A New Phenylazo-Based Fluorescent Probe for Sensitive Detection of Hypochlorous Acid in Aqueous Solution
Abstract
:1. Introduction
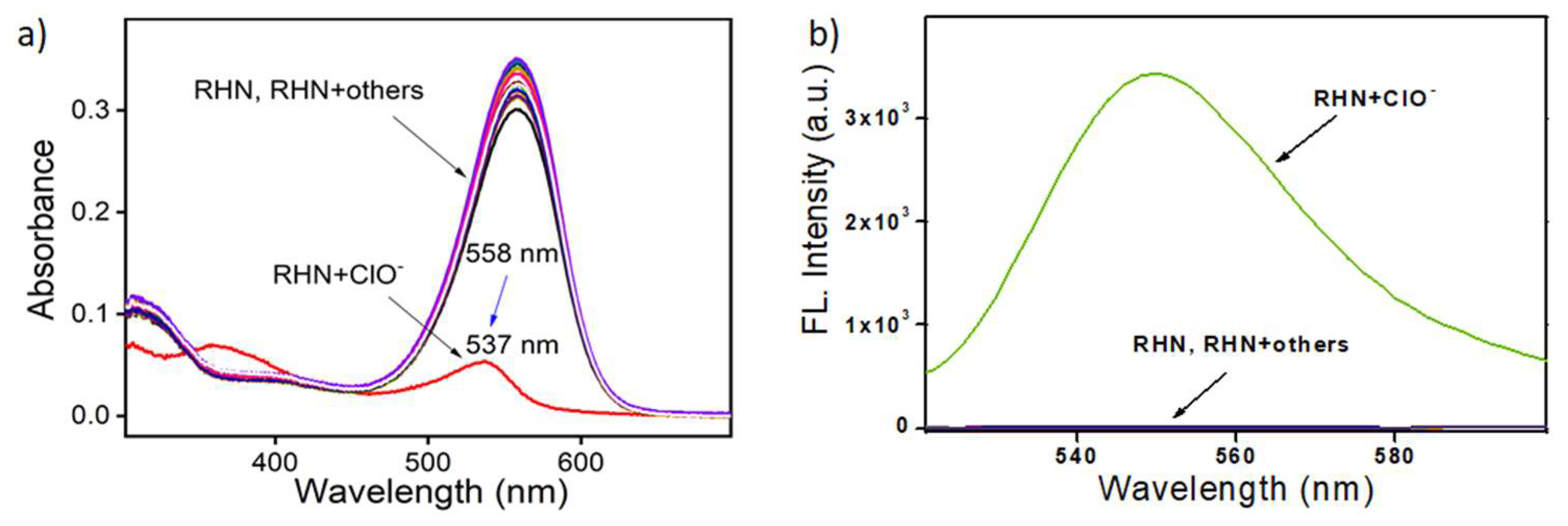
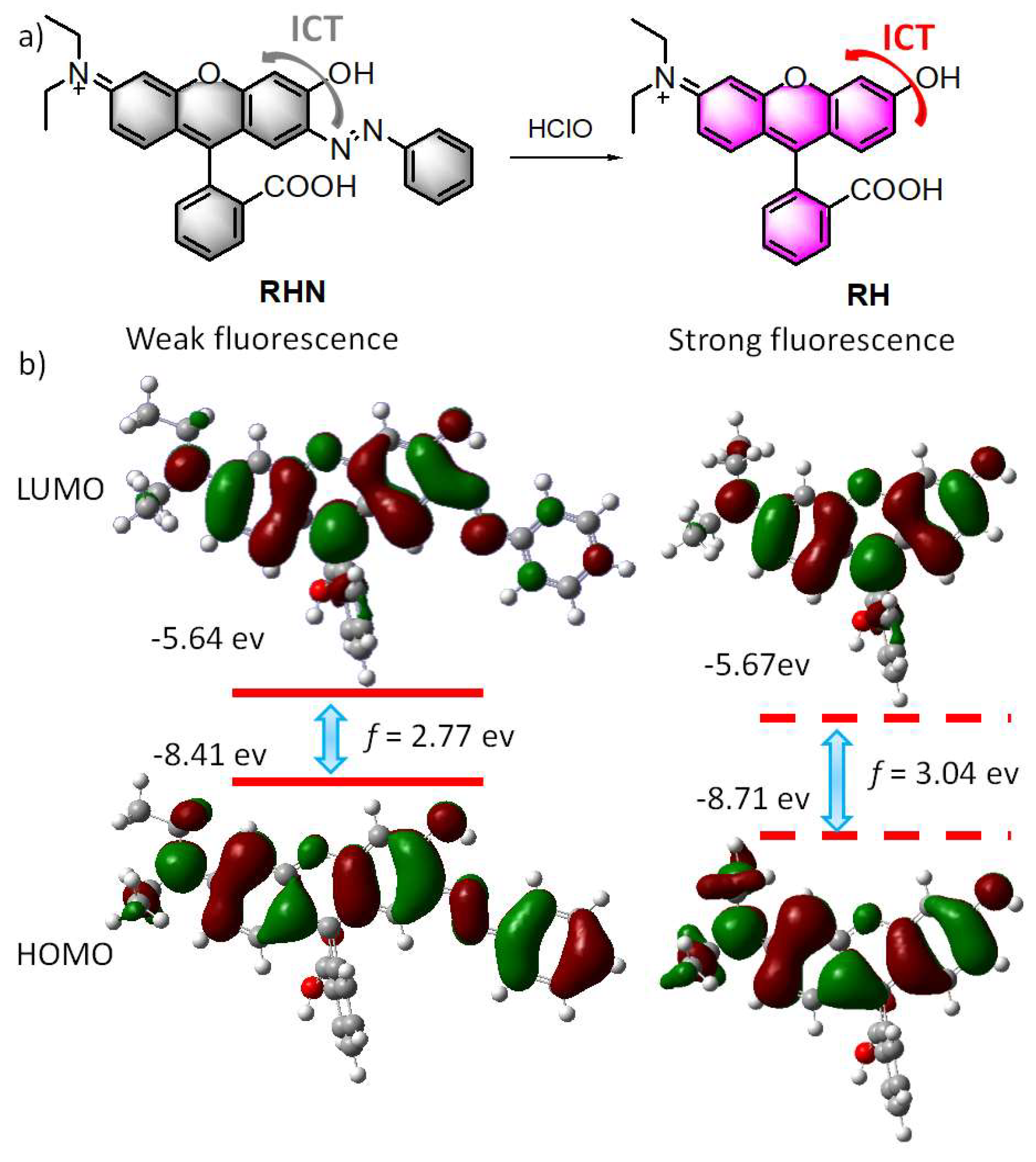
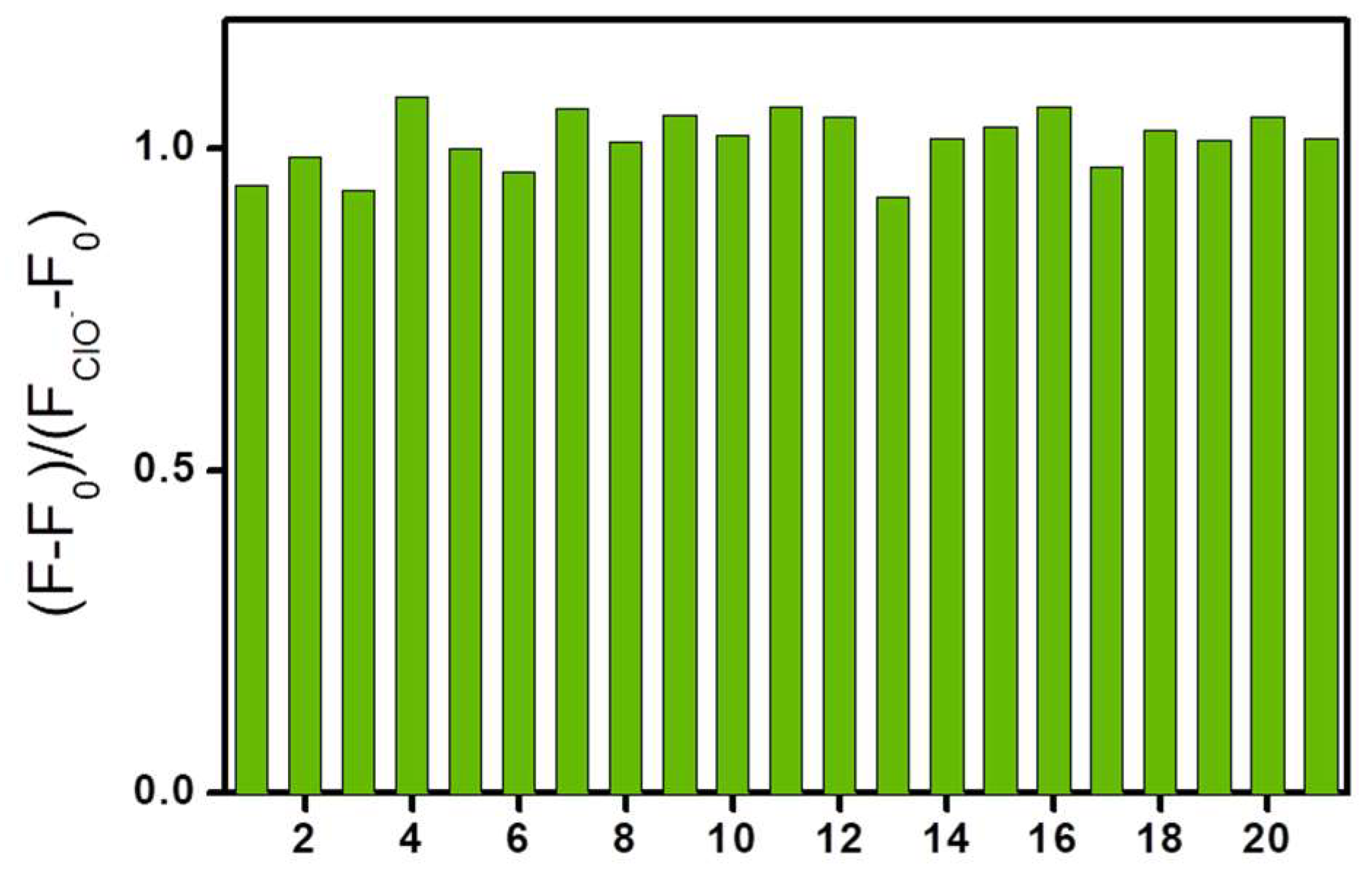
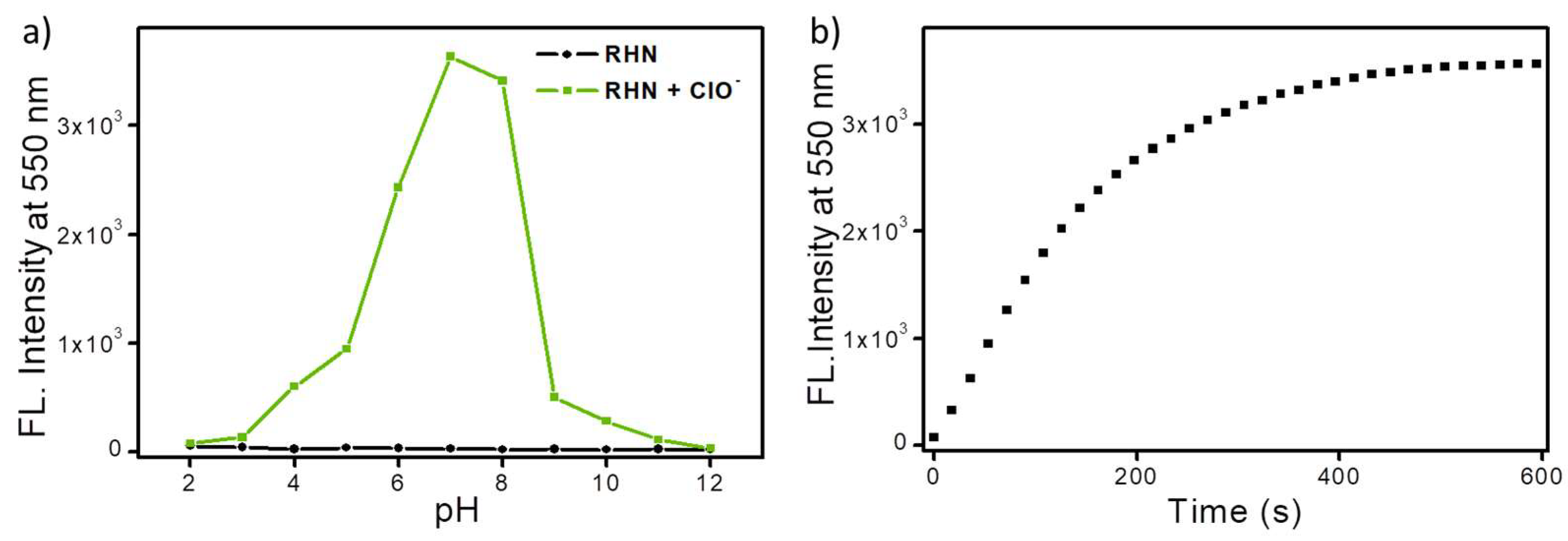
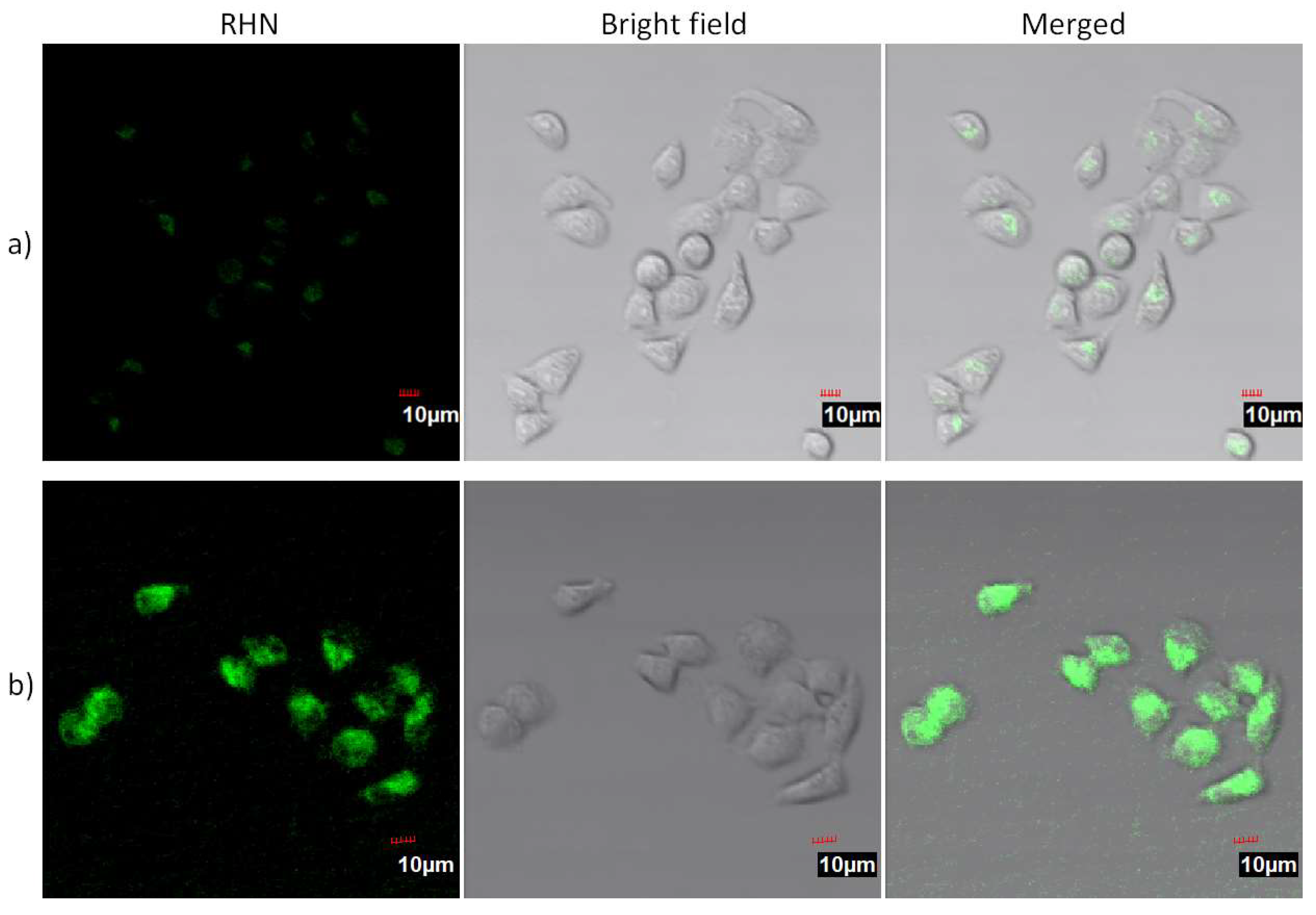
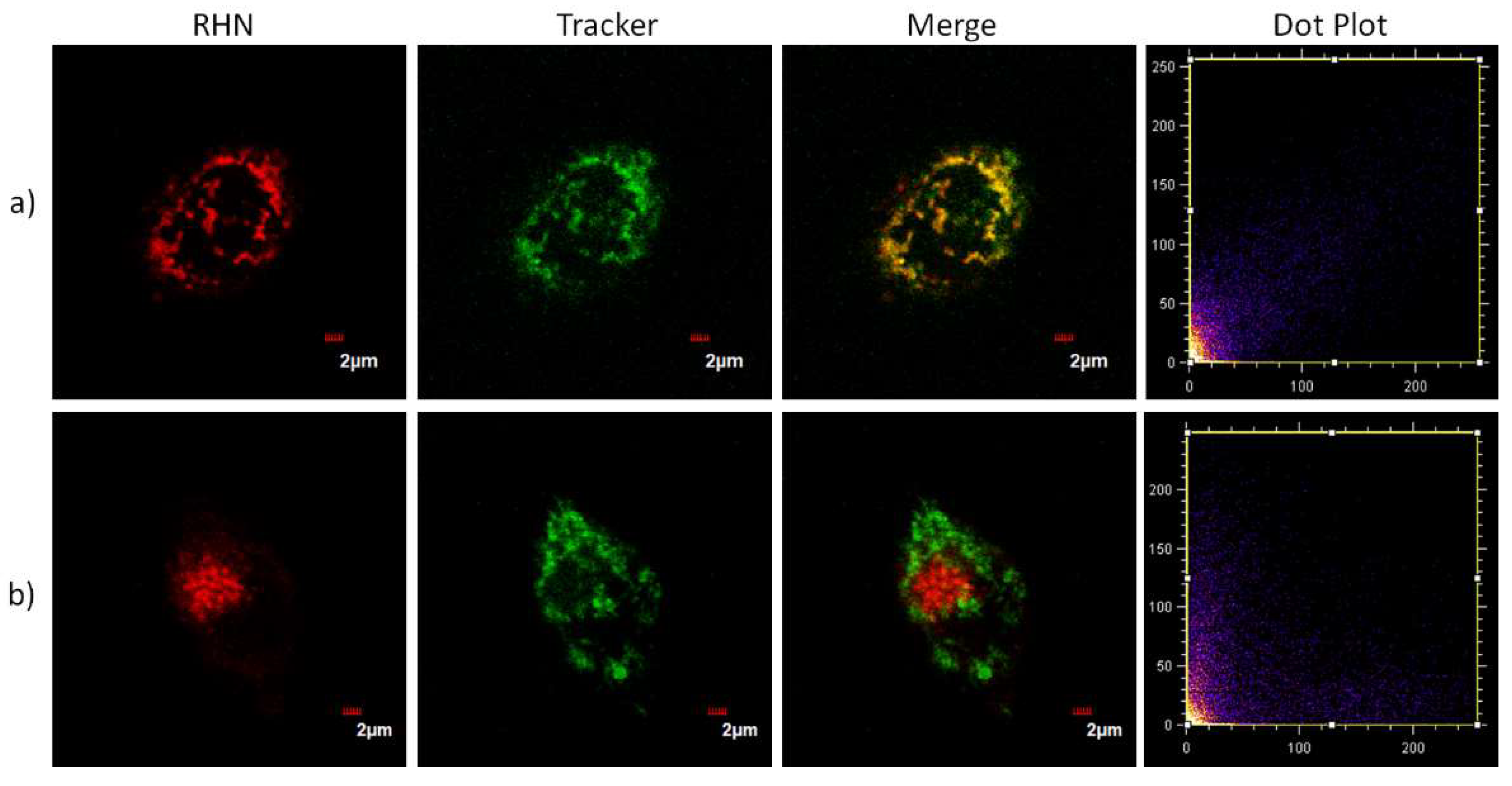
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.2. Standard MTT Assay
3.3. Cell Culture and Fluorescence Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, A.; Dunn, J.L.; Rateri, D.L.; Heinecke, J.W. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J. Clin. Investig. 1994, 94, 437–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, V.; Andrié, R.P.; Rudolph, T.K.; Friedrichs, K.; Klinke, A.; Hirsch-Hoffmann, B.; Schwoerer, A.P.; Lau, D.; Fu, X.; Klingel, K. Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat. Med. 2010, 16, 470–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, Y.W.; Whiteman, M.; Cheung, N.S. Chlorinative stress: An under appreciated mediator of neurodegeneration? Cell. Signal. 