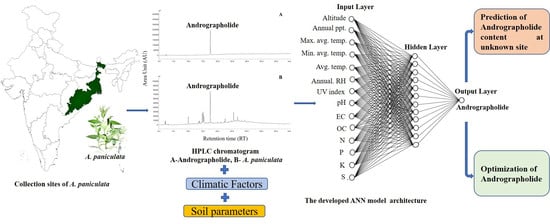
Application of a Multilayer Perceptron Artificial Neural Network for the Prediction and Optimization of the Andrographolide Content in Andrographis paniculata
Abstract
:1. Introduction
2. Results and Discussion
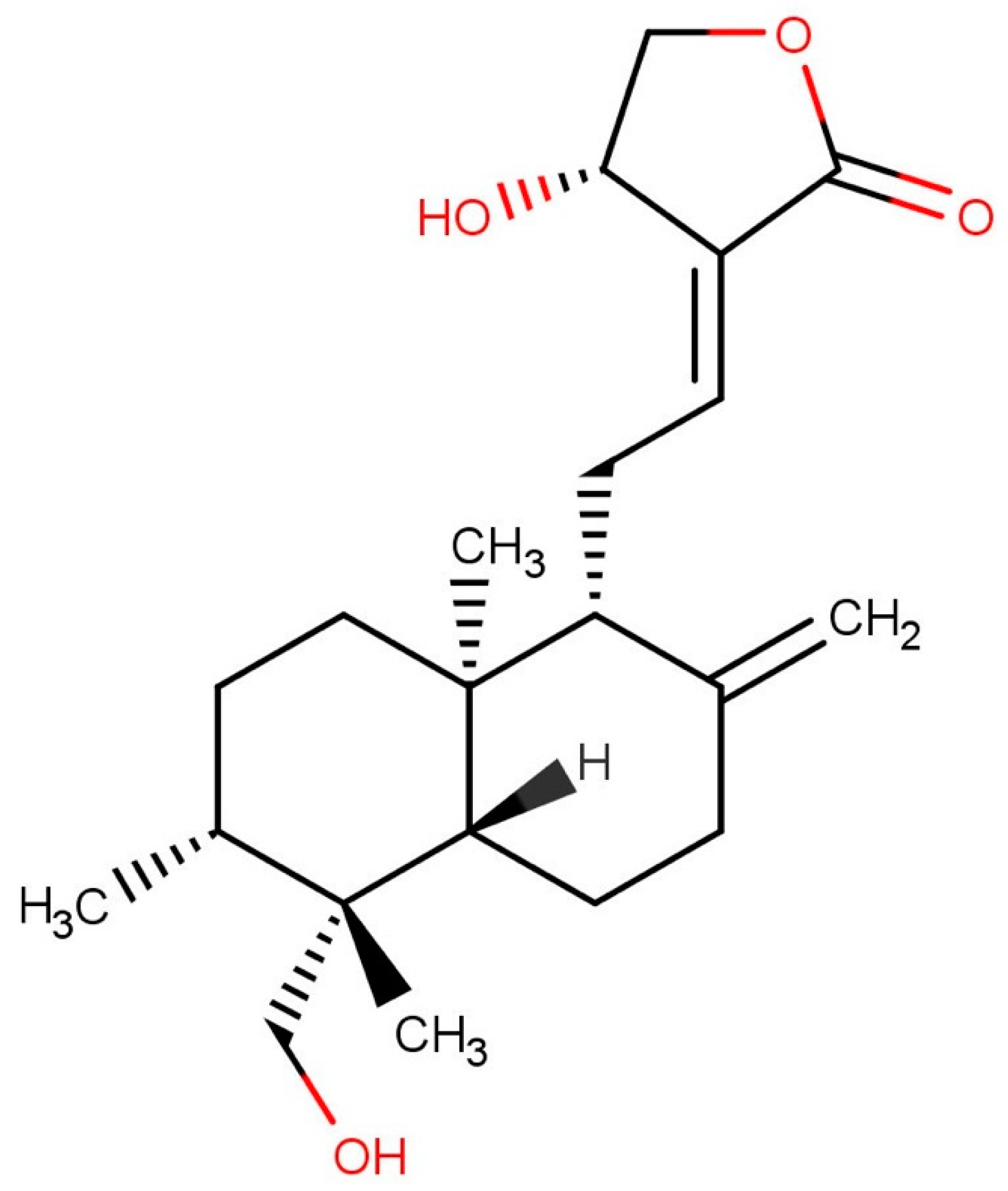
2.1. Variation in Andrographolide Content
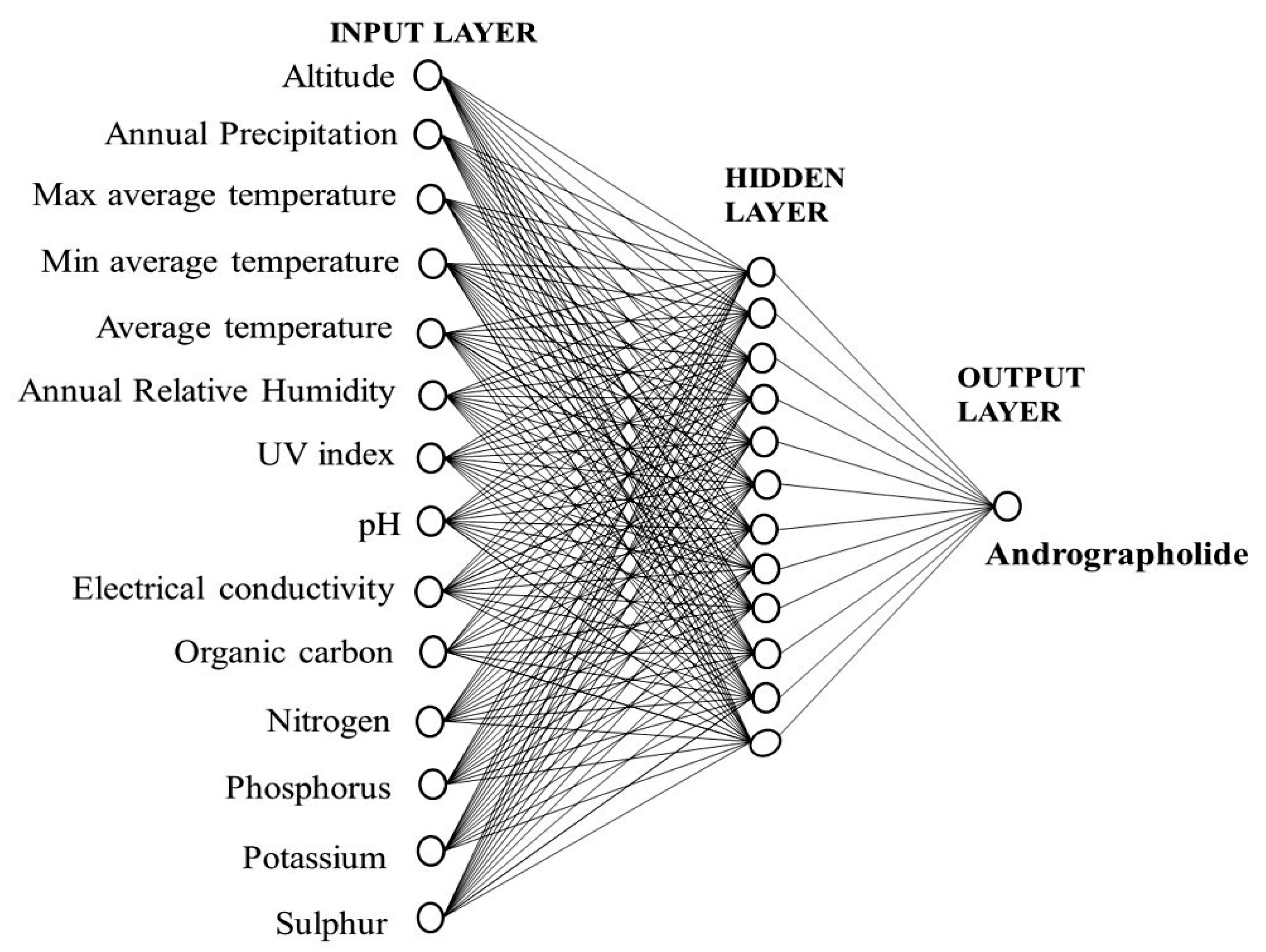
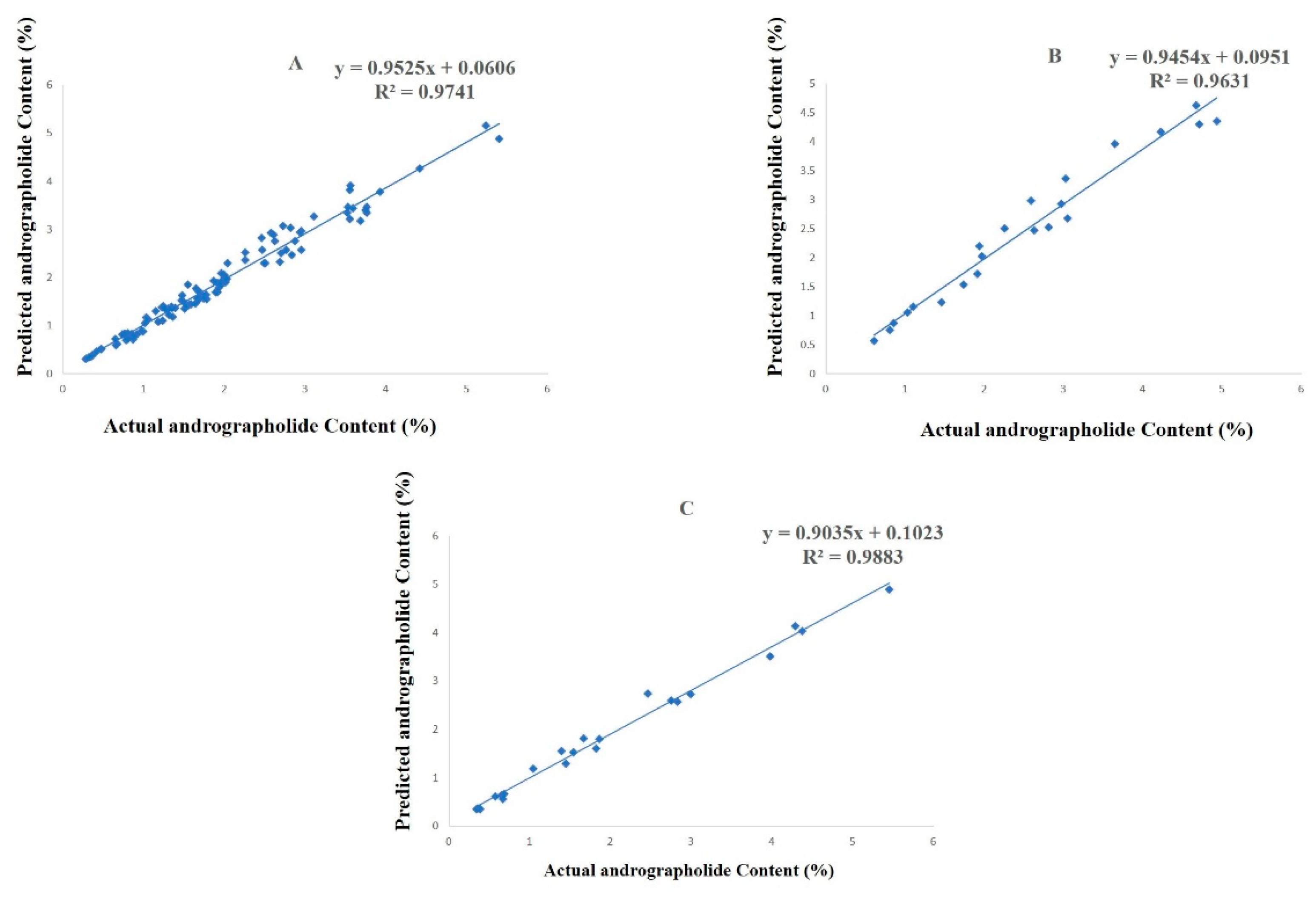
2.2. Development of the Artificial Neural Network (ANN) Model
2.3. Sensitivity Analysis
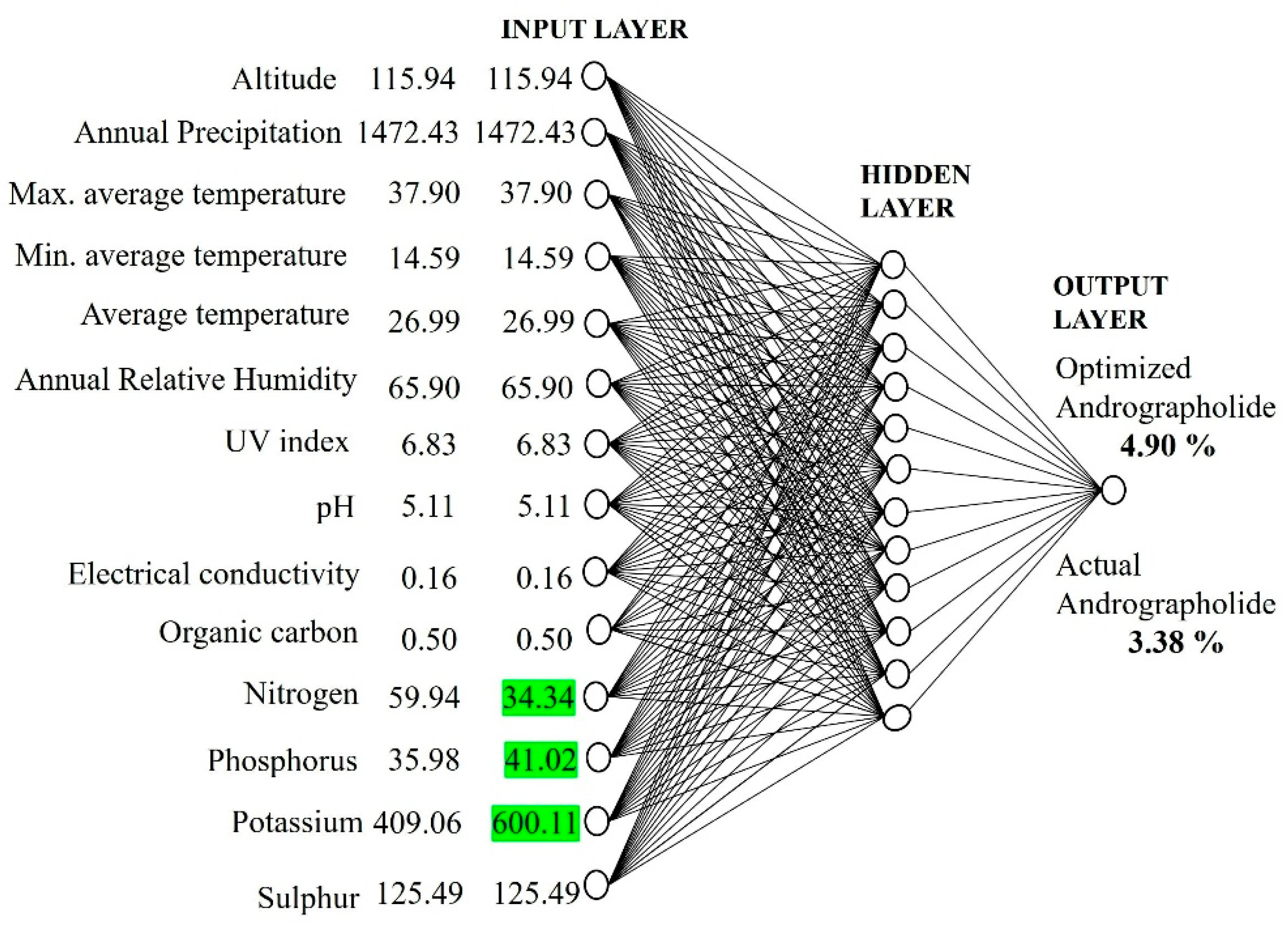
2.4. Optimization of Andrographolide Content
2.5. Application of the ANN Model at a New Site
3. Materials and Methods
3.1. Plant Samples and Chemical Reagents
3.2. Quantification of Andrographolide
3.2.1. Plant Samples and Standard Solution Preparation
3.2.2. HPLC Instrumentation and Chromatographic Conditions
3.2.3. Quantitative Estimation of Andrographolide
3.3. Quantitative Analysis of Soil and Climatic Factors
3.4. Artificial Neural Network Development
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ghosh, K.B.; Datta, K.A.; Mandal, A.; Dubey, K.P.; Halder, S. An overview on Andrographis paniculata (Burm. f.) Nees. Int. J. Res. Ayurveda Pharm. 2012, 3, 752–760. [Google Scholar] [CrossRef]
- Nyeem, M.A.B.; Mannan, M.A.; Nuruzzaman, M.; Kamrujjaman, K.M.; Das, S.K. Indigenous king of bitter (Andrographis paniculata): A review. J. Med. Plants 2017, 5, 318–324. [Google Scholar]
- Dhiman, A.; Jugnu, G.; Kavita, S.; Arun, N.; Sandeep, D. A review on medicinal prospectives of Andrographis paniculata Nees. J. Pharm. Sci. Innov. (JPSI) 2012, 1, 1–4. [Google Scholar]
- Kaskoos, R.A.; Ahamad, J. Evaluation of pharmacognostic features of aerial parts of Andrographis paniculata Wall. J. Pharmacogn. Phytochem. 2014, 3, 1–5. [Google Scholar]
