An Update on the Use of Molecularly Imprinted Polymers in Beta-Blocker Drug Analysis as a Selective Separation Method in Biological and Environmental Analysis
Abstract
1. Introduction
2. Methods of Synthesis of Molecularly Imprinted Polymers for Beta-Blocker Drugs
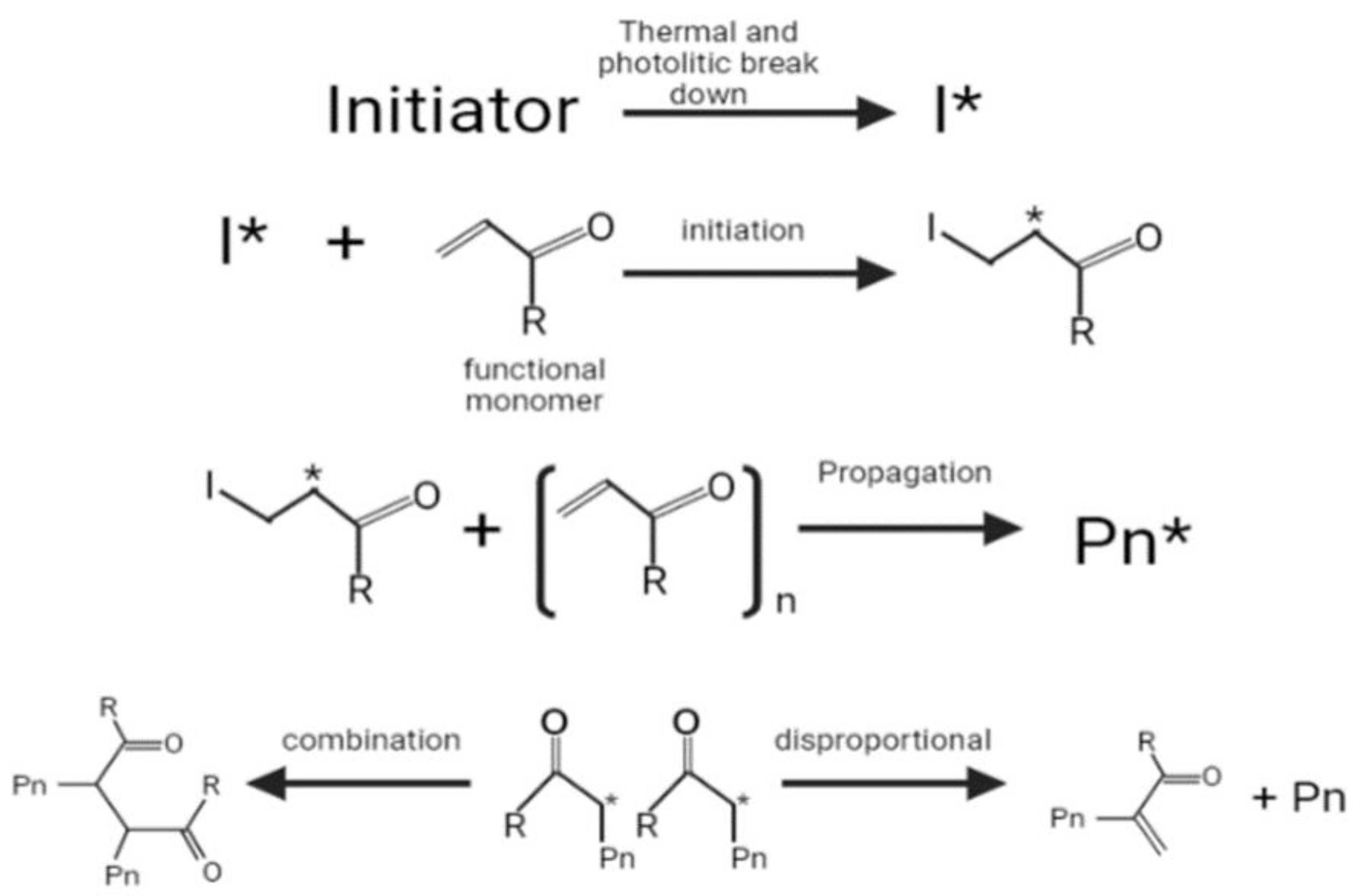
2.1. Bulk Polymerization Method
2.2. Precipitation Polymerization Method
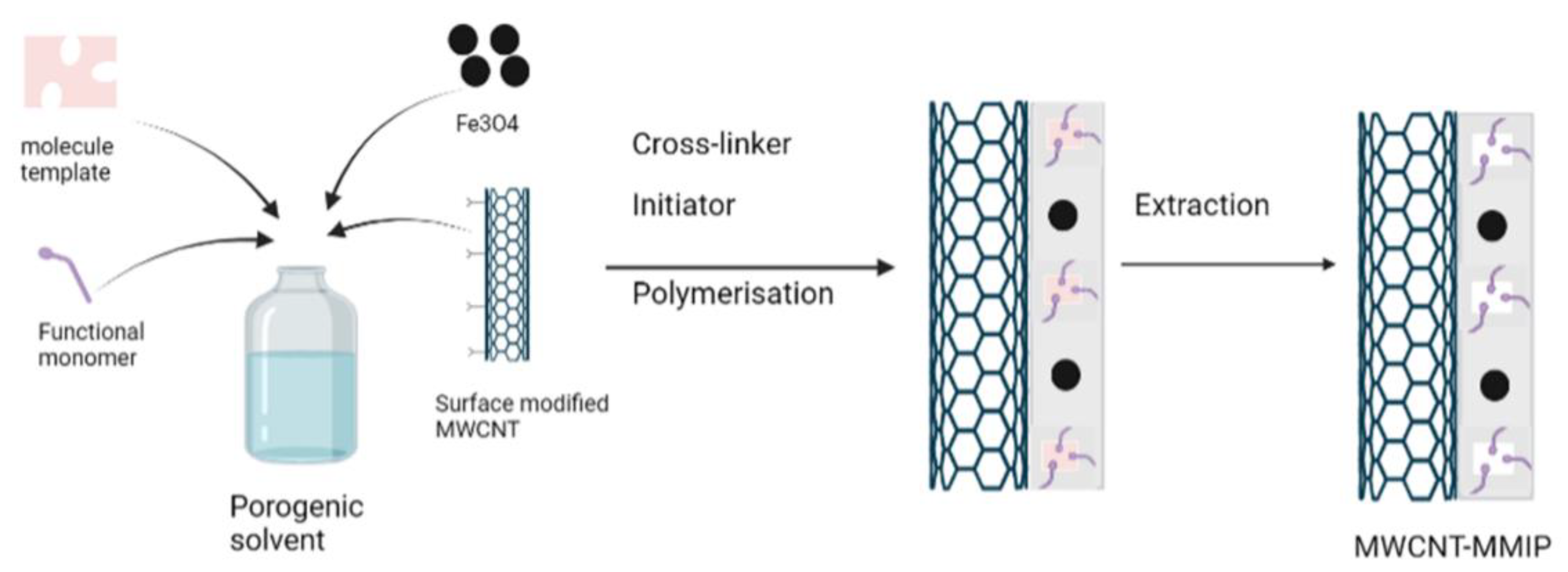
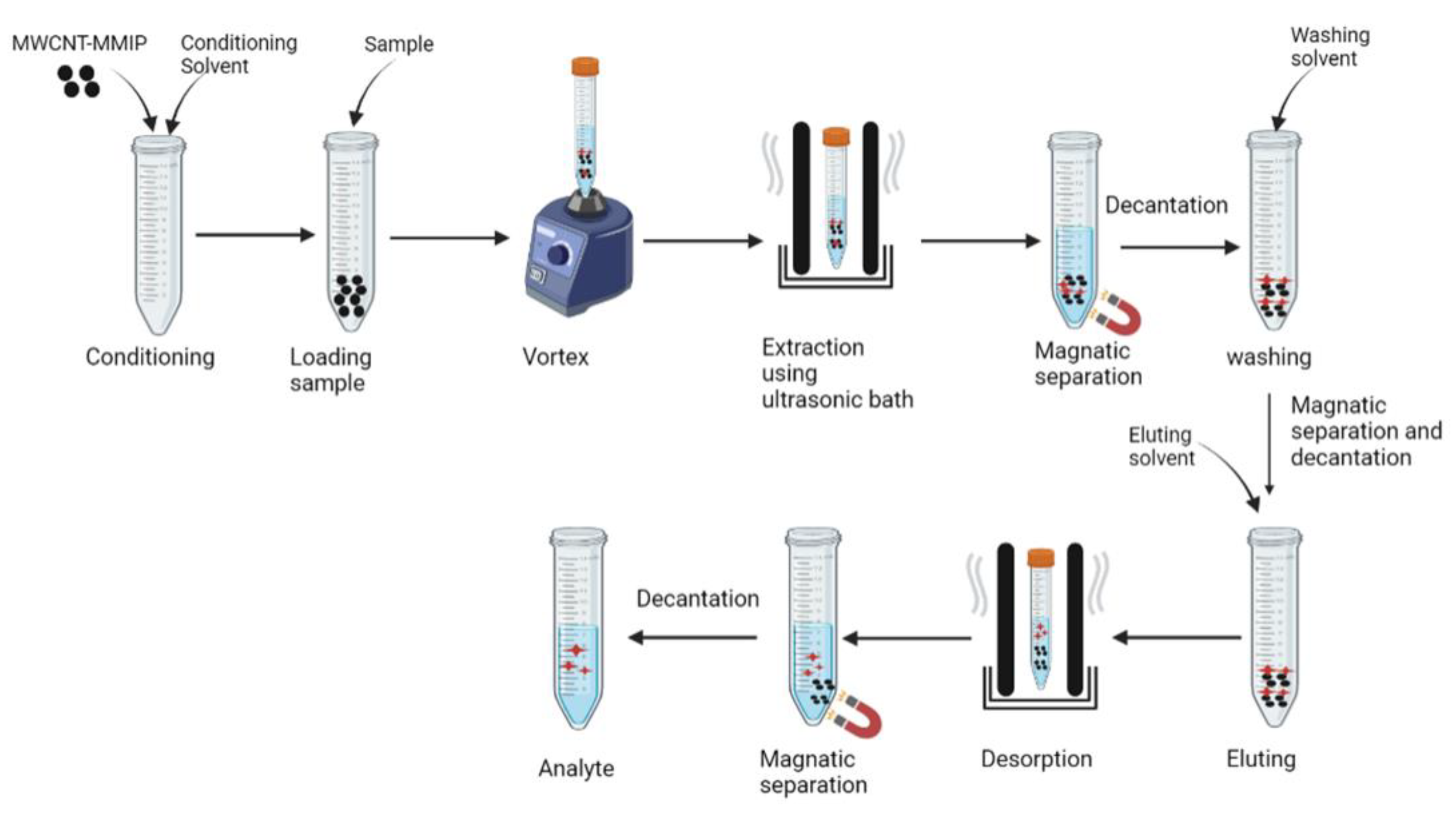