2007, 19, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.I.; Hawkins, C.L.; Davies, M.J. Hypochlorous acid-mediated oxidation of lipid components and antioxidants present in low-density lipoproteins: Absolute rate constants, product analysis, and computational modeling. Chem. Res. Toxicol. 2003, 16, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A.; Winterbourn, C. Myeloperoxidase: A key regulator of neutrophil oxidant production. Redox Rep. 1997, 3, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Schäferling, M. The art of fluorescence imaging with chemical sensors. Angew. Chem. Int. Ed. 2012, 51, 3532–3554. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, Z.; Wang, F.; Yang, G.; Zhang, Y.; Zhang, X. Multi-dimensional imaging of endogenous leucine aminopeptidase via fast response fluorescent read-out probe. Dyes Pigm. 2021, 187, 109145. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; Zhu, S. FRET-based small-molecule fluorescent probes: Rational design and bioimaging applications. Acc. Chem. Res. 2013, 46, 1462–1473. [Google Scholar] [CrossRef]
- Chen, W.-C.; Venkatesan, P.; Wu, S.-P. A highly selective turn-on fluorescent probe for hypochlorous acid based on hypochlorous acid-induced oxidative intramolecular cyclization of boron dipyrromethene-hydrazone. Anal. Chim. Acta 2015, 882, 68–75. [Google Scholar] [CrossRef]