- Sivananthan, M.; Elamaran, M. Medicinal and pharmacological properties of Andrographis paniculata. Int. J. Biomol. Biomed. 2013, 3, 1–12. [Google Scholar]
- Deng, W.L. Preliminary studies on the pharmacology of the Andrographis product dihydroandrographolide sodium succinate. Newsl. Chin. Herb. Med. 1978, 8, 26–28. [Google Scholar]
- Pholphana, N.; Rangkadilok, N.; Thongnest, S.; Ruchirawat, S.; Ruchirawat, M.; Satayavivad, J. Determination and variation of three active diterpenoids in Andrographis paniculata (Burm. f.) Nees. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2004, 15, 365–371. [Google Scholar] [CrossRef]
- Tang, W.; Eisenbrand, G. Andrographis paniculata (Burm. f.) Nees. In Chinese Drugs of Plant Origin; Springer: Berlin/Heidelberg, Germany, 1992; pp. 97–103. [Google Scholar]
- Bao, Z.; Guan, S.; Cheng, C.; Wu, S.; Wong, S.H.; Kemeny, D.M.; Leung, B.P.; Wong, W.F. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-κB pathway. Am. J. Respir. Crit. Care Med. 2009, 179, 657–665. [Google Scholar] [CrossRef]
- Lu, W.J.; Lee, J.J.; Chou, D.S.; Jayakumar, T.; Fong, T.H.; Hsiao, G.; Sheu, J.R. A novel role of andrographolide, an NF-kappa B inhibitor, on inhibition of platelet activation: The pivotal mechanisms of endothelial nitric oxide synthase/cyclic GMP. J. Mol. Med. 2011, 89, 1261–1273. [Google Scholar] [CrossRef]
- Shen, T.; Yang, W.S.; Yi, Y.S.; Sung, G.H.; Rhee, M.H.; Poo, H.; Kim, M.Y.; Kim, K.W.; Kim, J.H.; Cho, J.Y. AP-1/IRF-3 targeted anti-inflammatory activity of andrographolide isolated from Andrographis paniculata. Evid.-Based Complement. Altern. Med. 2013, 2013, 210736. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.D.; Lin, H.H.; Lee, Y.C.; Chao, J.K.; Lin, R.A.; Chen, J.H. Inhibition of cell-cycle progression in human colorectal carcinoma Lovo cells by andrographolide. Chem.