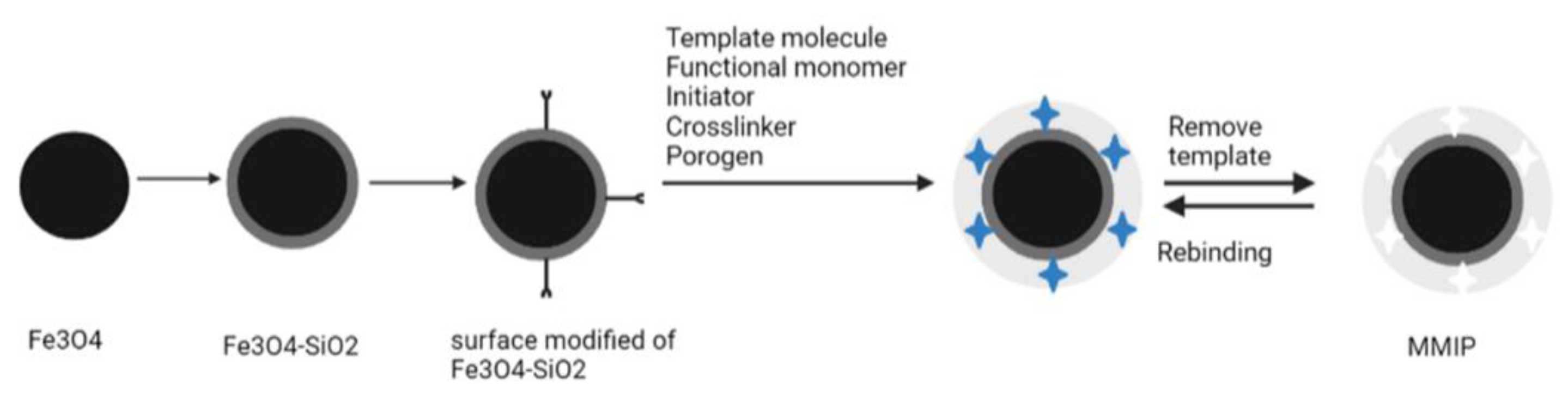
2.3. Surface Imprinted Polymerization
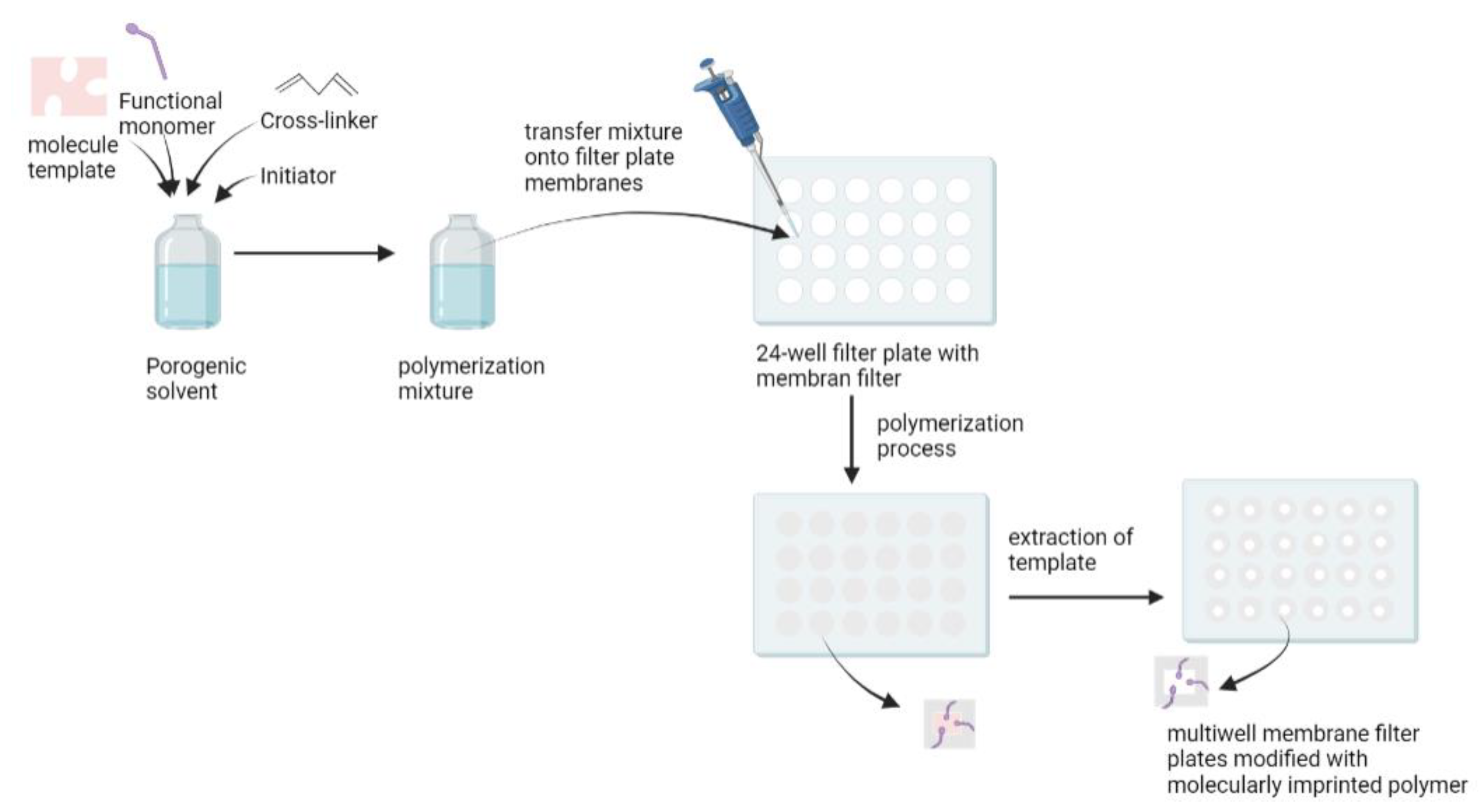
2.4. In Situ Polymerization
Monolithic Imprinted Polymerization
- The silanization process: a silicosteel column was hydrolyzed using acid and basic solution. Then, 3-(trimethoxysilyl)-propyl methacrylate was added to the hydrolyzed silicosteel column to start the salination reaction process. The reaction occurs between the methoxy and silanol groups of 3-(trimethoxysilyl)-propyl methacrylate on the wall surface of the silicosteel column. This reaction facilitated the formation of a covalent bond between the siliconsteel column and the monolithic polymer [104,105]
- Synthesis of the imprinted monolithic column: the components used in the polymerization process were dissolved and inserted into the siliconsteel column by syringe, and both ends of the column were closed. The in situ polymerization occurred in an oven at 80 °C for 18 h [102].