- Zheng, A.; Liu, H.; Peng, C.; Gao, X.; Xu, K.; Tang, B. A mitochondria-targeting near-infrared fluorescent probe for imaging hypochlorous acid in cells. Talanta 2021, 226, 122152. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Yu, H.; Zhu, W.; Yao, X.; Liu, W.; Yang, S.; Zhou, F.; Liu, Y. Near infrared fluorescent probe for in vivo bioimaging of endogenous hypochlorous acid. Dyes Pigm. 2021, 188, 109218. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, Z.-M.; Zhang, Y.-R.; Su, L.; Miao, J.-Y.; Zhao, B.-X. A lysosome-targeted ratiometric fluorescent probe for detection of hypochlorous acid in living cells. Sens. Actuators B Chem. 2017, 247, 736–741. [Google Scholar] [CrossRef]
- Jiao, C.; Liu, Y.; Pang, J.; Lu, W.; Zhang, P.; Wang, Y. A simple lysosome-targeted probe for detection of hypochlorous acid in living cells. J. Photochem. Photobiol. A Chem. 2020, 392, 112399. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, Y.; Chi, Y.; He, Y.; Chen, H.; Nemykin, V.N. A “reactive” turn-on fluorescence probe for hypochlorous acid and its bioimaging application. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 190–196. [Google Scholar] [CrossRef]
