Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC–MS and GC–MS and Chemometric Tools
Abstract
:1. Introduction
2. Results and Discussion
2.1. Secondary Metabolites Profiling Using UPLC-ESI-MS
2.1.1. Proanthocyanidins
Procyanidin
Prodelphinidins
2.1.2. Hydroxycinnamates (HCAs)
2.1.3. Flavonoids
2.1.4. Alkaloids
2.1.5. Lignans and Terpenes
2.1.6. Fatty Acids
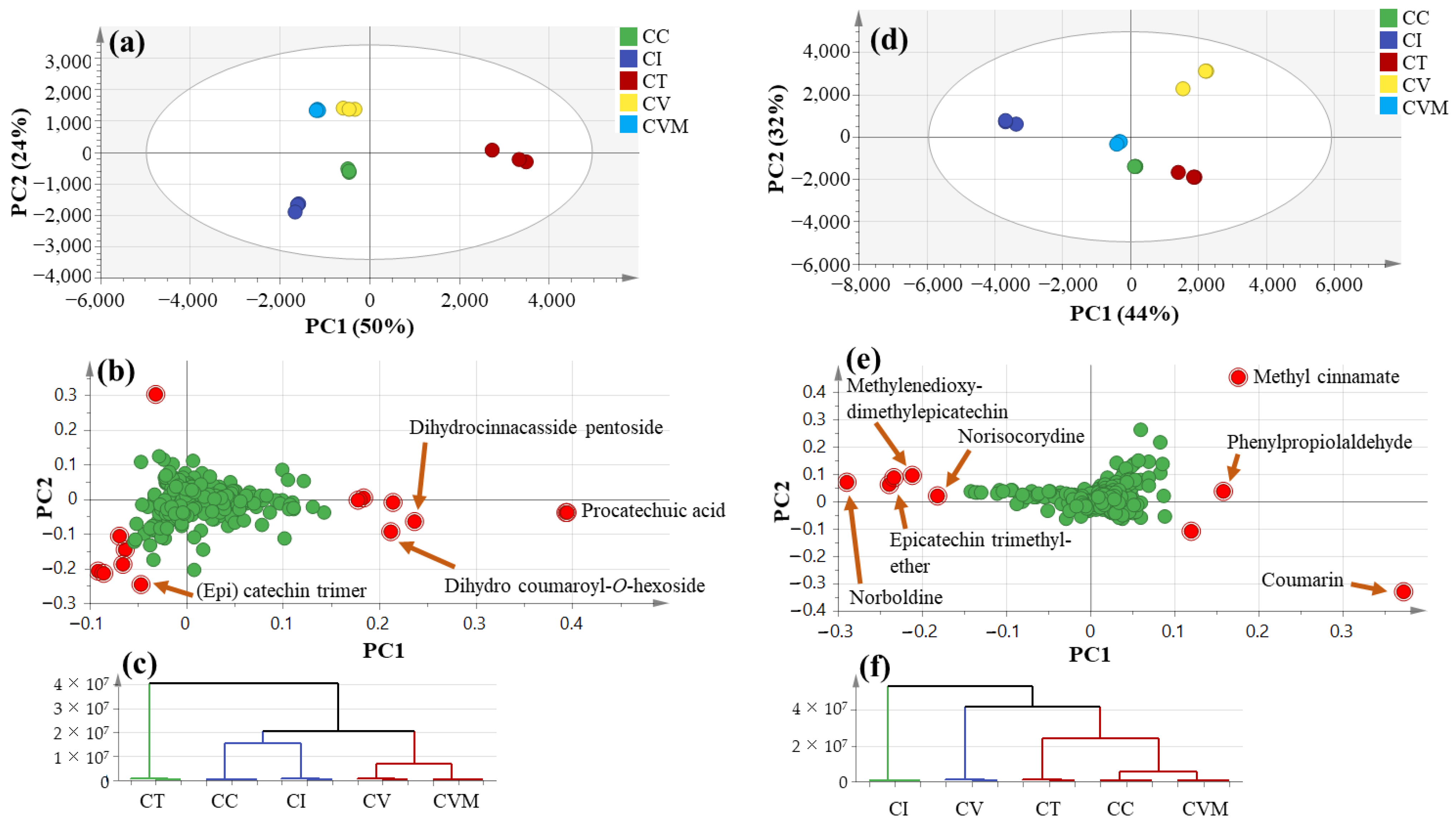
2.2. Multivariate Data Analysis of UPLC-ESI-MS Data
2.3. Primary Metabolites Profiling Using GC-MS
2.3.1. Sugars
2.3.2. Fatty Acids/Esters
2.3.3. Organic Acids
2.3.4. Phenolics
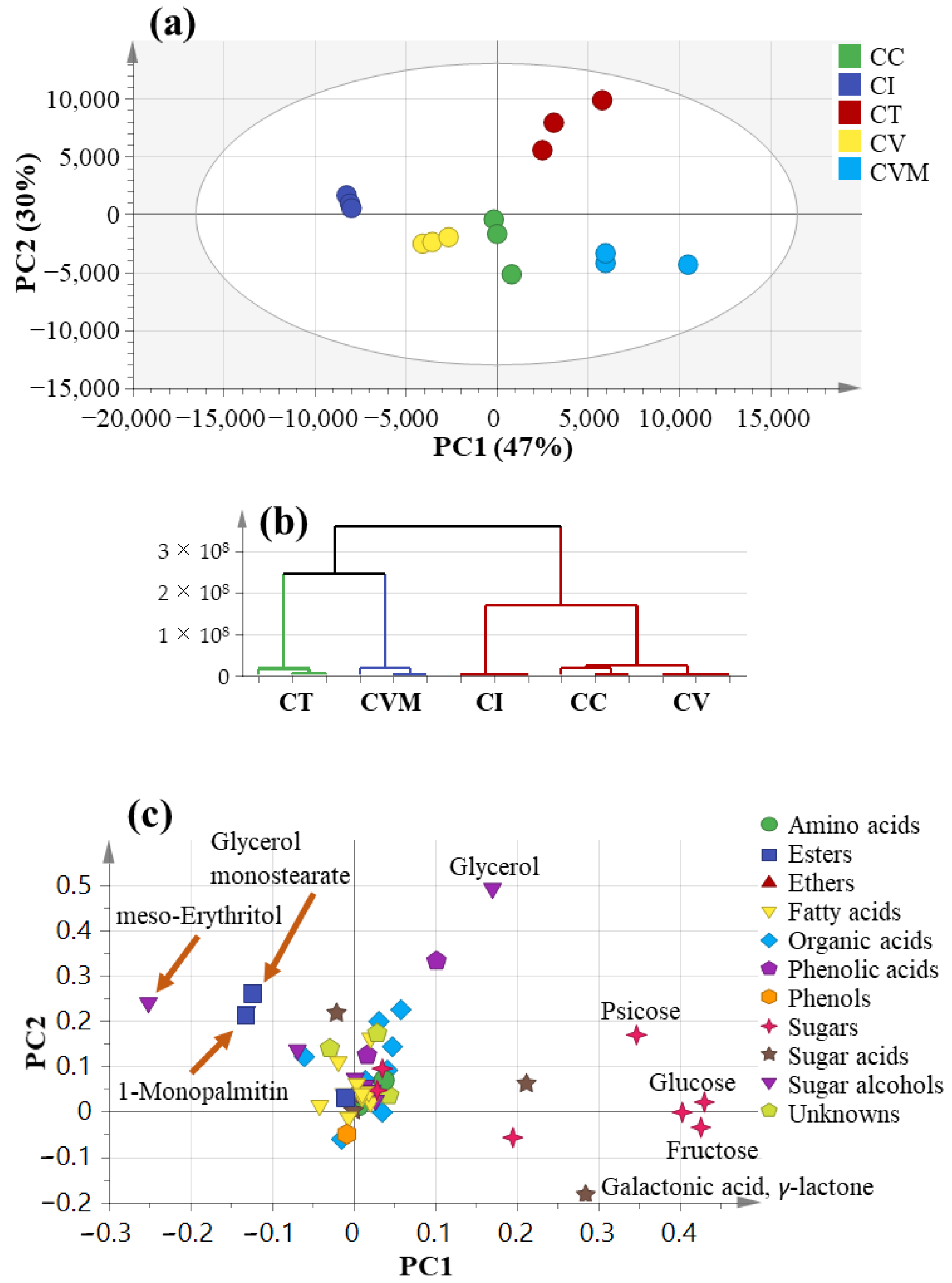
2.4. Multivariate Data Analysis Using GC-MS Data
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. UPLC-ESI-QTOF-MS Analysis and Metabolites Identification
3.4. GC-MS Analysis of Silylated Primary Metabolites and Identification
3.5. Multivariate Data (MVA) and Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Suriyagoda, L.; Mohotti, A.J.; Vidanarachchi, J.K.; Kodithuwakku, S.P.; Chathurika, M.; Bandaranayake, P.C.; Hetherington, A.M.; Beneragama, C.K. “Ceylon cinnamon”: Much more than just a spice. Plants People Planet 2021, 3, 319–336. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Rao, L.J.M. Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum. Crit. Rev. Food Sci. Nutr. 2011, 51, 547–562. [Google Scholar] [CrossRef]
- Hettiarachchi, I.; De Silva, D.; Esham, M.; Liyanagamage, T.; Abeysinghe, A.; Warnakulasooriya, S.; Harindra, W. An assessment of market landscape of cinnamon in Sri Lanka. J. Agric. Sci.–Sri Lanka 2020, 15, 198–206. [Google Scholar] [CrossRef]
- Gruenwald, J.; Freder, J.; Armbruester, N. Cinnamon and health. Crit. Rev. Food Sci. Nutr. 2010, 50, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Mokhtariniya, S. Essential oil composition of bark of Cinnamomum zeylanicum. J. Essent. Oil Bear. Plants 2016, 19, 786–789. [Google Scholar] [CrossRef]
- Chen, P.Y.; Yu, J.W.; Lu, F.L.; Lin, M.C.; Cheng, H.F. Differentiating parts of Cinnamomum cassia using LC-qTOF-MS in conjunction with principal component analysis. Biomed. Chromatogr. 2016, 30, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Avula, B.; Nanayakkara, N.D.; Zhao, J.; Khan, I.A. Cassia cinnamon as a source of coumarin in cinnamon-flavored food and food supplements in the United States. J. Agric. Food Chem. 2013, 61, 4470–4476. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, S.; Vasudeva, N. Chemical compositions of Cinnamomum tamala oil from two different regions of India. Asian Pac. J. Trop. Dis. 2012, 2, S761–S764. [Google Scholar] [CrossRef]
- Salleh, W.M.N.H.; Ahmad, F.; Yen, K.H.; Zulkifli, R.M. Essential oil compositions of Malaysian Lauraceae: A mini review. Pharm. Sci. 2016, 22, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Al-Sayed, H. Evaluation of antioxidant activity of some spices and their application in croissant and filling cream. Arab Univ. J. Agric. Sci. 2008, 16, 97–114. [Google Scholar] [CrossRef]
- Muhammad, D.R.A.; Dewettinck, K. Cinnamon and its derivatives as potential ingredient in functional food—A review. Int. J. Food Prop. 2017, 20 (Suppl. S2), 2237–2263. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Niu, X.; Ren, J. Analysis of cinnamon oil extracted by supercritical fluid. China Condiment 2011, 4, 76–86. [Google Scholar]
- Chuesiang, P.; Siripatrawan, U.; Sanguandeekul, R.; Yang, J.S.; McClements, D.J.; McLandsborough, L. Antimicrobial activity and chemical stability of cinnamon oil in oil-in-water nanoemulsions fabricated using the phase inversion temperature method. LWT 2019, 110, 190–196. [Google Scholar] [CrossRef]
- Hajimonfarednejad, M.; Ostovar, M.; Raee, M.J.; Hashempur, M.H.; Mayer, J.G.; Heydari, M. Cinnamon: A systematic review of adverse events. Clin. Nutr. 2019, 38, 594–602. [Google Scholar] [CrossRef]
- Bandara, T.; Uluwaduge, I.; Jansz, E. Bioactivity of cinnamon with special emphasis on diabetes mellitus: A review. Int. J. Food Sci. Nutr. 2012, 63, 380–386. [Google Scholar] [CrossRef]
- Camacho, S.; Michlig, S.; de Senarclens-Bezençon, C.; Meylan, J.; Meystre, J.; Pezzoli, M.; Markram, H.; Le Coutre, J. Anti-obesity and anti-hyperglycemic effects of cinnamaldehyde via altered ghrelin secretion and functional impact on food intake and gastric emptying. Sci. Rep. 2015, 5, 7919. [Google Scholar] [CrossRef] [Green Version]
- Batiha, G.E.-S.; Beshbishy, A.M.; Guswanto, A.; Nugraha, A.; Munkhjargal, T.; M Abdel-Daim, M.; Mosqueda, J.; Igarashi, I. Phytochemical characterization and chemotherapeutic potential of Cinnamomum verum extracts on the multiplication of protozoan parasites in vitro and in vivo. Molecules 2020, 25, 996. [Google Scholar] [CrossRef] [Green Version]
- Yap, P.; Krishnan, T.; Chan, K.-G.; Lim, S. Antibacterial mode of action of Cinnamomum verum bark essential oil, alone and in combination with piperacillin, against a multi-drug-resistant Escherichia coli strain. J. Microbiol. Biotechnol. 2015, 25, 1299–1306. [Google Scholar] [CrossRef]