- Removal of template molecule using a mobile phase that can elute the atenolol. In the study conducted by Hasanah et al., a methanol:acetic acid (90:10 v/v) mobile phase at a flow rate of 0.01–0.03 mL/minute was used to remove the atenolol and residual compound [102].
3. Green Chemistry Principle in Molecularly Imprinted Polymer for Beta-Blockers
4. Conclusions
- Developing MIPs for separation or extraction using molecule templates that have not been used so far, such as acebutolol, labetalol, alprenolol, metipranolol, metoprolol, betaxolol, nadolol, bunolol, carteolol, celiprolol, timolol, and esmolol.
- Studies about beta-blocker sensors, such as studies based on MIP technology are still lacking.
- Developing an in situ polymerization technique, as MIPs modified in the multi-well membrane filter can be further developed to obtain high throughput analysis.
- Developing MIPs for the extraction of beta-blockers in food samples, to obtain sorbents that are selective in their separation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiNicolantonio, J.J.; Fares, H.; Niazi, A.K.; Chatterjee, S.; D’Ascenzo, F.; Cerrato, E.; Biondi-Zoccai, G.; Lavie, C.J.; Bell, D.S.; O’Keefe, J.H. β-Blockers in Hypertension, Diabetes, Heart Failure and Acute Myocardial Infarction: A Review of the Literature. Open Hear 2015, 2, e000230. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef]
- Larochelle, P.; Tobe, S.W.; Lacourcière, Y. β-Blockers in Hypertension: Studies and Meta-Analyses over the Years. Can. J. Cardiol. 2014, 30, S16–S22. [Google Scholar] [CrossRef] [PubMed]
- Wells, B.G.; DiPiro, J.T.; Schwinghammer, T.L.; DiPiro, C.V. Pharmacotherapy Handbook, 10th ed.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- World Anti-Doping Agency. Prohibited List Wada 2020. World Anti-Doping Code 2020. Available online: https://www.wada-ama.org/sites/default/files/wada_2020_english_prohibited_list_0.pdf (accessed on 9 September 2021).
- Kern, S.; Baumgartner, R.; Helbling, D.E.; Hollender, J.; Singer, H.; Loos, M.J.; Schwarzenbach, R.P.; Fenner, K. A Tiered Procedure for Assessing the Formation of Biotransformation Products of Pharmaceuticals and Biocides during Activated Sludge Treatment. J. Environ. Monit. 2010, 12, 2100–2111. [Google Scholar] [CrossRef] [PubMed]
- World Anti-Doping Agency. 2018 Anti-Doping Testing. 2018. Available online: https://www.wada-ama.org/sites/default/files/resources/files/2018_testing_figures_report.pdf (accessed on 9 September 2021).
- Ramil, M.; El Aref, T.; Fink, G.; Scheurer, M.; Ternes, T.A. Fate of Beta Blockers in Aquatic-Sediment Systems: Sorption and Biotransformation. Environ. Sci. Technol. 2010, 44, 962–970. [Google Scholar] [CrossRef]
- Xu, J.; Sun, H.; Zhang, Y.; Alder, A.C. Occurrence and Enantiomer Profiles of β-Blockers in Wastewater and a Receiving Water Body and Adjacent Soil in Tianjin, China. Sci. Total Environ. 2019, 650, 1122–1130. [Google Scholar] [CrossRef]
- Wilde, M.L.; Mahmoud, W.M.M.; Kümmerer, K.; Martins, A.F. Oxidation-Coagulation of β-Blockers by K2FeVIO4 in Hospital Wastewater: Assessment of Degradation Products and Biodegradability. Sci. Total Environ. 2013, 452, 137–147. [Google Scholar] [CrossRef]
- Scheurer, M.; Ramil, M.; Metcalfe, C.D.; Groh, S.; Ternes, T.A. The Challenge of Analyzing Beta-Blocker Drugs in Sludge and Wastewater. Anal. Bioanal. Chem. 2010, 396, 845–856. [Google Scholar] [CrossRef]
- Maurer, M.; Escher, B.I.; Richle, P.; Schaffner, C.; Alder, A.C. Elimination of β-Blockers in Sewage Treatment Plants. Water Res. 2007, 41, 1614–1622. [Google Scholar] [CrossRef]
- Rosen, R.C.; Kostis, J.B.; Jekelis, A.W. Beta-Blocker Effects on Sexual Function in Normal Males. Arch. Sex. Behav. 1988, 17, 241–255. [Google Scholar] [CrossRef]
- el-Sayed, M.G.; el-Sayed, M.T.; Elazab Abd el, S.; Hafeiz, M.H.; el-Komy, A.A.; Hassan, E. Effects of Some Beta-Adrenergic Blockers on Male Fertility Parameters in Rats. Dtsch. Tierarztl. Wochenschr. 1998, 105, 10–12. [Google Scholar] [PubMed]
- Hernando, M.D.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. LC-MS Analysis of Basic Pharmaceuticals (Beta-Blockers and Anti-Ulcer Agents) in Wastewater and Surface Water. TrAC-Trends Anal. Chem. 2007, 26, 581–594. [Google Scholar] [CrossRef]
- Zhang, H.; Ihara, M.O.; Nakada, N.; Tanaka, H.; Ihara, M. Biological Activity-Based Prioritization of Pharmaceuticals in Wastewater for Environmental Monitoring: G Protein-Coupled Receptor Inhibitors. Environ. Sci. Technol. 2020, 54, 1720–1729. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.A.; Wasfi, I.A.; Al-Nassibi, S.S. Trace Determination of β-Blockers and Β2-Agonists in Distilled and Waste-Waters Using Liquid Chromatography-Tandem Mass Spectrometry and Solid-Phase Extraction. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 908, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, A.; Giebułtowicz, J.; Stankiewicz, U.; Wroczyński, P.; Nałecz-Jawecki, G. Determination of Selected Cardiovascular Active Compounds in Environmental Aquatic Samples-Methods and Results, a Review of Global Publications from the Last 10 Years. Chemosphere 2015, 138, 642–656. [Google Scholar] [CrossRef]
- Tambosi, J.L.; Yamanaka, L.Y.; José, H.J.; De Fátima Peralta Muniz Moreira, R.; Schröder, H.F. Recent Research Data on the Removal of Pharmaceuticals from Sewage Treatment Plants (STP). Quim. Nova 2010, 33, 411–420. [Google Scholar] [CrossRef]