- Gong, J.; Liu, C.; Cai, S.; He, S.; Zhao, L.; Zeng, X. Novel near-infrared fluorescent probe with a large Stokes shift for sensing hypochlorous acid in mitochondria. Org. Bio. Chem. 2020, 18, 7656–7662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.; Li, B.; Li, W.; Zhang, C.; Hou, X.; Jiang, J.; Dong, Y. Ratiometric fluorescent probes for capturing endogenous hypochlorous acid in the lungs of mice. Chem. Sci. 2018, 9, 8207–8212. [Google Scholar] [CrossRef] [Green Version]
- Ren, M.; Li, Z.; Nie, J.; Wang, L.; Lin, W. A photocaged fluorescent probe for imaging hypochlorous acid in lysosomes. Chem. Commun. 2018, 54, 9238–9241. [Google Scholar] [CrossRef]
- Tang, Z.; Ding, X.-L.; Liu, Y.; Zhao, Z.-M.; Zhao, B.-X. A new probe based on rhodamine B and benzothiazole hydrazine for sensing hypochlorite in living cells and real water samples. RSC Adv. 2015, 5, 99664–99668. [Google Scholar] [CrossRef]
- Ikeno, T.; Hanaoka, K.; Iwaki, S.; Myochin, T.; Murayama, Y.; Ohde, H.; Komatsu, T.; Ueno, T.; Nagano, T.; Urano, Y. Design and synthesis of an activatable photoacoustic probe for hypochlorous acid. Anal. Chem. 2019, 91, 9086–9092. [Google Scholar] [CrossRef]