-Biol. Interact. 2008, 174, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Tiwari, S.K.; Kakkar, A.; Pandey, A.K. Andrographolide content in Madhya Pradesh, India. Int. J. Pharma Bio Sci. 2010, 1, 2. [Google Scholar]
- Raina, A.P.; Sivaraj, N.; Kumar, A. Chemical diversity among Andrographis paniculata Nees (Kalmegh) and assessing climate suitable regions for elite germplasm distribution in India. Med. Plants-Int. J. Phytomed. Relat. Ind. 2016, 8, 294–302. [Google Scholar] [CrossRef]
- Pandey, A.K.; Mandal, A.K. Variation in morphological characteristics and andrographolide content in Andrographis paniculata (Burm. f.) Nees of Central India. Iran. J. Energy Environ. 2010, 1, 165–169. [Google Scholar]
- Basak, B.B.; Jat, R.S.; Gajbhiye, N.A.; Saha, A.; Manivel, P. Organic nutrient management through manures, microbes and biodynamic preparation improves yield and quality of Kalmegh (Andrograghis paniculata), and soil properties. J. Plant Nutr. 2020, 43, 548–562. [Google Scholar] [CrossRef]
- Verma, R.K.; Verma, S.K.; Pankaj, U.; Gupta, A.K.; Khan, K.; Shankar, K. Improvement in the yield and quality of kalmegh (Andrographis paniculata Nees) under the sustainable production system. Nat. Prod. Res. 2015, 29, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Pholphana, N.; Rangkadilok, N.; Saehun, J.; Ritruechai, S.; Satayavivad, J. Changes in the contents of four active diterpenoids at different growth stages in Andrographis paniculata (Burm. f.) Nees (Chuanxinlian). Chin. Med. 2013, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Giri, A.K.; Patel, R.K.; Mahapatra, S.S. Artificial neural network (ANN) approach for modelling of arsenic (III) biosorption from aqueous solution by living cells of Bacillus cereus biomass. Chem. Eng. J. 2011, 178, 15–25. [Google Scholar] [CrossRef]