- Escher, B.I.; Bramaz, N.; Richter, M.; Lienert, J. Comparative Ecotoxicological Hazard Assessment of Beta-Blockers and Their Human Metabolites Using a Mode-of-Action-Based Test Battery and a QSAR Approach. Environ. Sci. Technol. 2006, 40, 7402–7408. [Google Scholar] [CrossRef]
- Balizs, G.; Hewitt, A. Determination of Veterinary Drug Residues by Liquid Chromatography and Tandem Mass Spectrometry. Anal. Chim. Acta 2003, 492, 105–131. [Google Scholar] [CrossRef]
- Sai, F.; Hong, M.; Zhao, Y.; Chen, H.; Wu, Y. Simultaneous Detection of Residues of 25 Β2-Agonists and 23 β-Blockers in Animal Foods by High-Performance Liquid Chromatography Coupled with Linear Ion Trap Mass Spectrometry. J. Agric. Food Chem. 2012, 60, 1898–1905. [Google Scholar] [CrossRef]
- Spanakis, M.; Niopas, I. Determination of Atenolol in Human Plasma by HPLC with Fluorescence Detection: Validation and Application in a Pharmacokinetic Study. J. Chromatogr. Sci. 2013, 51, 128–132. [Google Scholar] [CrossRef]
- Kim, Y.H.; Choi, H.Y.; Noh, Y.H.; Lee, S.H.; Lim, H.S.; Kim, C.; Bae, K.S. Dose Proportionality and Pharmacokinetics of Carvedilol Sustained-Release Formulation: A Single Dose-Ascending 10-Sequence Incomplete Block Study. Drug Des. Devel. Ther. 2015, 9, 2911–2918. [Google Scholar] [PubMed]
- Yilmaz, B.; Arslan, S.; Asci, A. HPLC Method for Determination of Atenolol in Human Plasma and Application to a Pharmacokinetic Study in Turkey. J. Chromatogr. Sci. 2012, 50, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Arslan, S. Determination of Atenolol in Human Urine by Using HPLC. Sep. Sci. Plus 2018, 1, 4–10. [Google Scholar] [CrossRef]
- Rodina, T.A.; Mel’nikov, E.S.; Dmitriev, A.I.; Belkov, S.A.; Sokolov, A.V.; Arkhipov, V.V.; Prokof’ev, A.B. Simultaneous Determination of Metoprolol and Bisoprolol in Human Serum by HPLC-MS/MS for Clinical Drug Monitoring. Pharm. Chem. J. 2018, 51, 1111–1118. [Google Scholar] [CrossRef]
- Srikanth, M.V.; Janaki Ram, B.; Sunil, S.A.; Sreenivasa Rao, N.; Ramana Murthy, K.V. Development and Validation of HPLC Method for Estimation of Propranolol HCL in Human Plasma. J. Sci. Ind. Res. 2012, 71, 120–123. [Google Scholar]
- Zeeb, M.; Mirza, B. Ionic Liquid Phase Microextraction Combined with Fluorescence Spectrometry for Preconcentration and Quantitation of Carvedilol in Pharmaceutical Preparations and Biological Media. DARU J. Pharm. Sci. 2015, 23, 30. [Google Scholar] [CrossRef][Green Version]
- Mahmoudi, A.; Rajabi, M. Selective Determination of Some Beta-Blockers in Urine and Plasma Samples Using Continuous Flow Membrane Microextraction Coupled with High Performance Liquid Chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1128, 121768. [Google Scholar] [CrossRef]
- Talebpour, Z.; Taraji, M.; Adib, N. Stir Bar Sorptive Extraction and High Performance Liquid Chromatographic Determination of Carvedilol in Human Serum Using Two Different Polymeric Phases and an Ionic Liquid as Desorption Solvent. J. Chromatogr. A 2012, 1236, 1–6. [Google Scholar] [CrossRef]
- Itohda, A.; Tsutsumi, K.; Imai, H.; Iwao, M.; Kotegawa, T.; Ohashi, K. Determination of Celiprolol in Human Plasma Using High Performance Liquid Chromatography with Fluorescence Detection for Clinical Application. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 904, 8892. [Google Scholar] [CrossRef]
- Iancu, V.I.; Radu, G.L.; Scutariu, R. A New Analytical Method for the Determination of Beta-Blockers and One Metabolite in the Influents and Effluents of Three Urban Wastewater Treatment Plants. Anal. Methods 2019, 11, 4668–4680. [Google Scholar] [CrossRef]
- Farahmand, F.; Ghasemzadeh, B.; Naseri, A. Air-Assisted Liquid–Liquid Microextraction Using Floating Organic Droplet Solidification for Simultaneous Extraction and Spectrophotometric Determination of Some Drugs in Biological Samples through Chemometrics Methods. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2018, 188, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Ulu, S.T.; Aydoǧmuş, Z. An HPLC Method for the Determination of Bisoprolol in Human Plasma and Its Application to a Pharmacokinetic Study. J. Chromatogr. Sci. 2012, 50, 615–619. [Google Scholar] [PubMed]
- Bengtsson, C.; Johnson, G.; Regdh, C.G. Plasma Levels and Effects of Metoprolol on Blood Pressure and Heart Rate in Hypertensive Patients after an Acute Dose and between Two Doses during Long-Term Treatment. Clin. Pharmacol. Ther. 1975, 17, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Daneshmend, T.K.; Roberts, C.J.C. The influence of food on the oral and intravenous pharmacokinetics of a high clearance drug: A study with labetalol. Br. J. Clin. Pharmacol. 1982, 14, 73–78. [Google Scholar] [CrossRef]
- Gupta, P.K.; Lim, J.K.C.; Zoest, A.R.; Lam, F.C.; Hung, C.T. Relative Bioavailability of Oral Sustained-release and Regular-release Oxprenolol Tablets at Steady-state. Biopharm. Drug Dispos. 1991, 12, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J.; Scavone, J.M.; Harmatz, J.S.; Engelhardt, N.; Shader, R.I. Cognitive Effects of 13 -Adrenergic Antagonists after Single Doses: Pharmacokinetics and Pharmacodynamics of Propranolol, Atenolol, Lorazepam, and Placebo. Clin. Pharm. 1993, 53, 577–584. [Google Scholar]
- Gallegos, A.; Peavy, T.; Dixon, R.; Isseroff, R.R. Development of a Novel Ion-Pairing UPLC Method with Cation-Exchange Solid-Phase Extraction for Determination of Free Timolol in Human Plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1096, 228–235. [Google Scholar] [CrossRef]