- Liu, C.; Jiao, X.; He, S.; Zhao, L.; Zeng, X. A highly selective and sensitive fluorescent probe for hypochlorous acid and its lysosome-targetable biological applications. Talanta 2017, 174, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Yu, S.; Wang, Q.; Tobia, J.; Chen, H.; Li, Z.; Liu, X.; Zhang, Y. A BODIPY-Based Far-Red-Absorbing Fluorescent Probe for Hypochlorous Acid Imaging. ChemPhotoChem 2021. [Google Scholar] [CrossRef]
- Song, Z.; Yuan, Q.; Lv, P.; Chen, K. Research Progress of Small Molecule Fluorescent Probes for Detecting Hypochlorite. Sensors 2021, 21, 6326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, X.; Zhang, R.; Liu, Y.; Ren, X.; Xian, M.; Ye, Y.; Zhao, Y. Lysosomal-targeted two-photon fluorescent probe to sense hypochlorous acid in live cells. Anal. Chem. 2017, 89, 10384–10390. [Google Scholar] [CrossRef]
- Liu, J.; Yin, Z. A novel NIR-emissive probe with large Stokes shift for hypochlorite detection and imaging in living cells. Talanta 2019, 196, 352–356. [Google Scholar] [CrossRef]
- Chen, B.; Fu, H.; Lv, Y.; Li, X.; Han, Y. An oxidative cyclization reaction based fluorescent “Turn-On” probe for highly selective and rapid detection of hypochlorous acid. Tetrahedron Lett. 2018, 59, 1116–1120. [Google Scholar] [CrossRef]
- Yan, Y.-H.; He, X.-Y.; Su, L.; Miao, J.-Y.; Zhao, B.-X. A new FRET-based ratiometric fluorescence probe for hypochlorous acid and its imaging in living cells. Talanta 2019, 201, 330–334. [Google Scholar] [CrossRef]
- Xing, P.; Gao, K.; Wang, B.; Gao, J.; Yan, H.; Wen, J.; Li, W.; Xu, Y.; Li, H.; Chen, J. HEPES is not suitable for fluorescence detection of HClO: A novel probe for HClO in absolute PBS. Chem. Commun. 2016, 52, 5064–5066. [Google Scholar] [CrossRef]
- Kim, Y.K.; Ha, H.-H.; Lee, J.-S.; Bi, X.; Ahn, Y.-H.; Hajar, S.; Lee, J.-J.; Chang, Y.-T. Control of muscle differentiation by a mitochondria-targeted fluorophore. J. Am. Chem. Soc. 2010, 132, 576–579. [Google Scholar] [CrossRef]
- Gong, J.; Liu, C.; Jiao, X.; He, S.; Zhao, L.; Zeng, X. A near-infrared fluorescent probe based on a novel rectilinearly π-extended rhodamine derivative and its applications. J. Mater. Chem. B 2020, 8, 2343–2349. [Google Scholar] [CrossRef]