- Ainane, T.; Khammour, F.; Merghoub, N. Cosmetic bio-product based on cinnamon essential oil “Cinnamomum verum” for the treatment of mycoses: Preparation, chemical analysis and antimicrobial activity. MOJ Toxicol. 2019, 5, 5–8. [Google Scholar] [CrossRef]
- Afifi, S.M.; El-Mahis, A.; Heiss, A.G.; Farag, M.A. Gas chromatography–mass spectrometry-based classification of 12 fennel (Foeniculum vulgare Miller) varieties based on their aroma profiles and estragole levels as analyzed using chemometric tools. ACS Omega 2021, 6, 5775–5785. [Google Scholar] [CrossRef]
- Huang, Z.; Jia, S.; Zhang, L.; Liu, X.; Luo, Y. Inhibitory effects and membrane damage caused to fish spoilage bacteria by cinnamon bark (Cinnamomum tamala) oil. LWT 2019, 112, 108195. [Google Scholar] [CrossRef]
- Farag, M.A.; Labib, R.M.; Noleto, C.; Porzel, A.; Wessjohann, L.A. NMR approach for the authentication of 10 cinnamon spice accessions analyzed via chemometric tools. LWT 2018, 90, 491–498. [Google Scholar] [CrossRef]
- Farag, M.A.; Afifi, S.M.; Rasheed, D.M.; Khattab, A.R. Revealing compositional attributes of Glossostemon bruguieri Desf. root geographic origin and roasting impact via chemometric modeling of SPME-GC-MS and NMR metabolite profiles. J. Food Compos. Anal. 2021, 102, 104073. [Google Scholar] [CrossRef]
- Bayram, B.; Esatbeyoglu, T.; Schulze, N.; Ozcelik, B.; Frank, J.; Rimbach, G. Comprehensive analysis of polyphenols in 55 extra virgin olive oils by HPLC-ECD and their correlation with antioxidant activities. Plant Foods Hum. Nutr. 2012, 67, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Gad, H.A.; Heiss, A.G.; Wessjohann, L.A. Metabolomics driven analysis of six Nigella species seeds via UPLC-qTOF-MS and GC–MS coupled to chemometrics. Food Chem. 2014, 151, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Abu-Reidah, I.M.; Arráez-Román, D.; Warad, I.; Fernández-Gutiérrez, A.; Segura-Carretero, A. UHPLC/MS2-based approach for the comprehensive metabolite profiling of bean (Vicia faba L.) by-products: A promising source of bioactive constituents. Food Res. Int. 2017, 93, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.; Serag, A.; Farag, M.A. Emerging analytical tools for the detection of the third gasotransmitter H2S, a comprehensive review. J. Adv. Res. 2020, 27, 137–153. [Google Scholar] [CrossRef]
- Fang, N.; Yu, S.; Prior, R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002, 50, 3579–3585. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Hong, Z.-L.; Yang, W.-L.; Wu, M.-H.; Huang, J.-C.; Lee, J.-Y. A novel homosesquiterpenoid from the stems of Cinnamomum burmanii. Nat. Prod. Res. 2012, 26, 1218–1223. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, W.; Huang, M.; Xu, W.; Li, H.; Ye, M.; Zhang, X.; Chu, K. Qualitative and quantitative analysis of phenolic acids, flavonoids and iridoid glycosides in Yinhua Kanggan Tablet by UPLC-QqQ-MS/MS. Molecules 2015, 20, 12209–12228. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, B.K.; Sethi, M.L.; Lloyd, H.; Kapadia, G.J. Aporphine and tetrahydrobenzylisoquinoline alkaloids in Sassafras albidum. Phytochemistry 1976, 15, 1803–1804. [Google Scholar] [CrossRef]
- Mateos-Martín, M.L.; Fuguet, E.; Quero, C.; Pérez-Jiménez, J.; Torres, J.L. New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 1327–1336. [Google Scholar] [CrossRef]
- Jiang, J.; Feng, Z.; Wang, Y.; Zhang, P. New phenolics from the roots of Symplocos caudata W all. Chem. Pharm. Bull. 2005, 53, 110–113. [Google Scholar] [CrossRef] [Green Version]
- Lin, I.; Yeh, H.; Cham, T.; Chen, C. A new butanolide from the leaves of Cinnamomum reticulatum. Chem. Nat. Compd. 2011, 47, 43. [Google Scholar] [CrossRef]
- Ravindran, P.; Nirmal-Babu, K.; Shylaja, M. Cinnamon and Cassia: The Genus Cinnamomum; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Ngoc, T.M.; Lee, I.; Ha, D.T.; Kim, H.; Min, B.; Bae, K. Tyrosinase-inhibitory constituents from the twigs of Cinnamomum cassia. J. Nat. Prod. 2009, 72, 1205–1208. [Google Scholar] [CrossRef]
- Tolonen, A.; György, Z.; Jalonen, J.; Neubauer, P.; Hohtola, A. LC/MS/MS identification of glycosides produced by biotransformation of cinnamyl alcohol in Rhodiola rosea compact callus aggregates. Biomed. Chromatogr. 2004, 18, 550–558. [Google Scholar] [CrossRef]
- Nasrullah, A.A. Phytochemicals and Bioactivities of Cryptocarya nigra (Lauraceae)/Ayu Afiqah Binti Nasrullah. Ph.D. Thesis, University of Malaya, Kuala Lumpur, Malaysia, 2014. [Google Scholar]
- Summons, R.E. The Alkaloids of Some Australian and New Guinea Plants; Wollongong University College: Wollongong, NSW, Australia, 1971. [Google Scholar]
- Luo, Q.; Wang, S.-M.; Lu, Q.; Luo, J.; Cheng, Y.-X. Identification of compounds from the water soluble extract of Cinnamomum cassia barks and their inhibitory effects against high-glucose-induced mesangial cells. Molecules 2013, 18, 10930–10943. [Google Scholar] [CrossRef] [Green Version]