- Magiera, S.; Kolanowska, A.; Baranowski, J. Salting-out Assisted Extraction Method Coupled with Hydrophilic Interaction Liquid Chromatography for Determination of Selected β-Blockers and Their Metabolites in Human Urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1022, 93–101. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Jiang, S.; Guo, X. Magnetic Solid-Phase Extraction Based on Magnetic Multiwalled Carbon Nanotubes for the Simultaneous Enantiomeric Analysis of Five β-Blockers in the Environmental Samples by Chiral Liquid Chromatography Coupled with Tandem Mass Spectrometry. Talanta 2018, 180, 98–107. [Google Scholar] [CrossRef]
- Cheng, J.Q.; Liu, T.; Nie, X.M.; Chen, F.M.; Wang, C.S.; Zhang, F. Analysis of 27 β-Blockers and Metabolites in Milk Powder by High Performance Liquid Chromatography Coupled to Quadrupole Orbitrap High-Resolution Mass Spectrometry. Molecules 2019, 24, 820. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Pellegrini, M.; Rotolo, M.C.; Luna, A.; Falcon, M.; Mancini, R. Development and Validation of a Method for the Analysis of Bisoprolol and Atenolol in Human Bone. Molecules 2019, 24, 2400. [Google Scholar] [CrossRef] [PubMed]
- Khedr, A.; Khayyat, A.N.; El-Shorbagi, A.N.A.; Kammoun, A.K. A Sensitive Liquid Chromatography-Tandem Mass Spectrometric Method for Determination of Five β-Blockers after Labeling with Either Hydrazonoyl Chloride or Dansyl Chloride Reagent. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1160, 122383. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, S.; Sellitepe, H.E. Vortex Assisted Liquid-Liquid Microextraction Based on in Situ Formation of a Natural Deep Eutectic Solvent by Microwave Irradiation for the Determination of Beta-Blockers in Water Samples. J. Chromatogr. A 2021, 1642, 462007. [Google Scholar] [CrossRef] [PubMed]
- Ameri Akhtiar Abadi, M.; Masrournia, M.; Abedi, M.R. Simultaneous Extraction and Preconcentration of Three Beta (β)-Blockers in Biological Samples with an Efficient Magnetic Dispersive Micro-Solid Phase Extraction Procedure Employing in Situ Sorbent Modification. Microchem. J. 2021, 163, 105937. [Google Scholar] [CrossRef]
- Jouyban, A.; Ali Farajzadeh, M.; Afshar Mogaddam, M.R.; Khodadadeian, F.; Nemati, M.; Khoubnasabjafari, M. In-Situ Formation of a Hydrophobic Deep Eutectic Solvent Based on Alpha Terpineol and Its Application in Liquid-Liquid Microextraction of Three β-Blockers from Plasma Samples. Microchem. J. 2021, 170, 106687. [Google Scholar] [CrossRef]
- Stoschitzky, K.; Lindner, W. Specific and Nonspecific Effects of Beta Receptor Blockers: Stereoselectively Different Properties Exemplified by (R)- and (S)-Propranolol. Wien. Med. Wochenschr. 1990, 140, 156–162. [Google Scholar]
- Kujawska, M.; Trochimczuk, A.W. Molecularly Imprinted Polymeric Adsorbent for β-Blockers Removal Synthesized Using Functionalized MSU-F Silica as a Sacrificial Template. Sep. Sci. Technol. 2016, 51, 15–16. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Suryana, S.; Mutakin; Rahayu, D. Evaluation Performance of Molecularly Imprinted Polymer Prepared by Two Different Polymerization Method for Atenolol Recognition in Human Plasma. Asian J. Chem. 2017, 29, 2429–2433. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Liu, W.; Holdsworth, C.; Ye, L. Synthesis of Molecularly Imprinted Polymers Using a Functionalized Initiator for Chiral-Selective Recognition of Propranolol. Chirality 2020, 32, 370–377. [Google Scholar] [CrossRef]
- Liu, G.; Huang, X.; Li, L.; Xu, X.; Zhang, Y.; Lv, J.; Xu, D. Recent Advances and Perspectives of Molecularly Imprinted Polymer-Based Fluorescent Sensors in Food and Environment Analysis. Nanomaterials 2019, 9, 1030. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K.; Gokulakrishnan, K.; Prakasam, T. Preparation and Evaluation of Molecularly Imprinted Polymer Liquid Chromatography Column for the Separation of Cathine Enantiomers. Saudi Pharm. J. 2012, 20, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Kou, X.; Lei, J.; Su, H.; Ma, G.; Su, Z. Preparation, Characterization and Adsorption Performance of Molecularly Imprinted Microspheres for Erythromycin Using Suspension Polymerization. J. Chem. Technol. Biotechnol. 2012, 87, 635–642. [Google Scholar] [CrossRef]
- Bakhtiar, S.; Bhawani, S.A.; Shafqat, S.R. Synthesis and Characterization of Molecular Imprinting Polymer for the Removal of 2-Phenylphenol from Spiked Blood Serum and River Water. Chem. Biol. Technol. Agric. 2019, 6, 15. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Wang, J.; Sun, X.; Cao, R.; Sun, H.; Huang, C.; Chen, J. Molecularly Imprinted Polymer Microspheres Prepared by Pickering Emulsion Polymerization for Selective Solid-Phase Extraction of Eight Bisphenols from Human Urine Samples. Anal. Chim. Acta 2015, 872, 35–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, B.; An, F.; Xu, Z.; Zhang, T. Adsorption and Recognition Characteristics of Surface Molecularly Imprinted Polymethacrylic Acid/Silica toward Genistein. J. Chromatogr. A 2014, 1359, 26–34. [Google Scholar] [CrossRef]
- Morante-Zarcero, S.; Sierra, I. Simultaneous Enantiomeric Determination of Propranolol, Metoprolol, Pindolol, and Atenolol in Natural Waters by HPLC on New Polysaccharide-Based Stationary Phase Using a Highly Selective Molecularly Imprinted Polymer Extraction. Chirality 2012, 24, 1359. [Google Scholar] [CrossRef]
- SupelMIP® SPE-Beta-Blockers Bed wt 25 mg, volume 3 mL, pk of 50 | Sigma-Aldrich. Available online: https://www.sigmaaldrich.com/ID/en/product/supelco/53213-u (accessed on 18 April 2022).
