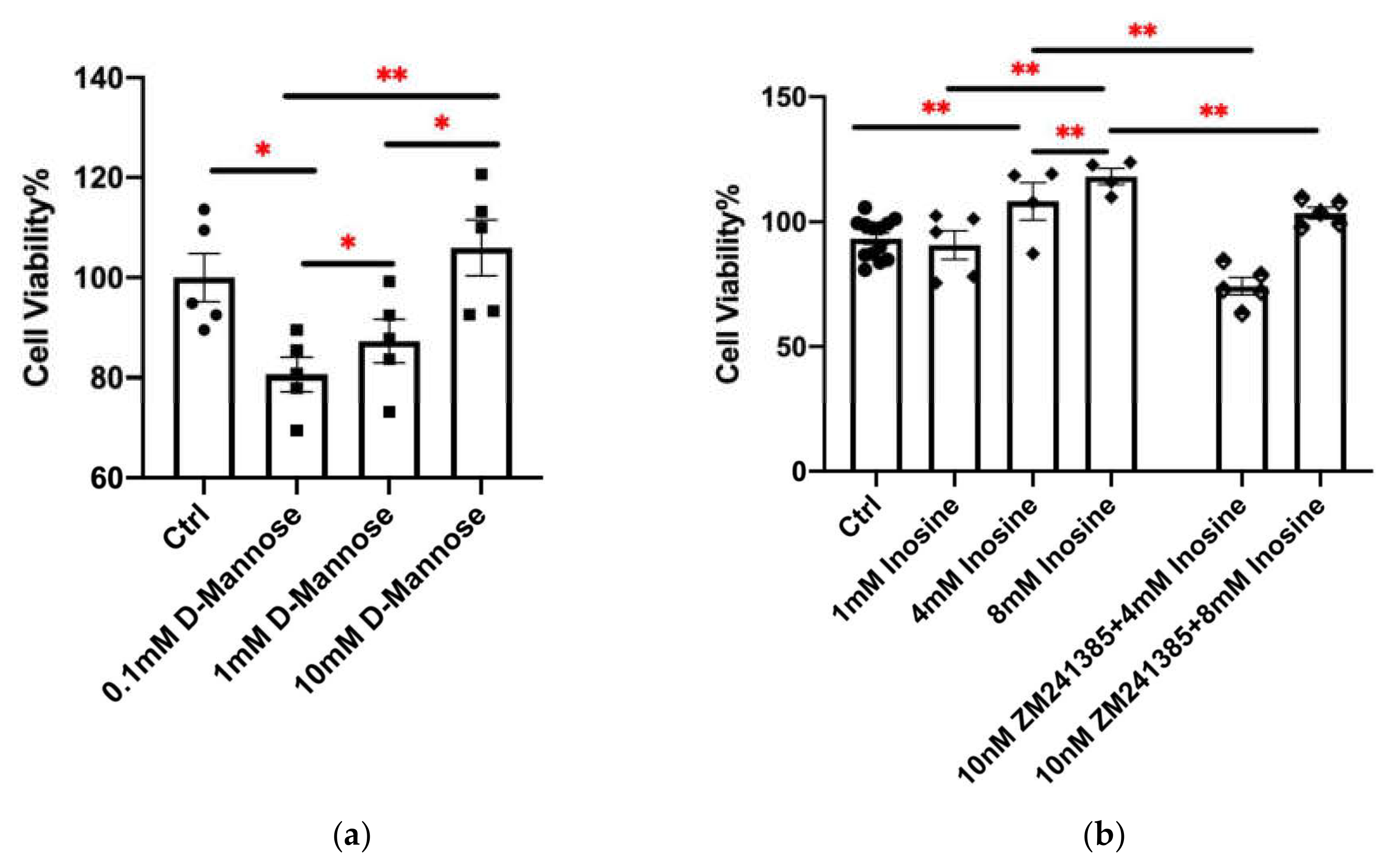
2.5. Comparison of Metabolites Secreted by Kp-R and Kp-S
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Bacterial Growth Kinetics In Vitro
4.3. Scanning Electron Microscopy Analysis
4.4. CPS Quantification of K. pneumoniae
4.5. Biofilm-Formation Assay
4.6. Antimicrobial Susceptibility Testing
4.7. Selection of Secreted Compounds
4.8. Strain Supernatants Collection
4.9. Cell Culture and K. pneumoniae Infection
4.10. Metabolites and Strain Supernatants Stimulation
4.11. Cell Viability Assay
4.12. Immunofluorescence Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Podschun, R.; Ullmann, U. Klebsiella spp. as Nosocomial Pathogens: Epidemiology, Taxonomy, Typing Methods, and Pathogenicity Factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.E.; Wertheim, H.; Zadoks, R.N.; Baker, S.; Whitehouse, C.A.; Dance, D.; Jenney, A.; Connor, T.R.; Hsu, L.Y.; Severin, J.; et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. USA 2015, 112, E3574–E3581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, Z.; Zhan, S.; Yang, Z.; Wei, D.; Zhang, J.; Li, J.; Kyaw, M.H. Disease burden of intensive care unit-acquired pneumonia in China: A systematic review and meta-analysis. Int. J. Infect. Dis. 2014, 29, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shi, Q.; Hu, H.; Hong, B.; Wu, X.; Du, X.; Akova, M.; Yu, Y. Emergence of ceftazidime/avibactam resistance in carbapenem-resistant Klebsiella pneumoniae in China. Clin. Microbiol. Infect. 2019, 26, 124.e1–124.e4. [Google Scholar] [CrossRef]
- Tiwari, V.; Meena, K.; Tiwari, M. Differential anti-microbial secondary metabolites in different ESKAPE pathogens explain their adaptation in the hospital setup. Infect. Genet. Evol. 2018, 66, 57–65. [Google Scholar] [CrossRef]
- Huang, R.Y.; Herr, D.R.; Moochhala, S. Manipulation of Alcohol and Short-Chain Fatty Acids in the Metabolome of Commensal and Virulent Klebsiella pneumoniae by Linolenic Acid. Microorganisms 2020, 8, 773. [Google Scholar] [CrossRef]
- Wyres, K.L.; Nguyen, T.N.T.; Lam, M.M.C.; Judd, L.M.; Chau, N.V.V.; Dance, D.A.B.; Ip, M.; Karkey, A.; Ling, C.L.; Miliya, T.; et al. Genomic surveillance for hypervirulence and multi-drug resistance in invasive Klebsiella pneumoniae from South and Southeast Asia. Genome Med. 2020, 12, 11. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Wang, Q.; Wang, X.; Chen, H.; Li, H.; Zhang, F.; Li, S.; Wang, R.; Wang, H. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: Geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob. Agents Chemother. 2016, 60, 6115–6120. [Google Scholar] [CrossRef]
- Rocker, A.; Lacey, J.A.; Belousoff, M.J.; Wilksch, J.J.; Strugnell, R.A.; Davies, M.R.; Lithgow, T. Global Trends in Proteome Remodeling of the Outer Membrane Modulate Antimicrobial Permeability in Klebsiella pneumoniae. mBio 2020, 11, e00603–e00620. [Google Scholar] [CrossRef]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Genet. 2006, 5, 48–56. [Google Scholar] [CrossRef]