- Tolonen, A.; Pakonen, M.; Hohtola, A.; Jalonen, J. Phenylpropanoid glycosides from Rhodiola rosea. J. Chem. Pharm. Bull. 2003, 51, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Hamed, A.I.; Al-Ayed, A.S.; Moldoch, J.; Piacente, S.; Oleszek, W.; Stochmal, A. Profiles analysis of proanthocyanidins in the argun nut (Medemia argun--an ancient Egyptian palm) by LC-ESI-MS/MS. J. Mass Spectrom. 2014, 49, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Marmet, C.; Actis-Goretta, L.; Renouf, M.; Giuffrida, F. Quantification of phenolic acids and their methylates, glucuronides, sulfates and lactones metabolites in human plasma by LC-MS/MS after oral ingestion of soluble coffee. J. Pharm. Biomed. Anal. 2014, 88, 617–625. [Google Scholar] [CrossRef]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Ali, S.E.; Hodaya, R.H.; El-Seedi, H.R.; Sultani, H.N.; Laub, A.; Eissa, T.F.; Abou-Zaid, F.O.; Wessjohann, L.A. Phytochemical profiles and antimicrobial activities of Allium cepa red cv. and A. sativum subjected to different drying methods: A comparative MS-based metabolomics. Molecules 2017, 22, 761. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Zhu, C.; Li, Y.; Tian, Y.; Lin, S.; Yuan, S.; Hu, J.; Hou, Q.; Chen, N.; Yang, Y. Lignans and neolignans from Sinocalamus affinis and their absolute configurations. J. Nat. Prod. 2011, 74, 1188–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, Z.; Zhang, Z.; Wang, Y.; Liu, X.; Wang, L.; Lin, R. Analysis of chemical constituents of Melastoma dodecandrum lour. by UPLC-ESI-Q-exactive focus-MS/MS. Molecules 2017, 22, 476. [Google Scholar] [CrossRef] [Green Version]
- Tine, Y.; Renucci, F.; Costa, J.; Wélé, A.; Paolini, J. A method for LC-MS/MS profiling of coumarins in Zanthoxylum zanthoxyloides (Lam.) B. Zepernich and Timler extracts and essential oils. Molecules 2017, 22, 174. [Google Scholar] [CrossRef] [Green Version]
- Shahwar, D.; Raza, M.A.; Shafiq-Ur-Rehman; Abbasi, M.A.; Atta-Ur-Rahman. An investigation of phenolic compounds from plant sources as trypsin inhibitors. J. Nat. Prod. Res. 2012, 26, 1087–1093. [Google Scholar] [CrossRef]
- Avula, B.; Smillie, T.J.; Wang, Y.-H.; Zweigenbaum, J.; Khan, I.A. Authentication of true cinnamon (Cinnamon verum) utilising direct analysis in real time (DART)-QToF-MS. Food Addit. Contam. Part A 2015, 32, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Fu, J.; Yao, X.-J.; Yang, J.; Liu, L.; Xie, T.-G.; Jiang, P.-C.; Jiang, Z.-H.; Zhu, G.-Y. Phenolic constituents isolated from the twigs of Cinnamomum cassia and their potential neuroprotective effects. J. Nat. Prod. 2018, 81, 1333–1342. [Google Scholar] [CrossRef]
- Wang, J.; Su, B.; Jiang, H.; Cui, N.; Yu, Z.; Yang, Y.; Sun, Y. Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): A review. Fitoterapia 2020, 146, 104675. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Fan, M.-X.; Wu, X.; Wang, H.-J.; Yang, J.; Si, N.; Bian, B.-L. Chemical profiling of the Chinese herb formula Xiao-Cheng-Qi decoction using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. Sci. 2013, 51, 273–285. [Google Scholar] [CrossRef] [Green Version]
- Agalar, H.G.; Çiftçi, G.A.; Göğer, F.; Kırımer, N. Activity guided fractionation of Arum italicum Miller Tubers and the LC/MS-MS profiles. Rec. Nat. Prod. 2017, 12, 64–75. [Google Scholar] [CrossRef]
- Tala, V.R.S.; Candida da Silva, V.; Rodrigues, C.M.; Nkengfack, A.E.; Campaner dos Santos, L.; Vilegas, W. Characterization of proanthocyanidins from Parkia biglobosa (Jacq.) G. Don.(Fabaceae) by flow injection analysis—Electrospray ionization ion trap tandem mass spectrometry and liquid chromatography/electrospray ionization mass spectrometry. Molecules 2013, 18, 2803–2820. [Google Scholar] [CrossRef] [Green Version]
- Serag, A.; Baky, M.H.; Döll, S.; Farag, M.A. UHPLC-MS metabolome based classification of umbelliferous fruit taxa: A prospect for phyto-equivalency of its different accessions and in response to roasting. RSC Adv. 2020, 10, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.-S.; Hou, W.-C.; Huang, L.-W.; Lee, T.-H. A new γ-lactone from the leaves of Cinnamomum kotoense. J. Nat. Prod. Res. 2006, 20, 1246–1250. [Google Scholar] [CrossRef]
- Kerwin, J.L.; Wiens, A.M.; Ericsson, L.H. Identification of fatty acids by electrospray mass spectrometry and tandem mass spectrometry. J. Mass Spectrom. 1996, 31, 184–192. [Google Scholar] [CrossRef]
- Zeng, J.; Xue, Y.; Shu, P.; Qian, H.; Sa, R.; Xiang, M.; Li, X.-N.; Luo, Z.; Yao, G.; Zhang, Y. Diterpenoids with immunosuppressive activities from Cinnamomum cassia. J. Nat. Prod. 2014, 77, 1948–1954. [Google Scholar] [CrossRef]
- Hema, R.; Kumaravel, S.; Martina, S. Chromatograph interfaced to a mass spectrometer analysis of Cinnamomum verum. J. Nat. Sci. 2010, 8, 152–155. [Google Scholar]
- Farag, M.A.; Abdelfattah, M.S.; Badr, S.E.A.; Wessjohann, L.A. Profiling the chemical content of Ficus lyrata extracts via UPLC-PDA-qTOF-MS and chemometrics. Nat. Prod. Res. 2014, 28, 1549–1556. [Google Scholar] [CrossRef]
