Synthesis, Theoretical Calculation, and Biological Studies of Mono- and Diphenyltin(IV) Complexes of N-Methyl-N-hydroxyethyldithiocarbamate
Abstract
:1. Introduction
2. Results and Discussion
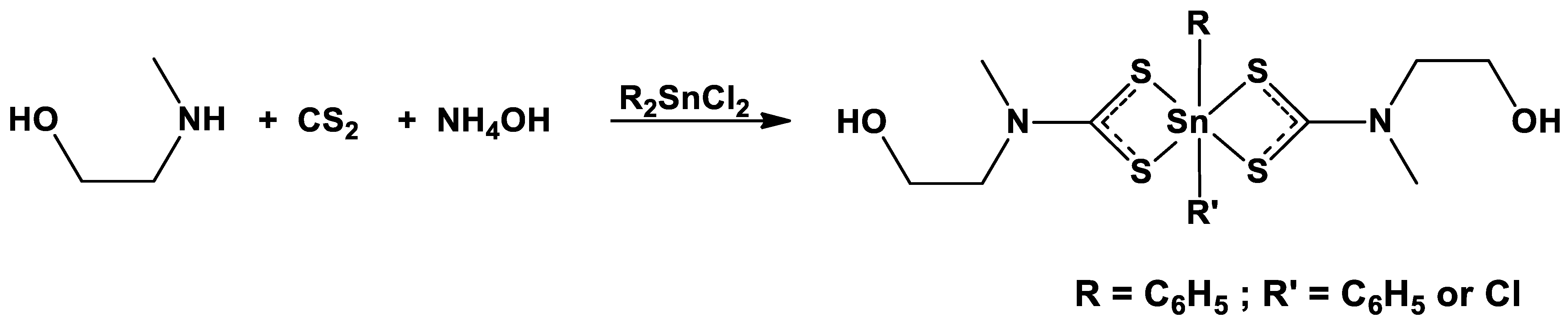
2.1. Synthesis and Characterization
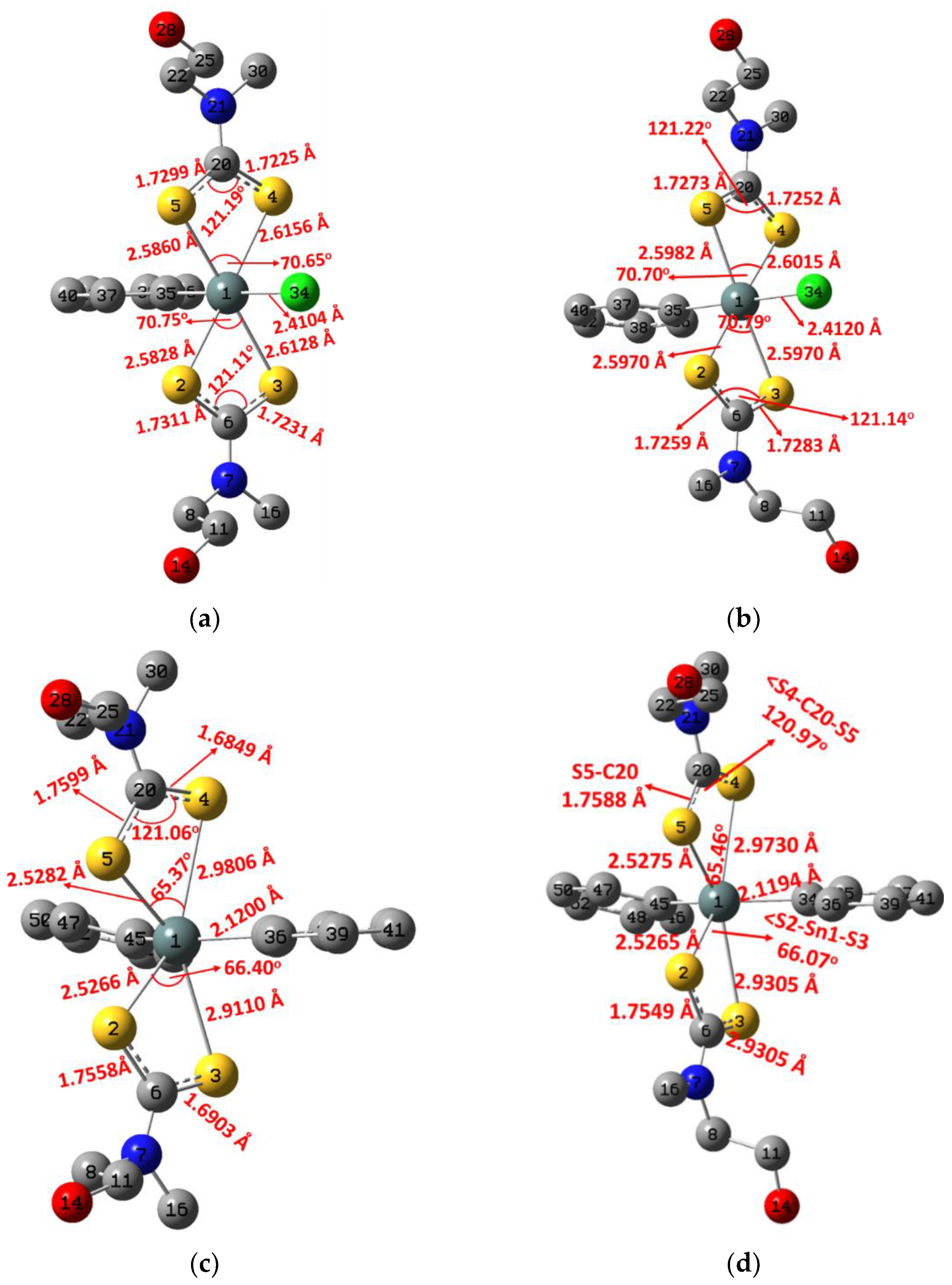
2.2. Molecular and Electronic Structures
2.3. Biological Study
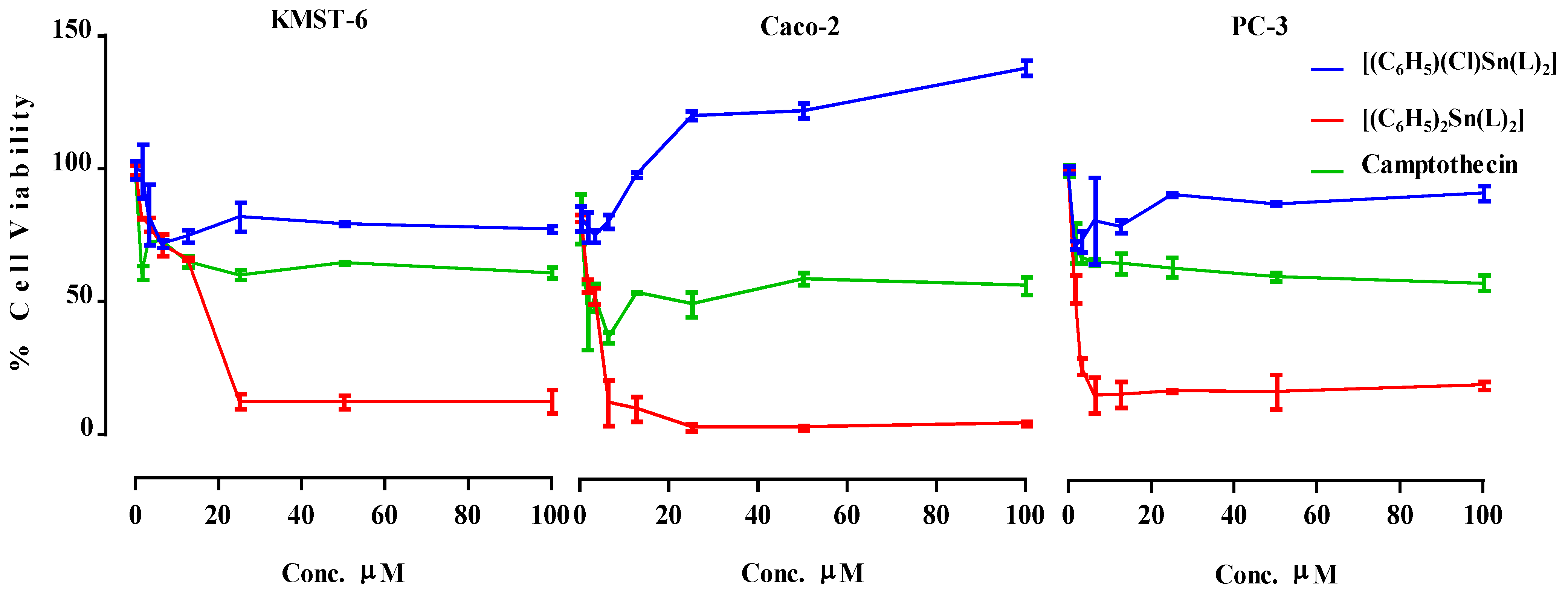
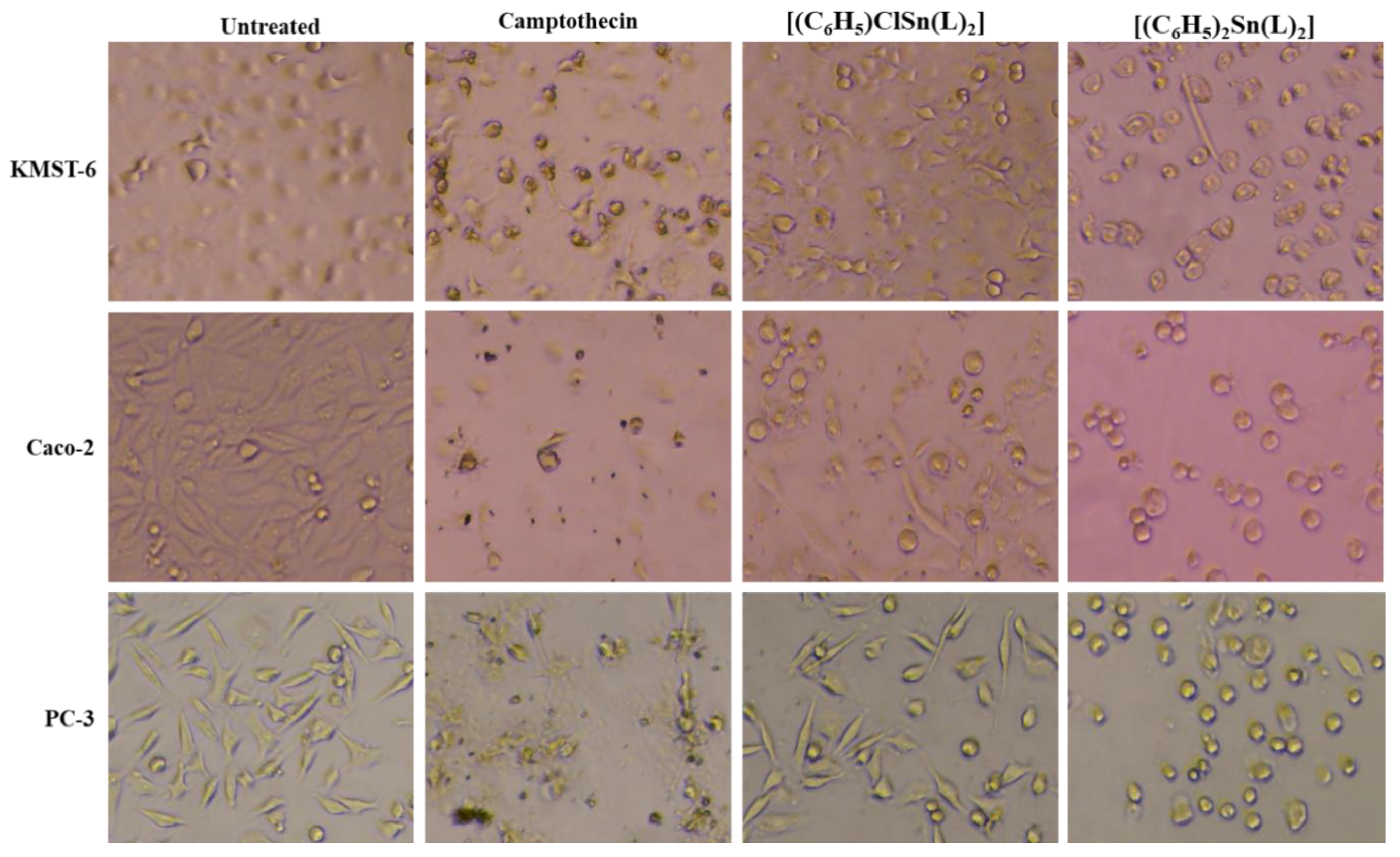
2.3.1. Cytotoxicity Study
2.3.2. In Vitro Anti-Inflammatory Assay
3. Materials and Methods
3.1. Synthesis of Organotin(IV) N-Methyl-N-hydroxyethyldithiocarbamate
3.2. Computational Details
3.3. Cytotoxicity Study
3.4. In Vitro Anti-Inflammatory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Adokoh, C.K. Therapeutic Potential of Dithiocarbamate Supported Gold Compounds. RSC Adv. 2020, 10, 2975–2988. [Google Scholar] [CrossRef] [Green Version]
- Gölcü, A. Transition Metal Complexes of Propranolol Dithiocarbamate: Synthesis, Characterization, Analytical Properties and Biological Activity. Transit. Met. Chem. 2006, 31, 405–412. [Google Scholar] [CrossRef]
- Al-Obaidy, G.S.; Ibraheem, K.R.; Mesher, M.F. Metal Complexes Derived from Dithiocarbamate Ligand: Formation, Spectral Characterization and Biological Activity. Syst. Rev. Pharm. 2020, 11, 360–368. [Google Scholar] [CrossRef]
- Fabretti, A.C.; Franchini, G.C.; Preti, C.; Tosi, G.; Zannini, P. Transition Metal Complexes with 2-Methyl-, 3-Methyl-, and 4-Methyl-Piperidine Dithiocarbamate as Ligands. Transit. Met. Chem. 1985, 10, 284–287. [Google Scholar] [CrossRef]
- Ahmed, A.J. Metal Complexes of Dithiocarbamate Derivatives and Its Biological Activity. Asian J. Chem. 2018, 30, 2595–2602. [Google Scholar] [CrossRef]
- Hogarth, G. Metal-Dithiocarbamate Complexes: Chemistry and Biological Activity. ChemInform 2013, 44. [Google Scholar] [CrossRef]