- Alam, M.A.; Naik, P.K. Impact of soil nutrients and environmental factors on podophyllotoxin content among 28 Podophyllum hexandrum populations of north western Himalayan region using linear and nonlinear approaches. Commun. Soil Sci. Plant Anal. 2009, 40, 2485–2504. [Google Scholar] [CrossRef]
- Sheikh, S.K.; Unde, M.G. Short term load forecasting using ANN technique. Int. J. Eng. Sci. Emerg. Technol. 2012, 1, 97–107. [Google Scholar] [CrossRef]
- Golovko, V.; Savitsky, Y.; Laopoulos, T.; Sachenko, A.; Grandinetti, L. Technique of learning rate estimation for efficient training of MLP. In Proceedings of the IEEE-INNS-ENNS International Joint Conference on Neural Networks. IJCNN 2000. Neural Computing: New Challenges and Perspectives for the New Millennium, Como, Italy, 27 July 2000; Volume 1, pp. 323–328. [Google Scholar]
- Jiang, D.; Zhang, Y.; Hu, X.; Zeng, Y.; Tan, J.; Shao, D. Progress in developing an ANN model for air pollution index forecast. Atmos. Environ. 2004, 38, 7055–7064. [Google Scholar] [CrossRef]
- Ray, A.; Halder, T.; Jena, S.; Sahoo, A.; Ghosh, B.; Mohanty, S.; Nayak, S. Application of artificial neural network (ANN) model for prediction and optimization of coronarin D content in Hedychium coronarium. Ind. Crops Prod. 2020, 146, 112186. [Google Scholar] [CrossRef]
- Singh, A.; Imtiyaz, M.; Isaac, R.K.; Denis, D.M. Comparison of soil and water assessment tool (SWAT) and multilayer perceptron (MLP) artificial neural network for predicting sediment yield in the Nagwa agricultural watershed in Jharkhand, India. Agric. Water Manag. 2012, 104, 113–120. [Google Scholar] [CrossRef]
- Padhiari, B.M.; Ray, A.; Champati, B.B.; Jena, S.; Sahoo, A.; Kuanar, A.; Halder, T.; Ghosh, B.; Naik, P.K.; Patnaik, J.; et al. Artificial neural network (ANN) model for prediction and optimization of bacoside A content in Bacopa monnieri: A statistical approach and experimental validation. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2022, 1–17. [Google Scholar] [CrossRef]
- Akbar, A.; Kuanar, A.; Joshi, R.K.; Sandeep, I.S.; Mohanty, S.; Naik, P.K.; Mishra, A.; Nayak, S. Development of prediction model and experimental validation in predicting the curcumin content of turmeric (Curcuma longa L.). Front. Plant Sci. 2016, 7, 1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.K.; Gulati, S.; Gupta, A.; Tripathi, Y.C. Variation in andrographolide content among different accessions of Andrographis paniculata. Pharma Innov. J. 2019, 8, 140–144. [Google Scholar]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [Green Version]
- Mishra, T. Climate change and production of secondary metabolites in medicinal plants: A review. Int. J. Herb. Med. 2016, 4, 27–30. [Google Scholar]
- Agatonovic-Kustrin, S.; Beresford, R. Basic concepts of artificial neural network (ANN) modeling and its application in pharmaceutical research. J. Pharm. Biomed. Anal. 2000, 22, 717–727. [Google Scholar] [CrossRef]
- Tusek, A.J.; Jurina, T.; Benković, M.; Valinger, D.; Belščak-Cvitanović, A.; Kljusurić, J.G. Application of multivariate regression and artificial neural network modelling for prediction of physical and chemical properties of medicinal plants aqueous extracts. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100229. [Google Scholar]