- Hasanah, A.N.; Suherman, M.; Susanti, I.; Pitaloka, I.; Mustarichie, R. Performance Evaluation of Atenolol Molecular Imprinted Polymer Using Two Different Polymerization and Two Different Porogen. Rasayan J. Chem. 2019, 12, 1269–1278. [Google Scholar] [CrossRef]
- Yan, H.; Kyung, H.R. Characteristic and Synthetic Approach of Molecularly Imprinted Polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Pratiwi, R.; Megantara, S.; Rahayu, D.; Pitaloka, I.; Hasanah, A.N. Comparison of Bulk and Precipitation Polymerization Method of Synthesis Molecular Imprinted Solid Phase Extraction for Atenolol Using Methacrylic Acid. J. Young Pharm. 2019, 11, 12–16. [Google Scholar] [CrossRef]
- Gorbani, Y.; Yılmaz, H.; Basan, H. Spectrofluorimetric Determination of Atenolol from Human Urine Using High-Affinity Molecularly Imprinted Solid-Phase Extraction Sorbent. Luminescence 2017, 32, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.T.M.; de Oliveira, H.L.; Silva, C.F.; Fonseca, M.C.; Pereira, T.F.D.; Nascimento, C.S.; de Figueiredo, E.C.; Borges, K.B. Efficient Molecularly Imprinted Polymer as a Pipette-Tip Solid-Phase Sorbent for Determination of Carvedilol Enantiomers in Human Urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061–1062, 399–410. [Google Scholar] [CrossRef]
- Tadi, K.K.; Motghare, R.V. Rational Synthesis of Pindolol Imprinted Polymer by Non-Covalent Protocol Based on Computational Approach. J. Mol. Model. 2013, 19, 3385–3396. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Karimi, M. Synthesis and Application of Molecularly Imprinted Polymer for Highly Selective Solid Phase Extraction Trace Amount of Sotalol from Human Urine Samples: Optimization by Central Composite Design (CCD). Med. Chem. Res. 2017, 26, 2477–2490. [Google Scholar] [CrossRef]
- Zhang, W.; She, X.; Wang, L.; Fan, H.; Zhou, Q.; Huang, X.; Tang, J.Z. Preparation, Characterization and Application of a Molecularly Imprinted Polymer for Selective Recognition of Sulpiride. Materials 2017, 10, 475. [Google Scholar] [CrossRef]
- Lafarge, C.; Bitar, M.; El Hosry, L.; Cayot, P.; Bou-Maroun, E. Comparison of Molecularly Imprinted Polymers (MIP) and Sol–Gel Molecularly Imprinted Silica (MIS) for Fungicide in a Hydro Alcoholic Solution. Mater. Today Commun. 2020, 24, 101157. [Google Scholar] [CrossRef]
- Gryshchenko, A.O.; Bottaro, C.S. Development of Molecularly Imprinted Polymer in Porous Film Format for Binding of Phenol and Alkylphenols from Water. Int. J. Mol. Sci. 2014, 15, 1338–1357. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Rahayu, D.; Pratiwi, R.; Rostinawati, T.; Megantara, S.; Saputri, F.A.; Puspanegara, K.H. Extraction of Atenolol from Spiked Blood Serum Using a Molecularly Imprinted Polymer Sorbent Obtained by Precipitation Polymerization. Heliyon 2019, 5, 01533. [Google Scholar] [CrossRef]
- Du, T.; Cheng, J.; Wu, M.; Wang, X.; Zhou, H.; Cheng, M. Pipette Tip-Based Molecularly Imprinted Monolith for Selective Micro-Solid-Phase Extraction of Methomyl in Environmental Water. Anal. Methods 2014, 6, 6375–6380. [Google Scholar] [CrossRef]
- Santos Da Silva, R.C.; Mano, V.; Pereira, A.C.; Costa De Figueiredo, E.; Borges, K.B. Development of Pipette Tip-Based on Molecularly Imprinted Polymer Micro-Solid Phase Extraction for Selective Enantioselective Determination of (−)-(2: S,4 R) and (+)-(2 R,4 S) Ketoconazole in Human Urine Samples Prior to HPLC-DAD. Anal. Methods 2016, 8, 4075–4085. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Yamazaki, T.; Chronakis, I.S.; Ye, L. Influence of Template/Functional Monomer/Cross-Linking Monomer Ratio on Particle Size and Binding Properties of Molecularly Imprinted Nanoparticles. J. Appl. Polym. Sci. 2012, 124, 1249–1255. [Google Scholar] [CrossRef]
- Fan, W.; He, M.; You, L.; Zhu, X.; Chen, B.; Hu, B. Water-Compatible Graphene Oxide/Molecularly Imprinted Polymer Coated Stir Bar Sorptive Extraction of Propranolol from Urine Samples Followed by High Performance Liquid Chromatography-Ultraviolet Detection. J. Chromatogr. A 2016, 1443, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sanbe, H.; Haginaka, J. Restricted Access Media-Molecularly Imprinted Polymer for Propranolol and Its Application to Direct Injection Analysis of β-Blockers in Biological Fluids. Analyst 2003, 128, 593–597. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Isac Moraes, G.; Da Silva, L.M.R.; Dos Santos-Neto, Á.J.; Florenzano, F.H.; Figueiredo, E.C. A New Restricted Access Molecularly Imprinted Polymer Capped with Albumin for Direct Extraction of Drugs from Biological Matrices: The Case of Chlorpromazine in Human Plasma. Anal. Bioanal. Chem. 2013, 405, 7687–7696. [Google Scholar] [CrossRef]