- Touriño, S.; Lizárraga, D.; Carreras, A.; Lorenzo, S.; Ugartondo, V.; Mitjans, M.; Vinardell, M.P.; Juliá, L.; Cascante, M.; Torres, J.L. Highly galloylated tannin fractions from witch hazel (Hamamelis virginiana) bark: Electron transfer capacity, in vitro antioxidant activity, and effects on skin-related cells. Chem. Res. Toxicol. 2008, 21, 696–704. [Google Scholar] [CrossRef]
- Carvalho, A.A.; Andrade, L.N.; de Sousa, É.B.V.; de Sousa, D.P. Antitumor phenylpropanoids found in essential oils. BioMed Res. Int. 2015, 2015, 392674. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63. [Google Scholar] [CrossRef]
- Chae, H.K.; Kim, W.; Kim, S.K. Phytochemicals of cinnamomi cortex: Cinnamic acid, but not cinnamaldehyde, attenuates oxaliplatin-induced cold and mechanical hypersensitivity in rats. Nutrients 2019, 11, 432. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Shi, Y.-Q.; Pan, X.-H.; Lu, Y.-H.; Cao, P. Antibacterial effects of cinnamon (Cinnamomum zeylanicum) bark essential oil on Porphyromonas gingivalis. Microb. Pathog. 2018, 116, 26–32. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.Y. Cinnamon subcritical water extract attenuates intestinal inflammation and enhances intestinal tight junction in a Caco-2 and RAW264. 7 co-culture model. Food Funct. 2019, 10, 4350–4360. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Tsai, K.-D.; Yang, S.-M.; Wong, H.-Y.; Chen, T.-W.; Cherng, J.; Cherng, J.-M. Cinnamomum verum ingredient 2-methoxycinnamaldehyde: A new antiproliferative drug targeting topoisomerase I and II in human lung squamous cell carcinoma NCI-H520 cells. Eur. J. Cancer Prev. 2017, 26, 314–323. [Google Scholar] [CrossRef]
- Yan, Y.-M.; Fang, P.; Yang, M.-T.; Li, N.; Lu, Q.; Cheng, Y.-X. Anti-diabetic nephropathy compounds from Cinnamomum cassia. J. Ethnopharmacol. 2015, 165, 141–147. [Google Scholar] [CrossRef]
- Custodio, D.L.; da Veiga Junior, V.F. Lauraceae alkaloids. RSC Adv. 2014, 4, 21864–21890. [Google Scholar] [CrossRef]
- Ozkan, G.; Kostka, T.; Esatbeyoglu, T.; Capanoglu, E. Effects of lipid-based encapsulation on the bioaccessibility and bioavailability of phenolic compounds. Molecules 2020, 25, 5545. [Google Scholar] [CrossRef]
- Farag, M.A.; Sharaf Eldin, M.G.; Kassem, H.; Abou el Fetouh, M. Metabolome classification of Brassica napus L. organs via UPLC–QTOF–PDA–MS and their anti-oxidant potential. Phytochem. Anal. 2013, 24, 277–287. [Google Scholar] [CrossRef]
- Chen, J.; Huang, W.; Zhang, T.; Lu, M.; Jiang, B. Anti-obesity potential of rare sugar D-psicose by regulating lipid metabolism in rats. Food Funct. 2019, 10, 2417–2425. [Google Scholar] [CrossRef]
- Grembecka, M. Sugar alcohols—Their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 104675. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, L.; Rywińska, A.; Gładkowski, W. Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehtiö, T.; Toivari, M.; Wiebe, M.G.; Harlin, A.; Penttilä, M.; Koivula, A. Production and applications of carbohydrate-derived sugar acids as generic biobased chemicals. Crit. Rev. Biotechnol. 2016, 36, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Yang, J. The influence of emulsifiers on the rheological properties of wheat flour dough and quality of fried instant noodles. LWT-Food Sci. Technol. 2013, 53, 61–69. [Google Scholar] [CrossRef]
- Lee, L.Y.; Chin, N.L.; Lim, C.H.; Yusof, Y.A.; Talib, R.A. Saturated distilled monoglycerides variants in gel-form cake emulsifiers. Agric. Agric. Sci. Procedia 2014, 2, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Ozdener, M.H.; Subramaniam, S.; Sundaresan, S.; Sery, O.; Hashimoto, T.; Asakawa, Y.; Besnard, P.; Abumrad, N.A.; Khan, N.A. CD36-and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology 2014, 146, 995–1005.e5. [Google Scholar] [CrossRef] [Green Version]
- Briggs, M.A.; Petersen, K.S.; Kris-Etherton, P.M. Saturated fatty acids and cardiovascular disease: Replacements for saturated fat to reduce cardiovascular risk. In Healthcare; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2017; p. 29. [Google Scholar]
- Zeng, K.; Thompson, K.E.; Yates, C.R.; Miller, D.D. Synthesis and biological evaluation of quinic acid derivatives as anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2009, 19, 5458–5460. [Google Scholar] [CrossRef]
- Mnafgui, K.; Derbali, A.; Sayadi, S.; Gharsallah, N.; Elfeki, A.; Allouche, N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet-induced obese rats. J. Food Sci. Technol. 2015, 52, 4369–4377. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical composition, antioxidant and antimicrobial activity of phenolic compounds extracted from wine industry by-products. Food Control 2018, 92, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Polášková, P.; Herszage, J.; Ebeler, S.E. Wine flavor: Chemistry in a glass. Chem. Soc. Rev. 2008, 37, 2478–2489. [Google Scholar] [CrossRef]
- Mrabti, H.N.; Jaradat, N.; Fichtali, I.; Ouedrhiri, W.; Jodeh, S.; Ayesh, S.; Cherrah, Y.; Faouzi, M.E.A. Separation, identification, and antidiabetic activity of catechin isolated from Arbutus unedo L. plant roots. Plants 2018, 7, 31. [Google Scholar] [CrossRef] [Green Version]
- Farag, M.A.; Porzel, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC–MS, LC–MS and 1D NMR techniques. Phytochemistry 2012, 76, 60–72. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Afifi, S.M.; Aly, M.S.; Ahmed, R.F.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Farag, M.A.; Elgamal, A.M.; Elshamy, A.I. Chemical profile of Launaea nudicaulis ethanolic extract and its antidiabetic effect in streptozotocin-induced rats. Molecules 2021, 26, 1000. [Google Scholar] [CrossRef]
- Buckingham, J. Dictionary of Natural Products on DVD; Chapman & Hall: New York, NY, USA, 2011; Volume 43. [Google Scholar]
- Farag, M.A.; Fathi, D.; Shamma, S.; Shawkat, M.S.A.; Shalabi, S.M.; El Seedi, H.R.; Afifi, S.M. Comparative metabolome classification of desert truffles Terfezia claveryi and Terfezia boudieri via its aroma and nutrients profile. LWT 2021, 142, 111046. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Reddy, I.R.; Duran, A.L.; Zhao, X.; Sumner, L.W. MET-IDEA: Data extraction tool for mass spectrometry-based metabolomics. Anal. Chem. 2006, 78, 4334–4341. [Google Scholar] [CrossRef]
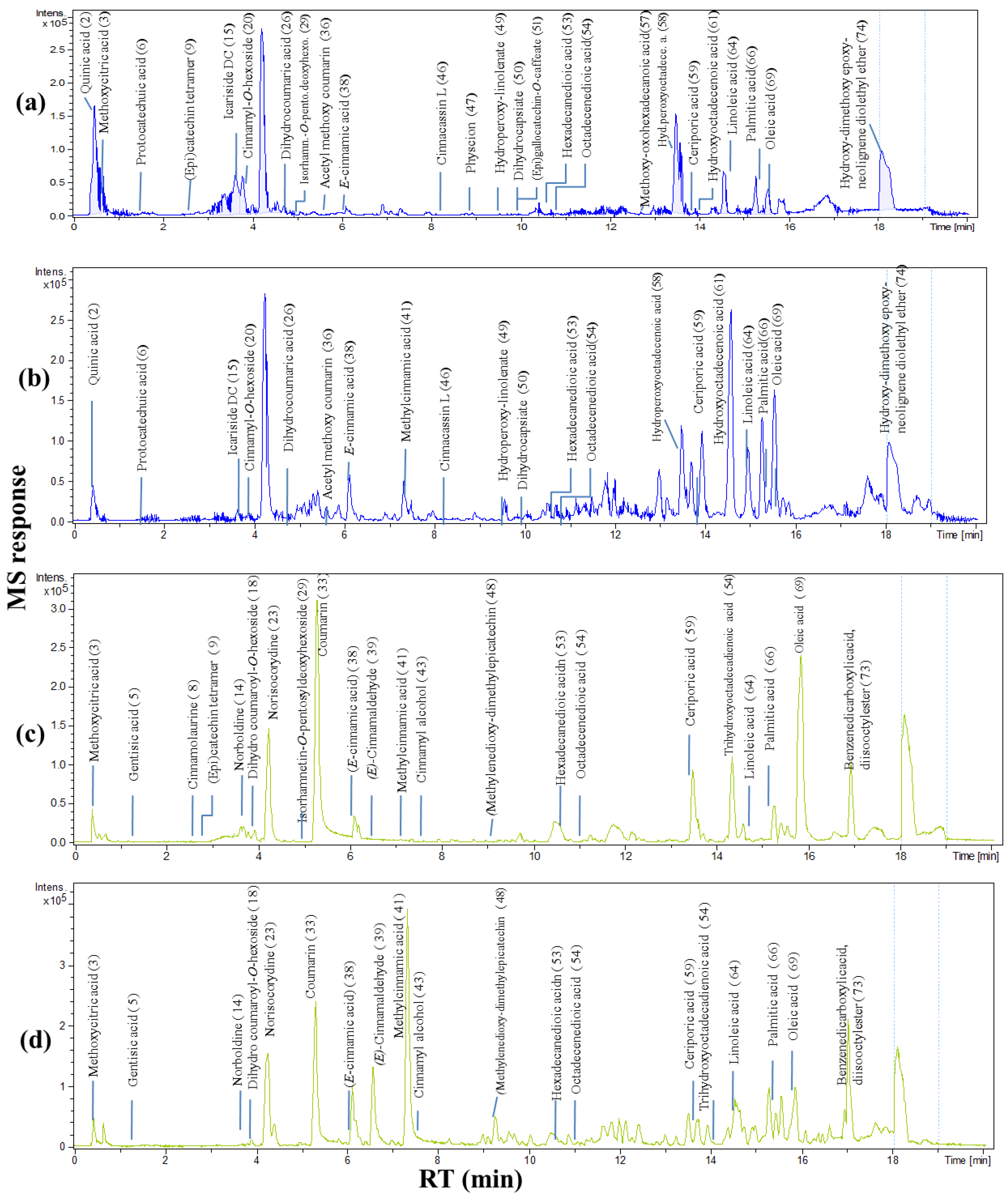
| No. | Rt | Compound Name | Chemical Class | UV | [M − H]−/ [M + H]+ | Molecular Formula | Error | MS/MS Fragments | Reference | CC | CI | CT | CV | CVM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 0.41 | Hexose | Sugar | 244 | 179.0561 | C6H11O6− | 3.1 | 161.0422 135.0323 | + | + | + | + | + | |
| L2 | 0.49 | Quinic acid | Phenolic acid | 222 264 | 191.0551 | C7H11O6− | 5.5 | 173.0418 129.0186 | [28] | + | + | + | + | + |
| L3 | 0.72 | Methoxycitric acid | Organic acid | 221.0302 223.0145 | C7H9O8− C7H11O8+ | 0.4 0.6 | 189.0034 145.0131 127.0038 | + | − | − | − | − | ||
| L4 | 1.55 | Protocatechuic acid hexoside | Phenolic acid | 223 280 | 315.0710 | C13H15O9− | 3.7 | 153.0185 109.0292 | [29] | − | − | + | − | − |
| L5 | 1.62 | Gentisic acid | Phenolic acid | 280 | 153.0188 155.0206 | C7H5O4− C7H7O4+ | 1.4 | 109.0291 | + | + | + | + | − | |
| L6 | 1.82 | Protocatechuic acid | Phenolic acid | 280 | 153.0189 | C7H5O4− | 0.4 | - | [30] | + | + | + | + | + |
| L7 | 2.41 | Protocatechualdehyde | Phenolic aldehyde | 137.0233 | C7H5O3− | 0.9 | - | [29] | + | + | + | + | + | |
| L8 | 2.62 | Cinnamolaurine | Alkaloid | 270 310 | 298.1433 | C18H19NO3+ | 1.7 | - | [31] | − | + | − | − | − |
| L9 | 2.88 | (Epi)catechin tetramer (EC-EC-EC-A-EC) | Proanthocyanidin | 234 275 | 1151.2454 1153.2626 | C60H47O24− C60H49O24+ | 0.8 | 863.1824 575.1204 | [32] | + | + | − | − | − |
| L10 | 3.20 | (Epi)catechin tetramer (EC-EC-A-EC-EC) | Proanthocyanidin | 234 275 | 1151.25 | C60H47O24− | −3.2 | 863.1870 573.1048 | + | − | − | − | − | |
| L11 | 3.27 | (Epi)catechin trimer A type (EC-A-EC-EC) | Proanthocyanidin | 234 280 | 863.1885 | C45H35O18− | −6.5 | 711.1297 573.1144 289.0711 | + | + | + | − | − | |
| L12 | 3.40 | (Epi)catechin trimer A type (EC-A-EC-EC) | Proanthocyanidin | 280 | 863.1853 865.1958 | C45H35O18− C45H37O18+ | 0.6 | 577.1333 427.1819 | + | + | + | − | − | |
| L13 | 3.43 | Dimethoxyphenol-O-pentosyl hexoside | Phenol | 214 | 447.1501 | C19H27O12− | 1.6 | 269.1029 161.0448 | [33] | + | + | + | + | + |
| L14 | 3.55 | Norboldine | Alkaloids | 220 280 310 | 312.1241 314.1382 | C18H18NO4− C18H20NO4+ | 2.7 | 297.0998 | [34] [35] | + | + | + | + | + |
| L15 | 3.62 | Phenylethyl-O-pentosyl hexoside (Icariside DC) | Hydroxycinnamates | 214 | 415.1237 | C19H27O10− | 0.4 | 269.1034 | [36] | + | − | + | + | + |
| L16 | 3.64 | Dihydrocinnacasside pentoside | Hydroxycinnamates | 214 | 459.1481 | C20H27O12− | 6 | 165.0552 | − | + | + | + | + | |
| L17 | 3.66 | Dihydro coumaroyl-O-pentosylhexoside | Hydroxycinnamates | 214 | 459.1506 | C20H27O12− | 0.5 | 415.1240 327.1078 165.0545 | + | − | − | + | + | |
| L18 | 3.67 | Dihydro coumaroyl-O-hexoside (Dihydomelilotoside) | Hydroxycinnamates | 214 | 327.1085 329.1034 | C15H19O8− C15H21O8+ | 0.7 | 281.1395 165.0544 | [36] | + | − | + | + | − |
| L19 | 3.78 | Dihydrocinnacasside (Hydroxyphenylpropanoy-O-hexoside) | Hydroxycinnamates | 214 | 327.1063 | C15H19O8− | 6.8 | 165.055 121.0675 | + | − | + | + | + | |
| L20 | 3.84 | Cinnamyl-O-hexoside | Hydroxycinnamates | 280 | 295.1155 | C15H19O6− | 10.7 | 251.1266 | [37] | − | − | + | + | − |
| L21 | 3.84 | Corydine | Alkaloids | 270 310 | 342.1678 | C20H24NO4+ | 6.3 | 297.1106 265.0842 | [38] [39] | + | + | + | + | + |
| L22 | 3.97 | Reticuline | Alkaloids | 280 | 330.1680 | C19H24NO4+ | 5.9 | 192.1014 | + | + | + | + | + | |
| L23 | 4.16 | Norisocorydine/ Boldine | Alkaloids | 270 310 | 328.1528 | C19H22NO4+ | 0.9 | − | + | + | + | + | + | |
| L24 | 4.50 | Cinnamyl-O-pentosylhexoside | Hydroxycinnamates | 251 280 | 427.1673 | C20H27O10− | −6.3 | 293.0854 233.0659 149.0447 | [40] [41] | + | − | − | − | − |
| L25 | 4.59 | (Epi) catechin trimer with double A linkage | Proanthocyanidins | 280 | 861.699 863.1811 | C45H33O18− C45H35O18+ | −2.7 | 595.1699 575.118 473.1653 | [42] | + | − | + | − | − |
| L26 | 4.60 | Dihydrocoumaric acid | Hydroxycinnamates | 310 | 165.0555 | C9H9O3− | 12.9 | 121.0657 | [43] | + | + | + | + | − |
| L27 | 4.63 | Dihydrocinnamyl-O-pentosyl hexoside | Hydroxycinnamates | 251 280 | 429.1726 | C20H31O12− | 1 | 297.1323 149.0440 | [41] | − | + | − | − | − |
| L28 | 4.68 | Naringenin di-O-hexoside | Flavonoids | 595.1699 597.1254 | C27H31O15− C27H33O15+ | −20.4 | 433.1139 271.0611 | [44] | − | + | + | − | − | |
| L29 | 4.85 | Isorhamnetin-O-pentosyldeoxyhexoside | Flavonoids | 256 354 | 593.1848 | C28H33O14− | 4.7 | 447.1285 315.0701 | + | − | + | − | − | |
| L30 | 4.93 | Luteolin-O-hexoside-C-hexoside | Flavonoids | 260 348 | 609.1998 | C27H29O16− | −5.2 | 447.0924 327.1076 | + | − | + | − | − | |
| L31 | 5.12 | Dipropylmalonic acid | Organic acid | 187.0973 189.0721 | C9H15O4− C9H17O4+ | 1.3 | 169.0866 143.1078 | + | + | + | + | + | ||
| L32 | 5.15 | Trimethoxy phenol | Phenol | 183.0655 185.0712 | C9H11O4− C9H13O4+ | 4.2 | 155.0708 139.0758 | + | + | + | + | + | ||
| L33 | 5.32 | Coumarin | Hydroxycinnamates | 273 312 | 147.0446 | C9H6O2+ | −3.8 | [45] | + | + | + | + | + | |
| L34 | 5.46 | Dihydroxy-tetramethoxy-epoxylignanolone | Lignans | 233 303 | 433.1479 | C22H25O9− | 5.6 | 418.1258 373.1270 285.0428 | [46] | + | + | + | + | + |
| L35 | 5.61 | Oxododecanedioic acid | Fatty acids | 243.1228 | C12H19O5− | 4 | 225.1129 181.1215 | [47] | − | + | + | + | + | |
| L36 | 5.78 | Acetyl methoxy coumarin | Hydroxycinnamates | 217.0501 | C12H9O4− | 2.5 | 185.0814 173.0600 | [48] | + | + | + | + | + | |
| L37 | 6.09 | Unknown | Catechins | 234 280 | 995.2414 | C51H47O21− | −29.1 | 705.1589 543.1298 289.0178 | − | − | + | − | − | |
| L38 | 6.13 | Cinnamic acid (E-cinnamic acid) | Hydroxycinnamates | 270 | 147.0435 149.0723 | C9H7O2− C9H9O2+ | 4.5 | 119.0503 | [49] | + | + | + | + | + |
| L39 | 6.50 | (E)-Cinnamaldehyde | Hydroxycinnamates | 133.0652 | C9H9O+ | −3.3 | - | [22] | + | + | + | + | + | |
| L40 | 7.11 | Unknown | nitrogenous compound | 242.1751 244.1574 | C13H24NO3− C13H26NO3+ | 4.5 | 225.1502 | + | + | + | + | + | ||
| L41 | 7.15 | Methylcinnamic acid | Hydroxycinnamates | 163.074 | C10H11O2+ | - | [50] | + | + | − | + | + | ||
| L42 | 7.33 | Methoxy cinnamaldehyde | Hydroxycinnamates | 163.076 | C10H11O2+ | 0.3 | - | + | + | + | + | + | ||
| L43 | 7.30 | Cinnamyl alcohol | Hydroxycinnamates | 135.081 | C9H11O+ | −3.8 | - | [50] | + | + | + | + | + | |
| L44 | 7.51 | Hydroxyl, dimethoxyphenyl, hydroxy methoxyphenyl propanediol | Phenol | 349.12 | C18H22O7− | 4.7 | 331.1177 293.1388 225.0767 | [51] | − | + | − | + | + | |
| L45 | 7.52 | Epicatechin trimethyl ether | Catechins | 333.1323 | C18H21O6+ | 2.8 | - | [52] | + | + | + | + | + | |
| L46 | 8.02 | Cinnacassin L | Lignans | 215 245 | 281.1168 | C17H18O3− | 5.6 | 207.1183 147.0448 | [51] | − | + | − | + | − |
| L47 | 8.80 | Physcion | Anthraquinon | 283.0606 | C16H11O5− | 2 | 269.0381 | [53] | − | + | − | − | + | |
| L48 | 9.00 | Methylenedioxy-dimethylepicatechin | Catechins | 234 280 | 331.117 | C18H19O6+ | 1.8 | - | + | + | + | + | + | |
| L49 | 9.43 | Hydroperoxy-linolenate | Fatty acid | 309.2047 311.2137 | C18H29O4− C18H31O4+ | 7.9 | 291.1967 265.2163 | − | + | + | + | − | ||
| L50 | 9.83 | Dihydrocapsiate (vanillyl-8-methylnonanate) | Methoxyphenols | 307.1919 | C18H27O4− | −1.5 | 265.1800 223.1331 209.1180 | [54] | + | + | + | + | − | |
| L51 | 9.92 | (Epi)gallocatechin-O-caffeate | Proanthocyanidins | 234 280 | 467.0982 | C24H19O10− | 0.3 | 313.2369 161.0243 | − | − | + | − | − | |
| L52 | 9.99 | (Epi)gallocatechin-(epi)catechin | Proanthocyanidins | 278 | 593.2696 | C30H41O12− | −15.6 | 467.0971 313.2373 305.1775 | [55] | − | − | + | − | − |
| L53 | 10.52 | Hexadecanedioic acid | Fatty acid | 285.2060 287.2163 | C16H30O4− C16H32O4+ | 4.4 | 267.1947 223.2042 | [56] | − | + | − | + | + | |
| L54 | 11.12 | Octadecenedioic acid | Fatty acid | 311.2205 313.2147 | C18H31O4− C18H33O4+ | 5.1 | 293 249.2234 | + | + | + | + | + | ||
| L55 | 11.36 | Hydroxylinoleic acid | Fatty acid | 221 | 295.2267 | C18H31O3− | 4.1 | 277.2161 195.1379 | − | − | + | + | + | |
| L56 | 12.30 | Emodin | Anthraquinone | 269.2091 | C15H10O5− | 225.2199 | [53] | + | + | + | + | + | ||
| L57 | 12.85 | Hexadecanedioic acid, monomethyl ester (Methoxy-oxohexadecanoic acid) | Fatty acid | 221 | 299.2060 | C17H31O4− | 1.4 | 255.2320 | + | + | + | − | + | |
| L58 | 12.96 | Hydroperoxyoctadecenoic acid | Fatty acid | 313.2373 | C18H33O4− | 4.2 | 269.2297 | [54] | + | + | + | + | + | |
| L59 | 13.97 | Ceriporic acid | Dicarboxylic acid | 351.2534 353.2431 | C21H35O4− C21H37O4+ | 1.9 | - | + | + | − | + | + | ||
| L60 | 14.02 | Trihydroxyoctadecadienoic acid | Fatty acid | 221 | 327.2177 329.1743 | C18H31O5− C18H33O5+ | 3.2 | 283.2266 | [54] | − | + | − | + | + |
| L61 | 14.25 | Hydroxyoctadecenoic acid | Fatty acid | 297.2405 | C18H33O3− | 10.1 | 253.2167 235.2058 | + | + | + | + | + | ||
| L62 | 14.28 | Cinnakotolactone | Lactone | 309.2426 311.2541 | C19H33O3− C19H35O3+ | 3.7 | - | [57] | + | − | + | + | + | |
| L63 | 14.39 | Cinnamyl cinnamate-O-pentoside | Hydroxycinnamates | 395.2781 | C23H39O5− | 2.2 | 263.2389 | − | + | − | − | − | ||
| L64 | 14.54 | Linoleic acid | Fatty acid | 221 | 279.232 281.2231 | C18H31O2− C18H33O2+ | 2 | 235.2051 | [58] | + | + | + | + | + |
| L65 | 14.99 | Isolinderanolide | Butanolides | 307.2272 309.2103 | C19H31O3− C19H33O3+ | 5.1 | - | [57] | + | + | + | + | + | |
| L66 | 15.25 | Palmitic acid | Fatty acid | 221 | 255.2328 257.2167 | C16H31O2− C16H33O2+ | 0.7 | - | [58] | + | + | + | + | + |
| L67 | 15.40 | Cinncassiol B | Diterpene | 399.1961 | C20H31O8− | 15.4 | - | [59] | + | + | + | + | + | |
| L68 | 15.53 | Cinncassiol A | Diterpene | 381.1724 | C20H29O7− | 51 | 337.1820 | + | + | + | + | + | ||
| L69 | 15.54 | Oleic acid | Fatty acid | 224 | 281.2483 283.2641 | C18H33O2− C18H35O2+ | 0.9 | - | [58] | + | + | + | + | + |
| L70 | 15.75 | Benzenedicarboxylic acid, bis(2-methylpropyl) ester | Fatty ester | 279.1587 | C16H23O4+ | 0.4 | - | [60] | + | + | + | + | + | |
| L71 | 15.81 | Methyl palmitate | Fatty acid | 221 | 271.2626 | C17H35O2+ | 0.6 | - | + | + | + | + | + | |
| L72 | 15.84 | Olealdehyde | Fatty aldehydes | 267.2677 | C18H35O+ | 2.1 | - | + | + | + | + | + | ||
| L73 | 17.03 | Benzenedicarboxylicacid, diisooctylester | Fatty ester | 391.2795 | C24H39O4+ | 0.4 | - | [60] | + | + | + | + | + | |
| L74 | 18.11 | Hydroxy-dimethoxy epoxy-neolignene diolethyl ether | Lignans | 205 303 | 385.1657 | C22H26O6− | 18.6 | - | [46] | − | + | + | + | − |
| No. | Rt (min) | RI | Identification | CC (a) | CI (b) | CT (c) | CV (d) | CVM (e) |
|---|---|---|---|---|---|---|---|---|
| Amino acids | ||||||||
| G1 | 12.218 | 1403 | L-Aspartic acid, 2TMS | 2.27 ± 0.36 | 2.03 ± 0.36 | 1.36 ± 0.15 | 2.27 ± 0.21 | 1.48 ± 0.08 |
| G2 | 12.652 | 1436 | β-Alanine, 3TMS * | 0.20 ± 0.04 | 0.18 ± 0.02 | 0.11 ± 0.00 | 0.23 ± 0.02 | 0.13 ± 0.01 |
| Total Amino acids | 2.47 | 2.21 | 1.47 | 2.49 | 1.60 | |||
| Esters | ||||||||
| G3 | 24.632 | 2602 | 1-Monopalmitin, 2TMS | 6.06 ± 3.88 | 10.43 ± 0.38 | 5.03 ± 1.06 | 9.03 ± 2.13 | 3.72 ± 1.79 |
| G4 | 26.106 | 2789 | Glycerol monostearate, 2TMS | 15.13 ± 5.57 | 21.73 ± 0.52 e | 12.01 ± 1.21 d | 23.96 ± 2.41 c | 11.46 ± 2.50 b |
| G5 | 26.322 | 2811 | Sebacic acid di(2-ethylhexyl) ester | 0.59 ± 0.01 | 0.61 ± 0.02 | 0.33 ± 0.01 | 0.63 ± 0.03 | 0.36 ± 0.06 |
| Total esters | 21.79 | 32.77 | 17.37 | 33.61 | 15.54 | |||
| Ethers | ||||||||
| G6 | 9.918 | 1253 | Diethylene glycol, 2TMS | 0.25 ± 0.02 | 0.25 ± 0.01 | 0.15 ± 0.01 | 0.27 ± 0.01 | 0.15 ± 0.02 |
| Total ethers | 0.25 | 0.25 | 0.15 | 0.27 | 0.15 | |||
| Fatty acids | ||||||||
| G7 | 17.733 | 1850 | Myristic acid, TMS * | 0.33 ± 0.01 | 0.59 ± 0.03 | 0.28 ± 0.01 | 0.47 ± 0.03 | 0.31 ± 0.03 |
| G8 | 18.784 | 1949 | Pentadecanoic acid, TMS | 0.09 ± 0.01 | 0.33 ± 0.02 | 0.07 ± 0.00 | 0.20 ± 0.03 | 0.07 ± 0.02 |
| G9 | 19.779 | 2047 | Palmitic Acid, TMS * | 2.33 ± 0.15 | 2.65 ± 0.06 | 2.48 ± 0.16 | 4.10 ± 0.36 | 1.77 ± 0.41 |
| G10 | 21.406 | 2216 | Linoleic acid, TMS * | 0.03 ± 0.00 | 0.09 ± 0.01 | 0.04 ± 0.01 | 0.22 ± 0.09 | 0.09 ± 0.05 |
| G11 | 21.44 | 2220 | Oleic Acid, TMS * | 0.69 ± 0.03 | 0.84 ± 0.08 | 0.92 ± 0.11 | 2.09 ± 0.74 | 0.67 ± 0.28 |
| G12 | 21.495 | 2226 | Elaidic acid TMS | 0.29 ± 0.01 | 0.24 ± 0.03 | 0.19 ± 0.04 | 0.39 ± 0.11 | 0.22 ± 0.04 |
| G13 | 21.655 | 2244 | Stearic acid, TMS | 3.35 ± 0.10 | 3.60 ± 0.04 | 2.11 ± 0.14 | 3.94 ± 0.20 | 2.08 ± 0.35 |
| G14 | 23.386 | 2444 | Arachidic acid, TMS | 0.22 ± 0.01 | 0.15 ± 0.01 | 0.14 ± 0.04 | 0.22 ± 0.04 | 0.11 ± 0.04 |
| G15 | 24.983 | 2646 | Behenic acid, TMS | 0.55 ± 0.07 | 0.28 ± 0.05 | 0.29 ± 0.12 | 0.41 ± 0.08 | 0.22 ± 0.12 |
| G16 | 26.466 | 2824 | Lignoceric acid, TMS | 0.25 ± 0.03 | 0.18 ± 0.01 | 0.17 ± 0.04 | 0.25 ± 0.02 | 0.13 ± 0.05 |
| Total fatty acids | 8.14 | 8.94 | 6.70 | 12.30 | 5.68 | |||
| Organic acids | ||||||||
| G17 | 7.06 | 1076 | Glycolic acid, 2TMS | 0.19 ± 0.03 | 0.24 ± 0.01 | 0.25 ± 0.04 | 0.25 ± 0.01 | 0.12 ± 0.02 |
| G18 | 7.549 | 1113 | Oxalic acid, 2TMS | 1.63 ± 0.45 | 1.57 ± 0.32 | 0.48 ± 0.07 | 1.33 ± 0.36 | 1.10 ± 0.39 |
| G19 | 8.265 | 1153 | 3-hydroxypropionic acid, 2TMS | 0.18 ± 0.04 | 0.11 ± 0.01 | 0.42 ± 0.02 | 0.16 ± 0.01 | 0.14 ± 0.02 |
| G20 | 9.766 | 1243 | 4-hydroxybutyric acid, 2TMS | 1.01 ± 0.25 | 0.82 ± 0.10 | 0.52 ± 0.04 | 0.98 ± 0.10 | 0.10 ± 0.01 |
| G21 | 10.983 | 1322 | Succinic acid, 2TMS | 0.60 ± 0.04 | 2.08 ± 0.00 | 0.82 ± 0.04 | 0.47 ± 0.02 | 0.36 ± 0.04 |
| G22 | 13.529 | 1502 | Malic acid, 3TMS * | 0.64 ± 0.01 | 1.41 ± 0.21 | 1.52 ± 0.13 | 1.50 ± 0.20 | 0.49 ± 0.04 |
| G23 | 14.269 | 1557 | (E)-Cinnamic acid, TMS * | 0.41 ± 0.05 | 0.21 ± 0.02 | 1.02 ± 0.10 | 0.10 ± 0.01 | 0.70 ± 0.08 |
| G24 | 17.418 | 1821 | Shikimic acid, 4TMS | 0.44 ± 0.05 | 0.37 ± 0.02 | 0.90 ± 0.01 | 0.25 ± 0.02 | 0.26 ± 0.04 |
| G25 | 18.099 | 1885 | Quinic acid, 5TMS | 1.10 ± 0.10 | 1.05 ± 0.03 | 2.03 ± 0.15 | 0.45 ± 0.08 | 0.44 ± 0.06 |