- Akbar, A.; Kuanar, A.; Patnaik, J.; Mishra, A.; Nayak, S. Application of artificial neural network modeling for optimization and prediction of essential oil yield in turmeric (Curcuma longa L.). Comput. Electron. Agric. 2018, 148, 160–178. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M.; Dubey, N.K. Role of macronutrients in plant growth and acclimation: Recent advances and future prospective. In Improvement of Crops in the Era of Climatic Changes; Ahmad, P., Wani, M.R., Azooz, M.M., Tran, L.-S.P., Eds.; Springer: New York, NY, USA, 2014; pp. 197–216. [Google Scholar]
- Spizek, J.; Tichy, P. Some aspects of overproduction of secondary metabolites. Folia Microbiol. 1995, 40, 43–50. [Google Scholar] [CrossRef]
- Chauhan, S.K.; Tiwari, G. Effect of sources and levels of nitrogen on partitioning of Andrographolide in Kalmegh [Andrographis paniculata (Burm. f.) Wall. ex Nees.]. Indian J. Plant Physiol. 2003, 8, 60–62. [Google Scholar]
- Radusiene, J.; Marksa, M.; Ivanauskas, L.; Jakštas, V.; Çalişkan, Ö.; Kurt, D.; Odabaşe, M.S.; Çirak, C. Effect of nitrogen on herb production, secondary metabolites and antioxidant activities of Hypericum pruinatum under nitrogen application. Ind. Crops Prod. 2019, 139, 111519. [Google Scholar] [CrossRef]
- Bryant, J.P.; Chapin III, F.S.; Klein, D.R. Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, H.; Sharma, S.; Pandey, R. Phosphorus nutrition: Plant growth in response to deficiency and excess. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 171–190. [Google Scholar]
- Chaturvedi, A.; Ghosh, C.; Tripathi, A.N. Effect of different levels of nitrogen and phosphorus on growth, yield and quality of kalmegh (Andrographis peniculata burn. F.). Int. J. Bio-Resour. Environ. Agric. Sci. (IJBEAS) 2016, 2, 261–268. [Google Scholar]
- Prajapati, K.; Modi, H.A. The importance of potassium in plant growth—A review. Indian J. Plant Sci. 2012, 1, 177–186. [Google Scholar]
- Dhanush, S.L.; Mallikarjuna, G.A.P.; Kademani, A.J.; Anilkumar, S.; Reddy, G.C. Effect of Nutrient Levels on Growth, Yield, Quality and Economics of Second Ratoon Crop in Kalmegh (Andrographis paniculata Nees.). Int. J. Pure Appl. Biosci. 2018, 6, 721–725. [Google Scholar]
- Bennett, O.L.; Rouse, R.D.; Ashley, D.A.; Doss, B.D. Yield, fiber quality and potassium content of irrigated cotton plants as affected by rates of potassium. Agron. J. 1965, 53, 296–299. [Google Scholar] [CrossRef]
- Champati, B.B.; Jena, S.; Ray, A.; Padhiari, B.M.; Haldar, T.; Mohanty, S.; Sahoo, A.; Kar, B.; Ghosh, B.; Nayak, S. Quality control and discrimination of Andrographis paniculata (Burm. f.) Nees based on High Performance Liquid Chromatography fingerprinting combined with chemometric approaches. Indian J. Pharm. Sci. 2021, 83, 1129–1143. [Google Scholar] [CrossRef]
- Subbiah, B.; Asija, G.L. A rapid procedure for estimation of available nitrogen in soils. Curr. Sci. 1956, 25, 259–260. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of total, organic, and available forms of phosphorus in soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeny, D.R. Methods of Soil Analysis. Part II. Chemical and Microbiological Properties, 2nd ed.; American Society of Agronomy, Monograph: Madison, WI, USA, 1982. [Google Scholar]
- Nelson, D.A.; Sommers, L. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 539–579. [Google Scholar]



| Parameters | Error Quotient | Rank |
|---|---|---|
| Nitrogen (kg/ha) | 5.247 | 1 |
| Phosphorus (kg/ha) | 3.660 | 2 |
| Potassium (kg/ha) | 2.009 | 3 |
| Sulfur (kg/ha) | 1.038 | 4 |
| pH | 1.038 | 5 |
| Electrical Conductivity (Ds/m) | 1.029 | 6 |
| Annual Precipitation (mm) | 1.018 | 7 |
| Organic Carbon (%) | 1.009 | 8 |