- Gasser, G.; Metzler-Nolte, N. The Potential of Organometallic Complexes in Medicinal Chemistry Current Opinion in Chemical Biology. Curr. Opin. Chem. Biol. 2012, 16, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasser, G.; Ott, I.; Metzler-Nolte, N. Organometallic Anticancer Compounds. J. Med. Chem. 2011, 54, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Buck-Koehntop, B.A.; Porcelli, F.; Lewin, J.L.; Cramer, C.J.; Veglia, G. Biological Chemistry of Organotin Compounds: Interactions and Dealkylation by Dithiols. J. Organomet. Chem. 2006, 691, 1748–1755. [Google Scholar] [CrossRef]
- Cervantes, J.; Zárraga, R.; Salazar-Hernández, C. Organotin Catalysts in Organosilicon Chemistry. Appl. Organomet. Chem. 2012, 26, 157–163. [Google Scholar] [CrossRef]
- Pellerito, L.; Nagy, L. Organotin(IV)n+ Complexes Formed with Biologically Active Ligands: Equilibrium and Structural Studies, and Some Biological Aspects. Coord. Chem. Rev. 2002, 224, 111–150. [Google Scholar] [CrossRef]
- Pellerito, C.; Nagy, L.; Pellerito, L.; Szorcsik, A. Biological Activity Studies on Organotin(IV)n+ Complexes and Parent Compounds. J. Organomet. Chem. 2006, 691, 1733–1747. [Google Scholar] [CrossRef]
- Awang, N.; FarahanaKamaludin, N.; Baba, I.; Chan, K.M.; Rajab, N.F.; Hamid, A. Synthesis, Characterization, and Antitumor Activity of New Organotin(IV) Methoxyethyldithiocarbamate Complexes. Orient. J. Chem. 2016, 32, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Kadu, R.; Roy, H.; Singh, V.K. Diphenyltin(IV) Dithiocarbamate Macrocyclic Scaffolds as Potent Apoptosis Inducers for Human Cancer HEP 3B and IMR 32 Cells: Synthesis, Spectral Characterization, Density Functional Theory Study and in Vitro Cytotoxicity. Appl. Organomet. Chem. 2015, 29, 746–755. [Google Scholar] [CrossRef]
- Awang, N.; Baba, I.; Mohd Yousof, N.S.A.; Kamaludin, N.F. Synthesis and Characterization of Organotin(IV) N-Benzyl-N-Isopropyldithiocarbamate Compounds: Cytotoxic Assay on Human Hepatocarcinoma Cells (HepG2). Am. J. Appl. Sci. 2010, 7, 1047–1052. [Google Scholar] [CrossRef]
- Bobinihi, F.F.; Onwudiwe, D.C.; Ekennia, A.C.; Okpareke, O.C.; Arderne, C.; Lane, J.R. Group 10 Metal Complexes of Dithiocarbamates Derived from Primary Anilines: Synthesis, Characterization, Computational and Antimicrobial Studies. Polyhedron 2019, 158, 296–310. [Google Scholar] [CrossRef]
- Ekennia, A.C.; Osowole, A.A.; Onwudiwe, D.C.; Babahan, I.; Ibeji, C.U.; Okafor, S.N.; Ujam, O.T. Synthesis, Characterization, Molecular Docking, Biological Activity and Density Functional Theory Studies of Novel 1,4-Naphthoquinone Derivatives and Pd(II), Ni(II) and Co(II) Complexes. Appl. Organomet. Chem. 2018, 32, e4310. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C.; Singh, M. Synthesis, Characterization, and Cytotoxicity Study of Organotin(IV) Complexes Involving Different Dithiocarbamate Groups. J. Mol. Struct. 2019, 1179, 366–375. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Saibu, G.M.; Olasunkanmi, L.O.; Fadaka, A.O.; Meyer, M.; Sibuyi, N.R.S.; Onwudiwe, D.C.; Oyedeji, A.O. Synthesis, Computational and Biological Studies of Alkyltin(IV) N-Methyl-N-Hydroxyethyl Dithiocarbamate Complexes. Heliyon 2021, 7, e07693. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.E.; Ellis, C.A.; Lewis, C.E.; Tiekink, E.R.T. 3D-, 2D- and 1D-Supramolecular Structures of {Zn[S2CN(CH2CH2OH)R]2}2 and Their {Zn[S2CN(CH2CH2OH)R]2}2(4,4′-Bipyridine) Adducts for R = CH2CH2OH, Me or Et: Polymorphism and Pseudo-Polymorphism. CrystEngComm 2007, 9, 930–940. [Google Scholar] [CrossRef]
- Howie, R.A.; De Lima, G.M.; Menezes, D.C.; Wardell, J.L.; Wardell, S.M.S.V.; Young, D.J.; Tiekink, E.R.T. The Influence of Cation upon the Supramolecular Aggregation Patterns of Dithiocarbamate Anions Functionalised with Hydrogen Bonding Capacity—The Prevalence of Charge-Assisted O–H⋯S Interactions. CrystEngComm 2008, 10, 1626–1637. [Google Scholar] [CrossRef]