- Abrão, L.C.C.; Maia, P.P.; Figueiredo, E.C. Determination of Tetracyclines by Solid-Phase Extraction with a Molecularly Imprinted Polymer and High-Performance Liquid Chromatography. Anal. Lett. 2014, 47, 2183–2194. [Google Scholar] [CrossRef]
- Santos, M.G.; Tavares, I.M.C.; Boralli, V.B.; Figueiredo, E.C. Direct Doping Analysis of Beta-Blocker Drugs from Urinary Samples by on-Line Molecularly Imprinted Solid-Phase Extraction Coupled to Liquid Chromatography/Mass Spectrometry. Analyst 2015, 140, 2696–2703. [Google Scholar] [CrossRef]
- Babooram, K. Brief Overview of Polymer Science. In Polymer Science and Nanotechnology; Elsevier: New York, NY, USA, 2020; pp. 3–10. [Google Scholar]
- Viltres-Portales, M.; Alberto, M.D.L.; Ye, L. Synthesis of Molecularly Imprinted Polymers Using an Amidine-Functionalized Initiator for Carboxylic Acid Recognition. React. Funct. Polym. 2021, 165, 104969. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Tristi, J.; Rahayu, D. Atenolol Imprinted Polymer with Butanol as a Porogenic Solvent: Using Two Polymerization Methods. Rasayan J. Chem. 2020, 13, 1313–1320. [Google Scholar] [CrossRef]
- Wei, S.; Molinelli, A.; Mizaikoff, B. Molecularly Imprinted Micro and Nanospheres for the Selective Recognition of 17β-Estradiol. Biosens. Bioelectron. 2006, 21, 1943–1951. [Google Scholar] [CrossRef]
- Verheyen, E.; Schillemans, J.P.; Van Wijk, M.; Demeniex, M.A.; Hennink, W.E.; Van Nostrum, C.F. Challenges for the Effective Molecular Imprinting of Proteins. Biomaterials. 2011, 32, 3008–3020. [Google Scholar] [CrossRef]
- Lu, C.H.; Zhou, W.H.; Han, B.; Yang, H.H.; Chen, X.; Wang, X.R. Surface-Imprinted Core-Shell Nanoparticles for Sorbent Assays. Anal. Chem. 2007, 79, 5457–5461. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Wang, N.; Trant, T.; Yuan, L.; Li, J.; Cai, Q. Surface Molecular Imprinting on Dye-(NH2)-SiO2 NPs for Specific Recognition and Direct Fluorescent Quantification of Perfluorooctane Sulfonate. Sens. Actuators B Chem. 2014, 195, 266–273. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, F.; Zeng, B. Synthesis of Water-Compatible Surface-Imprinted Polymer via Click Chemistry and RAFT Precipitation Polymerization for Highly Selective and Sensitive Electrochemical Assay of Fenitrothion. Biosens. Bioelectron. 2014, 62, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Luo, Z.; Ge, Y.; Guo, P.; Du, K.; Tang, W.; Du, W.; Zeng, A.; Chang, C.; Fu, Q. A Novel Surface Molecularly Imprinted Polymer as the Solid-Phase Extraction Adsorbent for the Selective Determination of Ampicillin Sodium in Milk and Blood Samples. J. Pharm. Anal. 2016, 6, 157–164. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.; Yu, P.; Chen, X. Preparation and Characteristics of Protein Molecularly Imprinted Membranes on the Surface of Multiwalled Carbon Nanotubes. Talanta 2010, 81, 162–166. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, Z.; Li, S.; Lu, Y.; Hu, Y.; Fan, H.; Zhai, X. Preparation of Magnetic Molecularly Imprinted Polymers for Selective Recognition and Determination of Clenbuterol in Pork Samples. J. Chem. 2020, 2020, 8820262. [Google Scholar] [CrossRef]
- Chang, L.; Ding, Y.; Li, X. Surface Molecular Imprinting onto Silver Microspheres for Surface Enhanced Raman Scattering Applications. Biosens. Bioelectron. 2013, 50, 106–110. [Google Scholar] [CrossRef]
- Ansari, S.; Masoum, S. A Multi-Walled Carbon Nanotube-Based Magnetic Molecularly Imprinted Polymer as a Highly Selective Sorbent for Ultrasonic-Assisted Dispersive Solid-Phase Microextraction of Sotalol in Biological Fluids. Analyst 2018, 143, 2862–2875. [Google Scholar] [CrossRef]
- Deng, X.; Li, W.; Wang, Y.; Ding, G. Recognition and Separation of Enantiomers Based on Functionalized Magnetic Nanomaterials. TrAC-Trends Anal. Chem. 2020, 124, 115804. [Google Scholar] [CrossRef]
- Li, X.S.; Zhu, G.T.; Luo, Y.B.; Yuan, B.F.; Feng, Y.Q. Synthesis and Applications of Functionalized Magnetic Materials in Sample Preparation. TrAC-Trends Anal. Chem. 2013, 45, 233–247. [Google Scholar] [CrossRef]
- Díaz-Faes López, T.; Díaz-García, M.E.; Badía-Laíño, R. Molecularly Imprinted Silica-Silver Nanowires for Tryptophan Recognition. Nanotechnology 2014, 25, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xie, L.; Guo, J.; Li, H.; Jiang, X.; