Solvent Casting and UV Photocuring for Easy and Safe Fabrication of Nanocomposite Film Dressings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gellan Gum Methacrylate (GG-MA)
2.3. Rheological Characterization
2.4. Film Preparation
2.5. Film Characterization
2.5.1. Measurement of Film Thickness
2.5.2. Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS)
2.5.3. Mechanical Characterization: Tensile Tests
2.5.4. Colorimetric Analysis
2.6. Swelling Studies
2.7. Release Studies
2.8. Antimicrobial Activity of Films Loaded with AgNPs
2.8.1. Antimicrobial Activity in Liquid Culture Media
2.8.2. Antimicrobial Activity in Solid Culture Media (Diffusion Test)
2.9. Statistical Analysis
3. Results and Discussion
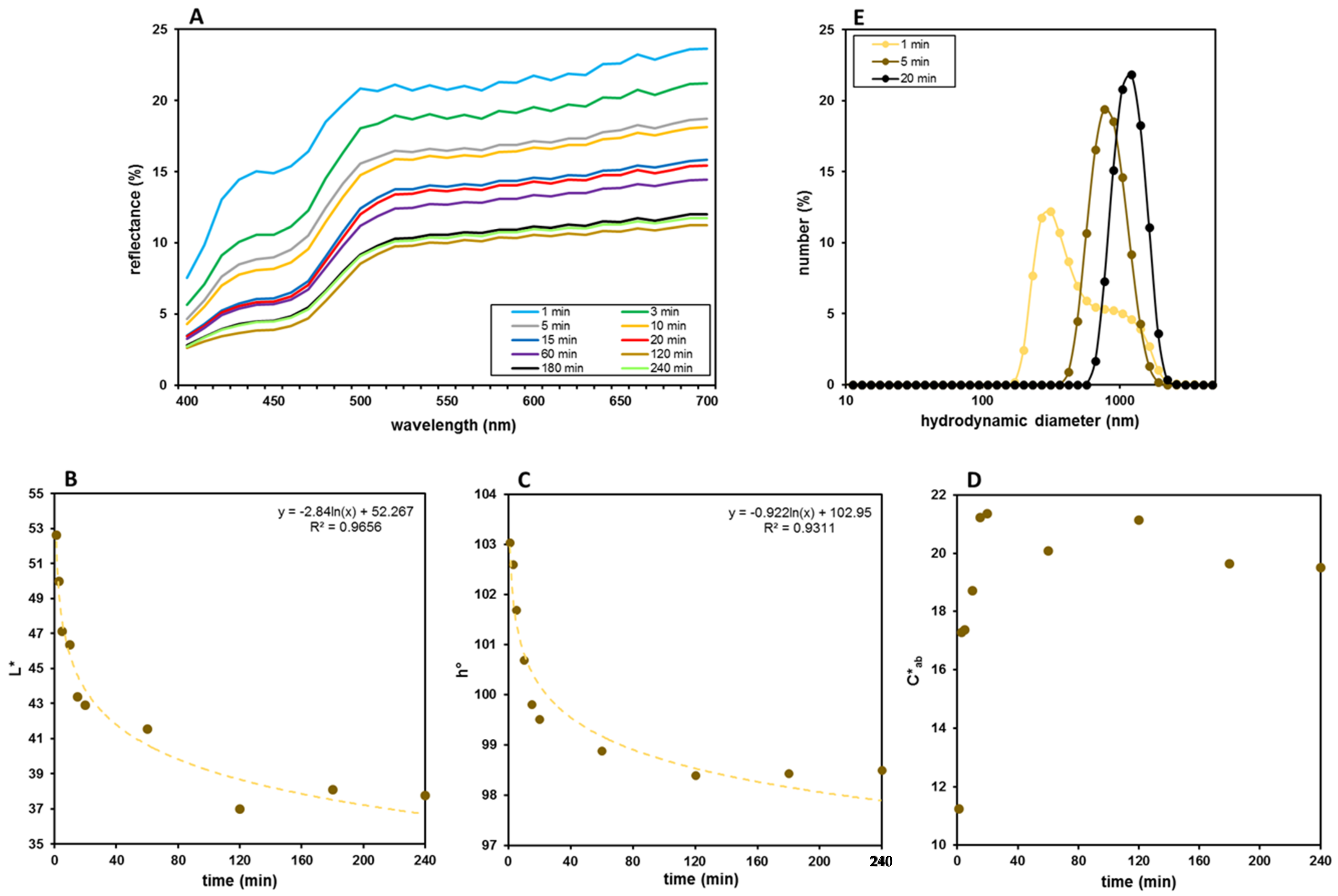
3.1. Film Preparation and Optimization
3.2. Film Characterization
3.3. Swelling Measurements
3.4. Release Studies