| Total organic acids | 6.20 | 7.86 | 7.96 | 5.49 | 3.70 | |||
| Phenolics | ||||||||
| G26 | 17.543 | 1832 | Protocatechuic acid, 3TMS | 0.63 ± 0.13 c,d | 0.26 ± 0.01c,d | 4.52 ± 0.27 a,b,d,e | 0.19 ± 0.17 a,b,c,e | 0.36 ± 0.02 c,d |
| G27 | 26.849 | 2859 | Catechin, 5TMS | 0.35 ± 0.09 | 0.35 ± 0.06 | 0.10 ± 0.03 | 1.40 ± 0.03 | 0.16 ± 0.03 |
| Total phenolics | 0.98 | 0.61 | 4.62 | 1.59 | 0.52 | |||
| Sugars | ||||||||
| G28 | 15.63 | 1667 | Arabinose, 4TMS | 0.62 ± 0.09 | 0.19 ± 0.04 | 0.56 ± 0.01 | 0.26 ± 0.03 | 0.13 ± 0.02 |
| G29 | 17.476 | 1826 | Psicofuranose, 5TMS | 1.96 ± 0.23 b,c,d,e | 0.11 ± 0.03 a,c,e | 1.06 ± 0.15 a,b,e | 0.92 ± 0.22 a,e | 2.94 ± 0.44 a,b,c,d |
| G30 | 17.557 | 1834 | Psicofuranose, 5TMS isomer | 5.46 ± 0.60 b,d,e | 0.58 ± 0.04 a,c,d,e | 6.86 ± 0.11 b,d | 3.01 ± 0.46 a,b,c,e | 7.54 ± 1.11 a,b,d |
| G31 | 17.662 | 1844 | Fructopyranose, 5TMS * | 8.45 ± 1.23 b,d,e | 0.45 ± 0.01 a,c,d,e | 6.30 ± 0.40 b,e | 4.21 ± 0.44 a,b,e | 12.93 ± 0.58 a,b,c,d |
| G32 | 18.45 | 1918 | Glucopyranose, 5TMS * | 8.10 ±1.01 b,c,d,e | 0.27 ± 0.01 a,c,d,e | 5.98 ±0.47 a,b,d,e | 3.73 ± 0.53 a,b,c,e | 10.97 ± 0.68 a,b,c,d |
| G33 | 19.347 | 2002 | Glucopyranose, 5TMS isomer | 10.01 ± 1.46 b,c,d,e | 0.37 ± 0.04 a,c,d,e | 7.43 ± 0.35 a,b,d,e | 4.63 ± 0.49 a,b,c,e | 12.51 ± 0.36 a,b,c,d |
| G34 | 25.318 | 2689 | Sucrose, 8TMS * | 2.35 ± 0.28 | 0.25 ± 0.04 | 0.83 ± 0.19 | 0.77 ± 0.36 | 0.25 ± 0.07 |
| Total sugars | 36.95 | 2.23 | 29.03 | 17.52 | 47.27 | |||
| Sugar acids | ||||||||
| G35 | 11.338 | 1343 | Glyceric acid, 3TMS | 0.25 ± 0.05 | 0.22 ± 0.01 | 0.16 ± 0.01 | 0.37 ± 0.02 | 0.15 ± 0.02 |
| G36 | 14.529 | 1577 | Erythronic acid, 4TMS | 0.03 ± 0.00 | 0.05 ± 0.01 | 0.06 ± 0.00 | 0.08 ± 0.01 | 0.01 ± 0.00 |
| G37 | 18.381 | 1909 | Galactonic acid, γ-lactone, 4TMS | 1.74 ± 0.33 b,d,e | 0.13 ± 0.04 a,c,e | 2.02 ± 0.12 b,d | 0.74 ± 0.15 a,c,e | 2.78 ± 0.42 a,b,d |
| G38 | 18.493 | 1923 | Galactonic acid, γ-lactone, 4TMS isomer | 0.11 ± 0.02 | 0.28 ± 0.09 | 0.71 ± 0.01 | 0.11 ± 0.02 | 9.03 ± 1.06 |
| G39 | 18.732 | 1944 | Talonic acid, γ-lactone, 4TMS | 0.03 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 |
| G40 | 19.627 | 2031 | Gluconic acid, 6TMS | 0.32 ± 0.02 | 1.87 ± 0.07 | 1.56 ± 0.27 | 0.61 ± 0.09 | 0.10 ± 0.02 |
| Total sugar acids | 2.47 | 2.56 | 4.52 | 1.94 | 12.09 | |||
| Sugar alcohols | ||||||||
| G41 | 10.434 | 1286 | Glycerol, 3TMS * | 3.60 ± 0.64 c | 2.56 ± 0.14 c,d | 11.29 ± 0.98 a,b,d,e | 4.63 ±0.38 b,c,e | 2.36 ±0.37 c,d |
| G42 | 13.721 | 1516 | L-Threitol, 4TMS | 0.04 ± 0.01 | 0.19 ± 0.01 | 0.20 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.01 |
| G43 | 13.819 | 1524 | meso-Erythritol, 4TMS | 1.43 ± 0.26 b,c,d | 15.96 ± 0.28 a,c,d,e | 3.84 ±0.12 a,b,d,e | 2.61 ± 0.06 a,b,c,e | 1.04 ± 0.14 b,c,d |
| G44 | 16.362 | 1729 | Arabitol, 5TMS | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.14 ± 0.01 | 0.04 ± 0.00 | 0.03 ± 0.00 |
| G45 | 16.502 | 1741 | Arabitol, 5TMS isomer | 0.54 ± 0.08 | 6.14 ± 0.24 | 2.13 ± 0.09 | 0.33 ± 0.02 | 0.13 ± 0.01 |
| G46 | 18.944 | 1965 | Mannitol, 6TMS | 0.11 ± 0.02 | 0.17 ± 0.04 | 0.42 ± 0.03 | 0.99 ± 0.11 | 0.28 ± 0.03 |
| G47 | 19.133 | 1982 | Myo-Inositol, 6TMS * | 0.08 ± 0.01 | 0.10 ± 0.00 | 0.12 ± 0.01 | 0.18 ± 0.02 | 0.13 ± 0.02 |
| G48 | 20.5 | 2121 | Myo-Inositol, 6TMS isomer | 0.79 ± 0.11 | 3.49 ± 0.11 | 1.29 ± 0.04 | 0.72 ± 0.09 | 0.78 ± 0.11 |
| Total sugar alcohols | 6.67 | 28.68 | 19.42 | 9.57 | 4.80 | |||
| Unknowns | ||||||||
| G49 | 10.903 | 1317 | Unknown 1 | 3.08 ± 0.59 | 2.45 ± 0.23 | 1.66 ± 0.14 | 3.43 ± 0.12 | 1.94 ± 0.22 |
| G50 | 11.596 | 1362 | Unknown 2 | 10.23 ± 0.67 | 10.46 ± 0.13 | 5.82 ± 0.36 | 11.25 ± 0.33 | 6.34 ± 0.89 |
| G51 | 19.881 | 2058 | Unknown 3 | 0.77 ± 0.13 | 0.98 ± 0.01 | 1.27 ± 0.01 | 0.54 ± 0.08 | 0.37 ± 0.06 |
| Total unknowns | 14.07 | 13.89 | 8.76 | 15.22 | 8.65 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.A.; Kabbash, E.M.; Mediani, A.; Döll, S.; Esatbeyoglu, T.; Afifi, S.M. Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC–MS and GC–MS and Chemometric Tools. Molecules 2022, 27, 2935. https://doi.org/10.3390/molecules27092935
Farag MA, Kabbash EM, Mediani A, Döll S, Esatbeyoglu T, Afifi SM. Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC–MS and GC–MS and Chemometric Tools. Molecules. 2022; 27(9):2935. https://doi.org/10.3390/molecules27092935
Chicago/Turabian StyleFarag, Mohamed A., Eman M. Kabbash, Ahmed Mediani, Stefanie Döll, Tuba Esatbeyoglu, and Sherif M. Afifi. 2022. "Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC–MS and GC–MS and Chemometric Tools" Molecules 27, no. 9: 2935. https://doi.org/10.3390/molecules27092935
APA StyleFarag, M. A., Kabbash, E. M., Mediani, A., Döll, S., Esatbeyoglu, T., & Afifi, S. M. (2022). Comparative Metabolite Fingerprinting of Four Different Cinnamon Species Analyzed via UPLC–MS and GC–MS and Chemometric Tools. Molecules, 27(9), 2935. https://doi.org/10.3390/molecules27092935