- Padhi, A.; Naik, S.K.; Sengupta, S.; Ganguli, G.; Sonawane, A. Expression of mycobacterium tuberculosis NLPC/p60 family protein Rv0024 induce biofilm formation and resistance against cell wall acting anti-tuberculosis drugs in Mycobacterium smegmatis. Microbes Infect. 2016, 18, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.A.; Pomianek, M.E.; Kraml, C.M.; Taylor, R.K.; Semmelhack, M.F.; Bassler, B. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature 2007, 450, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Galdiero, E.; Salvatore, M.; Maione, A.; de Alteriis, E.; Andolfi, A.; Salvatore, F.; Guida, M. GC-MS-Based Metabolomics Study of Single- and Dual-Species Biofilms of Candida albicans and Klebsiella pneumoniae. Int. J. Mol. Sci. 2021, 22, 3496. [Google Scholar] [CrossRef]
- Yelamanchi, S.D.; Kumar, S.T.A.; Mishra, A.; Prasad, T.S.K.; Surolia, A. Metabolite Dysregulation by Pranlukast in Mycobacterium tuberculosis. Molecules 2022, 27, 1520. [Google Scholar] [CrossRef]
- Pearcy, N.; Hu, Y.; Baker, M.; Maciel-Guerra, A.; Xue, N.; Wang, W.; Kaler, J.; Peng, Z.; Li, F.; Dottorini, T. Genome-scale metabolic models and machine learning reveal genetic determinants of antibiotic resistance in escherichia coli and unravel the underlying metabolic adaptation mechanisms. mSystems 2021, 6, e0091320. [Google Scholar] [CrossRef]
- Shon, A.S.; Bajwa, R.P.S.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef]
- Foschi, C.; Salvo, M.; Laghi, L.; Zhu, C.; Ambretti, S.; Marangoni, A.; Re, M.C. Impact of meropenem on Klebsiella pneumoniae metabolism. PLoS ONE 2018, 13, e0207478. [Google Scholar] [CrossRef]
- Wu, T.; Xu, F.; Su, C.; Li, H.; Lv, N.; Liu, Y.; Gao, Y.; Lan, Y.; Li, J. Alterations in the gut microbiome and cecal metabolome during klebsiella pneumoniae-induced pneumosepsis. Front. Immunol. 2020, 11, 1331. [Google Scholar] [CrossRef]
- Liu, D.; Chen, Z.; Yuan, Y.; Jing, H.; Zou, J.; Zhang, X.; Zeng, X.; Zhang, W.; Zou, Q.; Zhang, J. Innate Immune Effectors Play Essential Roles in Acute Respiratory Infection Caused by Klebsiella pneumoniae. J. Immunol. Res. 2020, 2020, 5291714. [Google Scholar] [CrossRef]
- Wan, X.; Li, J.; Wang, Y.; Yu, X.; He, X.; Shi, J.; Deng, G.; Zeng, X.; Tian, G.; Li, Y.; et al. H7N9 virus infection triggers lethal cytokine storm by activating gasdermin E-mediated pyroptosis of lung alveolar epithelial cells. Natl. Sci. Rev. 2021, 9, nwab137. [Google Scholar] [CrossRef] [PubMed]
- Rosett, W.; Hodges, G.R. Antimicrobial Activity of Heparin. J. Clin. Microbiol. 1980, 11, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Kuhel, D.G.; Németh, Z.H.; Mabley, J.G.; Stachlewitz, R.F.; Virág, L.; Lohinai, Z.; Southan, G.J.; Salzman, A.L.; Szabó, C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J. Immunol. 