3.5. Antimicrobial Activity of Films Loaded with AgNPs
3.5.1. Antimicrobial Activity in Solid Culture Media (Diffusion Test)
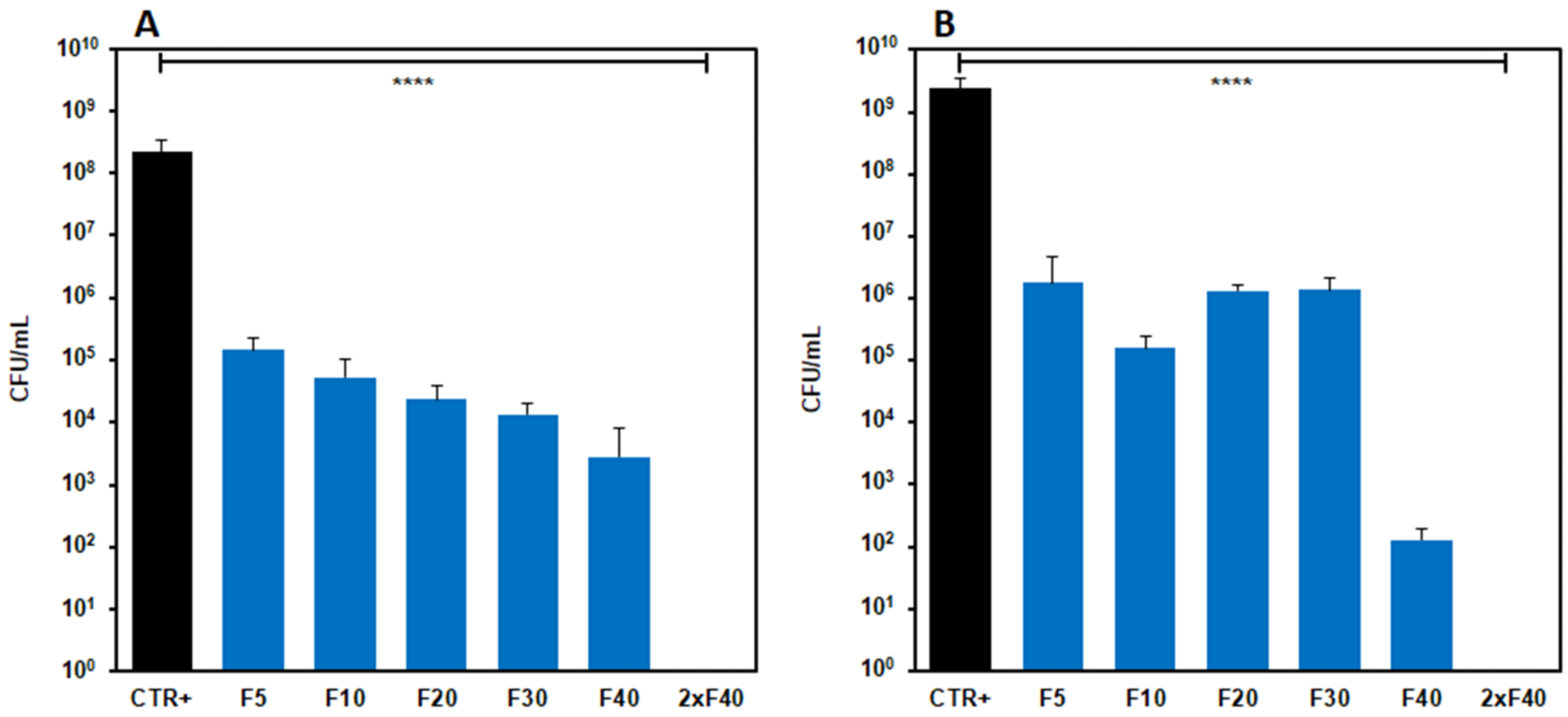
3.5.2. Antimicrobial Activity in Liquid Culture Media
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Kuwahara, R.; Uchino, M.; Ikeuchi, H.; Bando, T.; Sasaki, H.; Yasuhara, M.; Kimura, K.; Goto, Y.; Horio, Y.; Minagawa, T.; et al. Effect of Changing surgical instruments before wound closure to prevent wound infection in lower GI surgery: A randomized controlled trial. Dis. Colon Rectum 2022, 65, 100–107. [Google Scholar] [CrossRef]
- World Union of Wound Healing Societies (WUWHS), Florence Congress, Position Document. Management of Biofilm. Wounds International. Available online: https://www.woundsinternational.com/resources/details/position-document-management-biofilm (accessed on 23 September 2016).