| UV Index | 1.006 | 9 |
| Annual Relative Humidity | 1.004 | 10 |
| Average Temperature (°C) | 1.003 | 11 |
| Maximum Temperature (°C) | 1.002 | 12 |
| Altitude (m) | 0.999 | 13 |
| Minimum Temperature (°C) | 0.993 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Champati, B.B.; Padhiari, B.M.; Ray, A.; Halder, T.; Jena, S.; Sahoo, A.; Kar, B.; Kamila, P.K.; Panda, P.C.; Ghosh, B.; et al. Application of a Multilayer Perceptron Artificial Neural Network for the Prediction and Optimization of the Andrographolide Content in Andrographis paniculata. Molecules 2022, 27, 2765. https://doi.org/10.3390/molecules27092765
Champati BB, Padhiari BM, Ray A, Halder T, Jena S, Sahoo A, Kar B, Kamila PK, Panda PC, Ghosh B, et al. Application of a Multilayer Perceptron Artificial Neural Network for the Prediction and Optimization of the Andrographolide Content in Andrographis paniculata. Molecules. 2022; 27(9):2765. https://doi.org/10.3390/molecules27092765
Chicago/Turabian StyleChampati, Bibhuti Bhusan, Bhuban Mohan Padhiari, Asit Ray, Tarun Halder, Sudipta Jena, Ambika Sahoo, Basudeba Kar, Pradeep Kumar Kamila, Pratap Chandra Panda, Biswajit Ghosh, and et al. 2022. "Application of a Multilayer Perceptron Artificial Neural Network for the Prediction and Optimization of the Andrographolide Content in Andrographis paniculata" Molecules 27, no. 9: 2765. https://doi.org/10.3390/molecules27092765
APA StyleChampati, B. B., Padhiari, B. M., Ray, A., Halder, T., Jena, S., Sahoo, A., Kar, B., Kamila, P. K., Panda, P. C., Ghosh, B., & Nayak, S. (2022). Application of a Multilayer Perceptron Artificial Neural Network for the Prediction and Optimization of the Andrographolide Content in Andrographis paniculata. Molecules, 27(9), 2765. https://doi.org/10.3390/molecules27092765