2000, 164, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hoang, T.K.; Wang, T.; Ferris, M.; Taylor, C.M.; Tian, X.; Luo, M.; Tran, D.Q.; Zhou, J.; Tatevian, N.; et al. Resetting microbiota by lactobacillus reuteri inhibits T reg deficiency—Induced autoimmunity via adenosine A2A receptors. J. Exp. Med. 2016, 214, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Csóka, B.; Himer, L.; Selmeczy, Z.; Vizi, E.S.; Pacher, P.; Ledent, C.; Deitch, E.A.; Spolarics, Z.; Németh, Z.H.; Haskü, G. Adenosine A 2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008, 22, 3491–3499. [Google Scholar] [CrossRef]
- Ohta, A.; Gorelik, E.; Prasad, S.J.; Ronchese, F.; Lukashev, D.; Wong, M.K.K.; Huang, X.; Caldwell, S.; Liu, K.; Smith, P.; et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. USA 2006, 103, 13132–13137. [Google Scholar] [CrossRef]
- Munoz-Price, L.S.; Poirel, L.; Bonomo, R.A.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Scribano, D.; Sarshar, M.; Prezioso, C.; Lucarelli, M.; Angeloni, A.; Zagaglia, C.; Palamara, A.T.; Ambrosi, C. d-Mannose treatment neither affects uropathogenic escherichia coli properties nor induces stable FimH modifications. Molecules 2020, 25, 316. [Google Scholar] [CrossRef]
- Shi, B.; Xia, X. Changes in growth parameters of Pseudomonas pseudoalcaligenes after ten months culturing at increasing temperature. FEMS Microbiol. Ecol. 2003, 45, 127–134. [Google Scholar] [CrossRef][Green Version]
- Van Laar, T.A.; Chen, T.; You, T.; Leung, K.P. Sublethal Concentrations of Carbapenems Alter Cell Morphology and Genomic Expression of Klebsiella pneumoniae Biofilms. Antimicrob. Agents Chemother. 2015, 59, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Patel, S.; Abboud, C.; Diep, J.; Ly, N.S.; Pogue, J.; Kaye, K.S.; Li, J.; Rao, G.G. Polymyxin B in combination with meropenem against carbapenemase-producing Klebsiella pneumoniae: Pharmacodynamics and morphological changes. Int. J. Antimicrob. Agents 2016, 49, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Cortes, G. Role of Lung Epithelial Cells in Defense against Klebsiella pneumoniae Pneumonia. Infect. Immun. 2002, 70, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Sahly, H.; Podschun, R.; Oelschlaeger, T.A.; Greiwe, M.; Parolis, H.; Hasty, D.; Kekow, J.; Ullmann, U.; Ofek, I.; Sela, S. Capsule Impedes Adhesion to and Invasion of Epithelial Cells by Klebsiella pneumoniae. Infect. Immun. 2000, 68, 6744–6749. [Google Scholar] [CrossRef]
- Chua, M.D.; Liou, C.H.; Bogdan, A.C.; Law, H.T.; Yeh, K.M.; Lin, J.C.; Siu, L.K.; Guttman, J.A. Klebsiella pneumoniae disassembles host microtubules in lung epithelial cells. Cell. Microbiol. 2019, 21, e12977. [Google Scholar] [CrossRef]
- Moranta Mesquida, D.; Regueiro, V.; March, C.; Llobet, E.; Margareto, J.; Larrarte, E.; Garmendia, J.; Bengoechea, J.A. Klebsiella pneumoniae capsule polysaccharide impedes the expression of beta-defensins by airway epithelial cells. Infect. Immun. 2009, 78, 1135–1146. [Google Scholar] [CrossRef]