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Okur, M.E.; Karantas, I.D.; Şenyiğit, Z.; Üstündağ Okur, N.; Siafaka, P.I. Recent trends on wound management: New therapeutic choices based on polymeric carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2019, 29, 8–15. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khalili, S.; Nouri Khorasani, S.; Esmaeely Neisiany, R.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef] [Green Version]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Asadi, N.; Pazoki-Toroudi, H.; Del Bakhshayesh, A.R.; Akbarzadeh, A.; Davaran, S.; Annabi, N. Multifunctional hydrogels for wound healing: Special focus on biomacromolecular based hydrogels. Int. J. Biol. Macromol. 2020, 170, 728–750. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of appropriate wound dressing for various wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern wound dressings: Hydrogel dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Leyva-Gómez, G.; González-Torres, M.; Alcalá-Alcalá, S.; Bernal-Chávez, S.A.; Morales-Morfin, J.C.; González-Del Carmen, M.; Sharifi-Rad, J.; Figueroa-González, G.; Reyes-Hernández, O.D.; Del Prado-Audelo, M.L.; et al. Development of films from natural sources for infections during wound healing. Cell. Mol. Biol. (Noisy-le-grand) 2021, 67, 96–100. [Google Scholar] [CrossRef]
- Savencu, I.; Iurian, S.; Porfire, A.; Bogdan, C.; Tomuță, I. Review of advances in polymeric wound dressing films. React. Funct. Polym. 2021, 168, 105059. [Google Scholar] [CrossRef]
- Li, R.; Liu, K.; Huang, X.; Li, D.; Ding, J.; Liu, B.; Chen, X. Bioactive Materials Promote Wound Healing through Modulation of Cell Behaviors. Adv. Sci. 2022, 9, 2105152. [Google Scholar] [CrossRef]
- Udayakumar, G.P.; Muthusamy, S.; Selvaganesh, B.; Sivarajasekar, N.; Rambabu, K.; Banat, F.; Sivamani, S.; Sivakumar, N.; Hosseini-Bandegharaei, A.; Show, P.L. Biopolymers and composites: Properties, characterization and their applications in food, medical and pharmaceutical industries. J. Environ. Chem. Eng. 2021, 9, 105322. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based nanoparticles as antimicrobial agents: An overview. Nanomater. 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihai, M.M.; Dima, M.B.; Dima, B.; Holban, A.M. Nanomaterials for wound healing and infection control. Mater. 2019, 12, 2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nqakala, Z.B.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, M.; Onani, M.O.; Madiehe, A.M. Advances in nanotechnology towards Development of silver nanoparticle-based wound-healing agents. Int. J. Mol. Sci. 2021, 22, 11272. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Barańska-Rybak, W. Nanomaterials as a successor of antibiotics in antibiotic-resistant, biofilm infected wounds? Antibiotics 2021, 10, 941. [Google Scholar] [CrossRef]
- Paladini, F.; Pollini, M. Antimicrobial silver nanoparticles for wound healing application: Progress and future trends. Materials 2019, 12, 2540. [Google Scholar] [CrossRef] [Green Version]
- Badhwar, R.; Mangla, B.; Neupane, Y.R.; Khanna, K.; Popli, H. Quercetin loaded silver nanoparticles in hydrogel matrices for diabetic wound healing. Nanotechnology 2021, 32, 505102. [Google Scholar] [CrossRef]
- Alcântara, M.T.S.; Lincopan, N.; Santos, P.M.; Ramirez, P.A.; Brant, A.J.C.; Riella, H.G.; Lugão, A.B. Simultaneous hydrogel crosslinking and silver nanoparticle formation by using ionizing radiation to obtain antimicrobial hydrogels. Radiat. Phys. Chem. 2020, 169, 108777. [Google Scholar] [CrossRef]
- González-Sánchez, M.I.; Perni, S.; Tommasi, G.; Morris, N.G.; Hawkins, K.; López-Cabarcos, E.; Prokopovich, P. Silver nanoparticle based antibacterial methacrylate hydrogels potential for bone graft applications. Mater. Sci. Eng. C 2015, 50, 332–340. [Google Scholar] [CrossRef]
- Yuan, Y.; Ding, L.; Chen, Y.; Chen, G.; Zhao, T.; Yu, Y. Nano-silver functionalized polysaccharides as a platform for wound dressings: A review. Int. J. Biol. Macromol. 2022, 194, 644–653. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B. Ștefan; Andronescu, E. Inorganic nanoparticles and composite films for antimicrobial therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef]
- Shi, G.; Chen, W.; Zhang, Y.; Dai, X.; Zhang, X.; Wu, Z. An Antifouling hydrogel contained silver nanoparticles for modulating therapeutic immune response in chronic wound healing. Langmuir 2019, 35, 1837–1845. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, J.; Miao, Y.-E.; Huang, Y.; Liu, T. Catalytic and antibacterial activities of green-synthesized silver nanoparticles on electrospun polystyrene nanofiber membranes using tea polyphenols. Compos. B Eng. 2015, 79, 217–223. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevěčná, T.J.; Zbořil, R. Silver Colloid Nanoparticles: Synthesis, Characterization, and Their Antibacterial Activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound dressings functionalized with silver nanoparticles: Promises and pitfalls. Nanoscale 2020, 12, 2268–2291. [Google Scholar] [CrossRef]
- Krishnan, P.D.; Banas, D.; Durai, R.D.; Kabanov, D.; Hosnedlova, B.; Kepinska, M.; Fernandez, C.; Ruttkay-Nedecky, B.; Nguyen, H.V.; Farid, A.; et al. Silver Nanomaterials for Wound Dressing Applications. Pharmaceutics 2020, 12, 821. [Google Scholar] [CrossRef]
- Pangli, H.; Vatanpour, S.; Hortamani, S.; Jalili, R.; Ghahary, A. Incorporation of silver nanoparticles in hydrogel matrices for controlling wound infection. J. Burn Care Res. 2021, 42, 785–793. [Google Scholar] [CrossRef]
- Roppolo, I.; Doriguzzi Bozzo, A.; Castellino, M.; Chiappone, A.; Perrone, D.; Bejtka, K.; Bocchini, S.; Sangermano, M.; Chiolerio, A. Dual step irradiation process for in situ generation and patterning of silver nanoparticles in a photocured film. RSC Advances 2016, 6, 14832–14843. [Google Scholar] [CrossRef] [Green Version]
- Rahimi, M.; Noruzi, E.B.; Sheykhsaran, E.; Ebadi, B.; Kariminezhad, Z.; Molaparast, M.; Mehrabani, M.G.; Mehramouz, B.; Yousefi, M.; Ahmadi, R.; et al. Carbohydrate polymer-based silver nanocomposites: Recent progress in the antimicrobial wound dressings. Carbohydr. Polym. 2020, 231, 115696. [Google Scholar] [CrossRef]
- Paolicelli, P.; Petralito, S.; Varani, G.; Nardoni, M.; Pacelli, S.; Di Muzio, L.; Tirillò, J.; Bartuli, C.; Cesa, S.; Casadei, M.A. Effect of glycerol on the physical and mechanical properties of thin gellan gum films for oral drug delivery. Int. J. Pharm. 2018, 547, 226–234. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Avitabile, M.; Varani, G.; Di Muzio, L.; Cesa, S.; Tirillò, J.; Bartuli, C.; Nardoni, M.; Petralito, S.; et al. Design of a tunable nanocomposite double network hydrogel based on gellan gum for drug delivery applications. Eur. Polym. J. 2018, 104, 184–193. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Dreesen, I.; Kobayashi, S.; Vitalone, A.; Casadei, M.A. Injectable and photocross-linkable gels based on gellan gum methacrylate: A new tool for biomedical application. Int. J. Biol. Macromol. 2015, 72, 1335–1342. [Google Scholar] [CrossRef]
- Adrover, A.; Di Muzio, L.; Trilli, J.; Brandelli, C.; Paolicelli, P.; Petralito, S.; Casadei, M.A. Enhanced Loading Efficiency and Mucoadhesion Properties of Gellan Gum Thin Films by Complexation with Hydroxypropyl-β-Cyclodextrin. Pharmaceutics 2020, 12, 819. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Navarro-García, F.; Heras, A.; Acosta, N. Chitosan based films as supports for dual antimicrobial release. Carbohydr. Polym. 2016, 146, 402–410. [Google Scholar] [CrossRef]
- Chen, S.; Qin, J.; Du, J. Cross-linkable yet biodegradable polymer films. Acta Phys.-Chim. Sin. 2022, 38, 2006029. [Google Scholar] [CrossRef]
- Coutinho, D.F.; Sant, S.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified gellan gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [Green Version]
- Thiagamani, S.M.K.; Rajini, N.; Siengchin, S.; Rajulu, A.V.; Hariram, N.; Ayrilmis, N. Influence of silver nanoparticles on the mechanical, thermal and antimicrobial properties of cellulose-based hybrid nanocomposites. Compos. B Eng. 2019, 165, 516–525. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, H.; Gao, M.; Zhang, M.; Liu, P.; Liu, X. Cellulose nanocrystals/silver nanoparticles: In-situ preparation and application in PVA films. Holzforschung 2019, 74, 523–528. [Google Scholar] [CrossRef]
- Tsuboi, A.; Nakamura, K.; Kobayashi, N. Multicolor electrochromism showing three primary color states (cyan-magenta-yellow) based on size- and shape-controlled silver nanoparticles. Chem. Mater. 2014, 26, 6477–6485. [Google Scholar] [CrossRef]
- Richardson, T.J.; Slack, J.L.; Armitage, R.D.; Kostecki, R.; Farangis, B.; Rubin, M.D. Switchable mirrors based on nickel-magnesium films. Appl. Phys. Lett. 2001, 78, 3047–3049. [Google Scholar] [CrossRef] [Green Version]
- Prosposito, P.; Burratti, L.; Venditti, I. Silver Nanoparticles as Colorimetric Sensors for Water Pollutants. Chemosensors 2020, 8, 26. [Google Scholar] [CrossRef] [Green Version]
- Chartarrayawadee, W.; Charoensin, P.; Saenma, J.; Rin, T.; Khamai, P.; Nasomjai, P.; On Too, C. Green synthesis and stabilization of silver nanoparticles using Lysimachia foenum-graecum Hance extract and their antibacterial activity. Green Process. Synth. 2020, 9, 107–118. [Google Scholar] [CrossRef]
- Greenman, J.; Thorn, R.M.; Saad, S.; Austin, A.J. In vitro diffusion bed, 3-day repeat challenge ‘capacity’ test for antimicrobial wound dressings. Int. Wound J. 2006, 3, 322–3299. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.Y.; Zhu, X.; Mukherjee, D.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Pristine gellan gum-collagen interpenetrating network hydrogels as mechanically enhanced anti-inflammatory biologic wound dressings for burn wound therapy. ACS Appl. Bio. Mater. 2021, 4, 70–1482. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Pan, Y.J.; Li, S.K.; Xing, L.; Niu, X. Doubly crosslinked biodegradable hydrogels based on gellan gum and chitosan for drug delivery and wound dressing. Int. J. Biol. Macromol. 2020, 164, 2204–2214. [Google Scholar] [CrossRef]
- Dohle, E.; Scherrieble, A.; Doser, M.; Al-Maawi, S.; Hoss, M.; Dauner, M.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Co-culture model for cutaneous wound healing to assess a porous fiber-based drug delivery system. Tissue Eng. Part C Methods 2020, 26, 75–484. [Google Scholar] [CrossRef]
- Li, W.; Jian, X.; Zou, Y.; Wu, L.; Huang, H.; Li, H.; Hu, D.; Yu, B. The fabrication of a gellan gum-based hydrogel loaded with magnesium ions for the synergistic promotion of skin wound healing. Front. Bioeng. Biotechnol. 2021, 9, 709679. [Google Scholar] [CrossRef]






| Sample | GG-MA (% w/v) | Gly (% w/v) | AgNO3 (mg/mL) | Casted Volume (mL) |
|---|---|---|---|---|
| A5 | 2.0 | 3.0 | 5 | 6 |
| A10 | 2.0 | 3.0 | 10 | 6 |
| A20 | 2.0 | 3.0 | 20 | 6 |
| A30 | 2.0 | 3.0 | 30 | 6 |
| A40 | 2.0 | 3.0 | 40 | 6 |
| A50 | 2.0 | 3.0 | 50 | 6 |
| B40 | 2.0 | 4.0 | 40 | 6 |
| C40 | 2.0 | 2.0 | 40 | 6 |
| D40 | 1.5 | 2.0 | 40 | 6 |
| E40 | 1.5 | 1.0 | 40 | 6 |
| F5 | 1.5 | 1.0 | 5 | 12 |
| F10 | 1.5 | 1.0 | 10 | 12 |
| F20 | 1.5 | 1.0 | 20 | 12 |
| F30 | 1.5 | 1.0 | 30 | 12 |
| F40 | 1.5 | 1.0 | 40 | 12 |
| Sample | Thickness (μm) | Tensile Modulus (MPa) | Tensile Strength (MPa) |
|---|---|---|---|
| F5 | 72.2 ± 2.6 | 2.13 ± 0.24 | 0.41 ± 0.08 |
| F10 | 97.0 ± 6.4 | 2.70 ± 0.28 | 0.45 ± 0.07 |
| F20 | 116.0 ± 9.0 | 3.05 ± 0.24 | 0.47 ± 0.06 |
| F30 | 130.8 ± 6.4 | 3.21 ± 0.17 | 0.59 ± 0.03 |
| F40 | 152.3 ± 10.6 | 3.43 ± 0.30 | 0.61 ± 0.04 |
| Film Samples | L* | a* | b* | C*ab | h° |
|---|---|---|---|---|---|
| F5 | 19.68 | −0.02 | 8.62 | 8.62 | 91.34 |
| F10 | 21.39 | 0.02 | 9.88 | 9.95 | 83.04 |
| F20 | 24.34 | 1.02 | 12.78 | 13.03 | 78.75 |
| F30 | 34.83 | 1.21 | 13.88 | 13.91 | 85.72 |
| F40 | 38.16 | 2.54 | 11.10 | 11.11 | 89.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Muzio, L.; Simonetti, P.; Carriero, V.C.; Brandelli, C.; Trilli, J.; Sergi, C.; Tirillò, J.; Cairone, F.; Cesa, S.; Radocchia, G.; et al. Solvent Casting and UV Photocuring for Easy and Safe Fabrication of Nanocomposite Film Dressings. Molecules 2022, 27, 2959. https://doi.org/10.3390/molecules27092959
Di Muzio L, Simonetti P, Carriero VC, Brandelli C, Trilli J, Sergi C, Tirillò J, Cairone F, Cesa S, Radocchia G, et al. Solvent Casting and UV Photocuring for Easy and Safe Fabrication of Nanocomposite Film Dressings. Molecules. 2022; 27(9):2959. https://doi.org/10.3390/molecules27092959
Chicago/Turabian StyleDi Muzio, Laura, Prisca Simonetti, Vito Cosimo Carriero, Chiara Brandelli, Jordan Trilli, Claudia Sergi, Jacopo Tirillò, Francesco Cairone, Stefania Cesa, Giulia Radocchia, and et al. 2022. "Solvent Casting and UV Photocuring for Easy and Safe Fabrication of Nanocomposite Film Dressings" Molecules 27, no. 9: 2959. https://doi.org/10.3390/molecules27092959
APA StyleDi Muzio, L., Simonetti, P., Carriero, V. C., Brandelli, C., Trilli, J., Sergi, C., Tirillò, J., Cairone, F., Cesa, S., Radocchia, G., Schippa, S., Petralito, S., Paolicelli, P., & Casadei, M. A. (2022). Solvent Casting and UV Photocuring for Easy and Safe Fabrication of Nanocomposite Film Dressings. Molecules, 27(9), 2959. https://doi.org/10.3390/molecules27092959