- Cai, S.; Liu, Q.; Liu, C.; He, S.; Zhao, L.; Zeng, X.; Gong, J. Rational design of large Stokes shift xanthene-benzothiozolium dyad for probing cysteine in mitochondria. J. Mater. Chem. B 2022, 10, 1265–1271. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Liu, C.; He, S.; Zhao, L.; Zeng, X.; Zhou, J.; Gong, J. A New Phenylazo-Based Fluorescent Probe for Sensitive Detection of Hypochlorous Acid in Aqueous Solution. Molecules 2022, 27, 2978. https://doi.org/10.3390/molecules27092978
Liu Q, Liu C, He S, Zhao L, Zeng X, Zhou J, Gong J. A New Phenylazo-Based Fluorescent Probe for Sensitive Detection of Hypochlorous Acid in Aqueous Solution. Molecules. 2022; 27(9):2978. https://doi.org/10.3390/molecules27092978
Chicago/Turabian StyleLiu, Qiuchen, Chang Liu, Song He, Liancheng Zhao, Xianshun Zeng, Jin Zhou, and Jin Gong. 2022. "A New Phenylazo-Based Fluorescent Probe for Sensitive Detection of Hypochlorous Acid in Aqueous Solution" Molecules 27, no. 9: 2978. https://doi.org/10.3390/molecules27092978
APA StyleLiu, Q., Liu, C., He, S., Zhao, L., Zeng, X., Zhou, J., & Gong, J. (2022). A New Phenylazo-Based Fluorescent Probe for Sensitive Detection of Hypochlorous Acid in Aqueous Solution. Molecules, 27(9), 2978. https://doi.org/10.3390/molecules27092978