- Cano, V.; Moranta, D.; Llobet-Brossa, E.; Bengoechea, J.A.; Garmendia, J. Klebsiella pneumoniae triggers a cytotoxic effect on airway epithelial cells. BMC Microbiol. 2009, 9, 156. [Google Scholar] [CrossRef]
- Cortés, G.; Borrell, N.; de Astorza, B.; Gómez, C.; Sauleda, J.; Albertí, S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of Pneumonia. Infect. Immun. 2002, 70, 2583–2590. [Google Scholar] [CrossRef]
- Shankar-Sinha, S.; Valencia, G.A.; Janes, B.K.; Rosenberg, J.K.; Whitfield, C.; Bender, R.A.; Standiford, T.J.; Younger, J.G. The Klebsiella pneumoniae O antigen contributes to bacteremia and lethality during murine Pneumonia. Infect. Immun. 2004, 72, 1423–1430. [Google Scholar] [CrossRef]
- Lawlor, M.S.; Hsu, J.; Rick, P.D.; Miller, V.L. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol. Microbiol. 2010, 58, 1054–1073. [Google Scholar] [CrossRef] [PubMed]
- Nassif, X.; Sansonetti, P.J. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect. Immun. 1986, 54, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Lee, E.J.; Lee, J.H.; Jun, S.H.; Choi, C.W.; Kim, S.I.; Kang, S.S.; Hyun, S. Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune response. FEMS Microbiol. Lett. 2012, 331, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Treat, L.P.; Sepúlveda, V.E.; Miller, V.L. The small protein rmpd drives hypermucoviscosity in Klebsiella pneumoniae. mBio 2020, 11, e01750-20. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sun, P.; Vamathevan, J.; Li, Y.; Ingraham, K.; Palmer, L.; Huang, J.; Brown, J.R. Comparative genomics of Klebsiella pneumoniae strains with different antibiotic resistance profiles. Antimicrob. Agents Chemother. 2011, 55, 4267–4276. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Choi, W.; Buckley, A.; Shashikanth, N.; Joseph, N.E.; Wang, Y.; Joseph, N.E.; Wang, Y.; Warren, M.H.; Buschmann, M.M.; et al. Zo-1 Interactions with F-Actin and Occludin Direct Epithelial Polarization and Single Lumen Specification in 3d Culture. J. Cell Sci. 2017, 130, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mathema, B.; Chavda, K.D.; DeLeo, F.; Bonomo, R.A.; Kreiswirth, B.N. Carbapenemase-producing Klebsiella pneumoniae: Molecular and genetic decoding. Trends Microbiol. 2014, 22, 686–696. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Chen, L.; Porcella, S.F.; Martens, C.A.; Kobayashi, S.D.; Porter, A.R.; Chavda, K.D.; Jacobs, M.R.; Mathema, B.; Olsen, R.J.; et al. Molecular dissection of the evolution of carbapenem-resistant multilocus sequence type 258 Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2014, 111, 4988–4993. [Google Scholar] [CrossRef]
- Lopez, L.L.; Rusconi, B.; Gildersleeve, H.; Qi, C.; McLaughlin, M.; Scheetz, M.H.; Seshu, J.; Eppinger, M. Genome Sequences of Five Clinical Isolates of Klebsiella pneumoniae. Genome Announc. 2016, 4, e00040-16. [Google Scholar] [CrossRef]
- Zhou, K.; Lokate, M.; Deurenberg, R.H.; Tepper, M.; Arends, J.P.; Raangs, E.G.C.; Lo-Ten-Foe, J.; Grundmann, H.; Rossen, J.; Friedrich, A.W. Use of whole-genome sequencing to trace, control and characterize the regional expansion of extended-spectrum β-lactamase producing ST15 Klebsiella pneumoniae. Sci. Rep. 2016, 6, 20840. [Google Scholar] [CrossRef]
- Kállai, A.; Kelemen, M.; Molnár, N.; Tropotei, A.; Hauser, B.; Iványi, Z.; Gál, J.; Ligeti, E.; Kristóf, K.; Lőrincz, M. MICy: A Novel Flow Cytometric Method for Rapid Determination of Minimal Inhibitory Concentration. Microbiol. Spectr. 2021, 9, e0090121. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.; Sato, M.; Sato, C. Biofilm formation of staphylococcus epidermidis imaged using atmospheric scanning electron microscopy. Anal. Bioanal. Chem. 2021, 413, 7549–7558. [Google Scholar] [CrossRef] [PubMed]
- Fleeman, R.M.; Macias, L.A.; Brodbelt, J.S.; Davies, B.W. Defining principles that influence antimicrobial peptide activity against capsulated Klebsiella pneumoniae. Proc. Natl. Acad. Sci. USA 2020, 117, 27620–27626. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, A.P.; Dorsey, C.W.; Edelmann, R.E.; Actis, L.A. Attachment to and biofilm formation on abiotic surfaces by Acinetobacter baumannii: Involvement of a novel chaperone-usher pili assembly system. Microbiology 2003, 149, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Wayne, P.A.; Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. 2018. Available online: https://clsi.org/standards/products/microbiology/documents/m100/2018 (accessed on 9 March 2022).
- Wiegand, I.; Geiss, H.K.; Mack, D.; Stürenburg, E.; Seifert, H. Detection of Extended-Spectrum Beta-Lactamases among Enterobacteriaceae by Use of Semiautomated Microbiology Systems and Manual Detection Procedures. J. Clin. Microbiol. 2007, 45, 1167–1174. [Google Scholar] [CrossRef][Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhou, Z.; Xiao, W.; Tang, Y.; Guan, W.; Wang, J.; Shu, F.; Shen, J.; Gu, S.; Zhang, L.; et al. Inosine and D-Mannose Secreted by Drug-Resistant Klebsiella pneumoniae Affect Viability of Lung Epithelial Cells. Molecules 2022, 27, 2994. https://doi.org/10.3390/molecules27092994
Zhang Y, Zhou Z, Xiao W, Tang Y, Guan W, Wang J, Shu F, Shen J, Gu S, Zhang L, et al. Inosine and D-Mannose Secreted by Drug-Resistant Klebsiella pneumoniae Affect Viability of Lung Epithelial Cells. Molecules. 2022; 27(9):2994. https://doi.org/10.3390/molecules27092994
Chicago/Turabian StyleZhang, Yuhan, Ziwei Zhou, Wenxuan Xiao, Yuting Tang, Wei Guan, Jiang Wang, Farui Shu, Jiaqi Shen, Shaoyan Gu, Lu Zhang, and et al. 2022. "Inosine and D-Mannose Secreted by Drug-Resistant Klebsiella pneumoniae Affect Viability of Lung Epithelial Cells" Molecules 27, no. 9: 2994. https://doi.org/10.3390/molecules27092994
APA StyleZhang, Y., Zhou, Z., Xiao, W., Tang, Y., Guan, W., Wang, J., Shu, F., Shen, J., Gu, S., Zhang, L., Wang, Q., & Xie, L. (2022). Inosine and D-Mannose Secreted by Drug-Resistant Klebsiella pneumoniae Affect Viability of Lung Epithelial Cells. Molecules, 27(9), 2994. https://doi.org/10.3390/molecules27092994