- Muhammad, N.; Ali, S.; Butler, I.S.; Meetsma, A. New Mononuclear Organotin(IV) 4-Benzhydrylpiperazine-1-Carbodithioates: Synthesis, Spectroscopic Characterization, X-Ray Structures and in Vitro Antimicrobial Activities. Inorg. Chim. Acta 2011, 373, 187–194. [Google Scholar] [CrossRef]
- Tamilvanan, S.; Gurumoorthy, G.; Thirumaran, S.; Ciattini, S. Synthesis, Characterization, Cytotoxicity and Antimicrobial Studies on Bi(III) Dithiocarbamate Complexes Containing Furfuryl Group and Their Use for the Preparation of Bi2O3 nanoparticles. Polyhedron 2017, 121, 70–79. [Google Scholar] [CrossRef]
- Sathiyaraj, E.; Srinivasan, T.; Thirumaran, S.; Velmurugan, D. Synthesis and Spectroscopic Characterization of Ni(II) Complexes Involving Functionalised Dithiocarbamates and Triphenylphosphine: Anagostic Interaction in (N-Cyclopropyl-N-(4-Fluorobenzyl)Dithiocarbamato-S,S′) (Thiocyanato-N)(Triphenylphosphine)Nickel(II). J. Mol. Struct. 2015, 1102, 203–209. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C.; Hosten, E.C. Synthesis, Characterization and the Use of Organotin(IV) Dithiocarbamate Complexes as Precursor to Tin Sulfide Nanoparticles by Heat up Approach. J. Mol. Struct. 2019, 1195, 395–402. [Google Scholar] [CrossRef]
- Muthalib, A.F.A.; Baba, I. New Mono-Organotin (IV) Dithiocarbamate Complexes. AIP Conf. Proc. 2014, 1614, 237–243. [Google Scholar] [CrossRef]
- Prakasam, B.A.; Ramalingam, K.; Baskaran, R.; Bocelli, G.; Cantoni, A. Synthesis, NMR Spectral and Single Crystal X-Ray Structural Studies on Ni(II) Dithiocarbamates with NiS2PN, NiS2PC, NiS2P2 Chromophores: Crystal Structures of (4-Methylpiperazinecarbodithioato)(Thiocyanato-N) (Triphenylphosphine)Nickel(II) and Bis(Triphen). Polyhedron 2007, 26, 1133–1138. [Google Scholar] [CrossRef]
- Srinivasan, N.; Thirumaran, S.; Ciattini, S. Effect of position of methyl substituent in piperidinedithiocarbamate on the ZnS4N chromophore: Synthesis, spectral, valence-bond parameters and single crystal X-ray structural studies on bis(2-methylpiperidinecarbodithioato-S,S′)-(pyridine)zinc(II) and bis(4-methylpiperidinecarbodithioato-S,S′)(pyridine)zinc(II). J. Mol. Struct. 2009, 936, 234–238. [Google Scholar] [CrossRef]
- Arul Prakasam, B.; Ramalingam, K.; Bocelli, G.; Cantoni, A. NMR and Fluorescence Spectral Studies on Bisdithiocarbamates of Divalent Zn, Cd and Their Nitrogenous Adducts: Single Crystal X-Ray Structure of (1,10-Phenanthroline)Bis(4-Methylpiperazinecarbodithioato) Zinc(II). Polyhedron 2007, 26, 4489–4493. [Google Scholar] [CrossRef]
- Khan, H.N.; Ali, S.; Shahzadi, S.; Helliwell, M. Synthesis and Spectral Characterization of Chloro-Organotin(IV) Complexes of S-Donor Ligand: Crystal Structure of Chloro-t-Dibutyltin[4-Methyl-1-Piperidine]Thiocarboxylate. Russ. J. Inorg. Chem. 2012, 57, 665–670. [Google Scholar] [CrossRef]
- Khan, N.; Farina, Y.; Mun, L.K.; Rajab, N.F.; Awang, N. Syntheses, Characterization, X-Ray Diffraction Studies and in Vitro Antitumor Activities of Diorganotin(IV) Derivatives of Bis(p-Substituted-N-Methylbenzylaminedithiocarbamates). Polyhedron 2015, 85, 754–760. [Google Scholar] [CrossRef]
- Barba, V.; Arenaza, B.; Guerrero, J.; Reyes, R. Synthesis and Structural Characterization of Diorganotin Dithiocarbamates from 4-(Ethylaminomethyl)Pyridine. Heteroat. Chem. 2012, 23, 422–428. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Tin Dithiocarbamates: Applications and Structures. Appl. Organomet. Chem. 2008, 22, 533–550. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. (Eds.) CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781315380476. [Google Scholar]
- Lu, T.; Chen, F. Calculation of Molecular Orbital Composition. Acta Chim. Sin.-Chin. Ed. 2011, 69, 2393. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Sirajuddin, M.; Ali, S.; Khalid, N.; Tahir, M.N.; Shah, N.A.; Rasheed, Z.; Khan, M.R. Organotin(IV) Derivatives of o-Isobutyl Carbonodithioate: Synthesis, Spectroscopic Characterization, X-Ray Structure, HOMO/LUMO and in Vitro Biological Activities. Polyhedron 2016, 104, 80–90. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Onwudiwe, D.C. Organotin(IV) Dithiocarbamate Complexes: Chemistry and Biological Activity. Molecules 2018, 23, 2571. [Google Scholar] [CrossRef] [Green Version]
- Sedaghat, T.; Aminian, M.; Azarkish, M. New Bis-Diphenyltin(IV) Complexes With Oxalyldihydrazone Derivatives: Synthesis, Characterization And Antibacterial Activity. Phosphorus Sulfur Silicon Relat. Elem. 2015, 190, 352–359. [Google Scholar] [CrossRef]
- Nath, M.; Vats, M.; Roy, P. Mode of Action of Tin-Based Anti-Proliferative Agents: Biological Studies of Organotin(IV) Derivatives of Fatty Acids. J. Photochem. Photobiol. B Biol. 2015, 148, 88–100. [Google Scholar] [CrossRef]
- Wong, R.S.Y. Role of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) in Cancer Prevention and Cancer Promotion. Adv. Pharmacol. Sci. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Rani, P.; Pal, D.; Hegde, R.R.; Hashim, S.R. Anticancer, Anti-Inflammatory, and Analgesic Activities of Synthesized 2-(Substituted Phenoxy) Acetamide Derivatives. Biomed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Nath, M.; Pokharia, S.; Eng, G.; Song, X.; Kumar, A. New Triorganotin(IV) Derivatives of Dipeptides as Anti-Inflammatory–Antimicrobial Agents. Eur. J. Med. Chem. 2005, 40, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Nath, M.; Vats, M.; Roy, P. Tri- and Diorganotin(IV) Complexes of Biologically Important Orotic Acid: Synthesis, Spectroscopic Studies, In Vitro Anti-Cancer, DNA Fragmentation, Enzyme Assays and in Vivo Anti-Inflammatory Activities. Eur. J. Med. Chem. 2013, 59, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron Affinities of the First-row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef] [Green Version]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. III. The Atoms Aluminum through Argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; computer program; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. NBO 7.0: New vistas in localized and delocalized chemical bonding theory. J. Comput. Chem. 2019, 40, 2234–2241. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Atomic dipole moment corrected hirshfeld population method. J. Theor. Comput. Chem. 2012, 11, 163–183. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Chandra, S.; Chatterjee, P.; Dey, P. Evaluation of Anti-Inflammatory Effects of Green Tea and Black Tea: A Comparative in Vitro Study. J. Adv. Pharm. Technol. Res. 2012, 3, 136–138. [Google Scholar] [CrossRef] [PubMed]

| [(C6H5)(Cl)Sn(L)2] | [(C6H5)2Sn(L)2] | |||
|---|---|---|---|---|
| E(2) (kJ/mol) | E(2) (kJ/mol) | |||
| Donor → Acceptor Interactions | Syn-Syn | Anti-Anti | Syn-Syn | Anti-Anti |
| BD(1) S2-C6 → LP*Sn(1) | 42.43 | 41.97 | 41.09 | 40.67 |
| BD(1) S3-C6 → LP*Sn(1) | 40.92 | 40.96 | 25.94 | 25.52 |
| BD(1) S4-C20 → LP*Sn(1) | 40.58 | 41.59 | 23.35 | 23.68 |
| BD(1) S5-C20 → LP*Sn(1) | 42.13 | 41.55 | 39.96 | 39.96 |
| LP(3)S2 → LP*(2)Sn1 | 202.97 | 256.10 | 586.85 | 285.77 |
| Composition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Atom | [(C6H5)(Cl)Sn(L)2] | [(C6H5)2Sn(L)2] | ||||||
| HOMO | LUMO | HOMO−1 | LUMO+1 | HOMO | LUMO | HOMO−1 | LUMO+1 | |
| Sn1 | - | 39.26 | - | 3.53 | - | 3.10 | - | 1.51 |
| S2 | 10.96 | 7.43 | 8.80 | 5.80 | 10.82 | 6.81 | 4.91 | 5.31 |
| S3 | 27.07 | 7.27 | 19.70 | 5.50 | 18.23 | 11.21 | 50.60 | 7.59 |
| S4 | 8.36 | 7.48 | 8.48 | 10.54 | 44.10 | 9.28 | 28.66 | 11.56 |
| S5 | 20.89 | 7.31 | 24.37 | 9.98 | 14.01 | 4.55 | 2.25 | 7.00 |
| C6 | - | 1.11 | - | 15.05 | - | 24.40 | 1.13 | 17.38 |
| N7 | 1.24 | - | - | 4.76 | - | 7.44 | - | 5.05 |
| C20 | - | - | - | 27.58 | - | 18.46 | - | 23.23 |
| N21 | 1.08 | - | - | 8.43 | - | 5.60 | - | 6.51 |
| Cl34 | 17.12 | 11.90 | - | - | - | - | - | - |
| C34 | - | - | - | - | 1.13 | - | 2.62 | - |
| C35 | 3.79 | 7.53 | 2.11 | - | 1.12 | - | - | - |
| C36 | 1.20 | 1.09 | 6.15 | - | - | - | - | - |
| C37 | - | 1.09 | 8.03 | - | - | - | - | - |
| C38 | - | - | 9.41 | - | - | - | - | - |
| C40 | 1.12 | - | 7.76 | - | - | - | - | - |
| C42 | 2.77 | - | - | - | - | - | - | 2.00 |
| C45 | - | - | - | - | 1.21 | - | 2.70 | - |
| C46 | - | - | - | - | 1.09 | - | - | - |
| C52 | - | - | - | - | 1.40 | - | - | - |
| Samples | KMST-6 (µM) | Caco-2 (µM) | PC-3 (µM) |
|---|---|---|---|
| [(C6H5)2Sn(L)2] | >100 | >100 | >100 |
| [(C6H5)2Sn(L)2] | 11.81 ± 0.15 | 4.937 ± 0.12 | 1.630 ± 0.10 |
| Camptothecin | 59.91 ± 0.21 | >100 | 24.41 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeyemi, J.O.; Olasunkanmi, L.O.; Fadaka, A.O.; Sibuyi, N.R.S.; Oyedeji, A.O.; Onwudiwe, D.C. Synthesis, Theoretical Calculation, and Biological Studies of Mono- and Diphenyltin(IV) Complexes of N-Methyl-N-hydroxyethyldithiocarbamate. Molecules 2022, 27, 2947. https://doi.org/10.3390/molecules27092947
Adeyemi JO, Olasunkanmi LO, Fadaka AO, Sibuyi NRS, Oyedeji AO, Onwudiwe DC. Synthesis, Theoretical Calculation, and Biological Studies of Mono- and Diphenyltin(IV) Complexes of N-Methyl-N-hydroxyethyldithiocarbamate. Molecules. 2022; 27(9):2947. https://doi.org/10.3390/molecules27092947
Chicago/Turabian StyleAdeyemi, Jerry O., Lukman O. Olasunkanmi, Adewale O. Fadaka, Nicole R. S. Sibuyi, Adebola O. Oyedeji, and Damian C. Onwudiwe. 2022. "Synthesis, Theoretical Calculation, and Biological Studies of Mono- and Diphenyltin(IV) Complexes of N-Methyl-N-hydroxyethyldithiocarbamate" Molecules 27, no. 9: 2947. https://doi.org/10.3390/molecules27092947
APA StyleAdeyemi, J. O., Olasunkanmi, L. O., Fadaka, A. O., Sibuyi, N. R. S., Oyedeji, A. O., & Onwudiwe, D. C. (2022). Synthesis, Theoretical Calculation, and Biological Studies of Mono- and Diphenyltin(IV) Complexes of N-Methyl-N-hydroxyethyldithiocarbamate. Molecules, 27(9), 2947. https://doi.org/10.3390/molecules27092947










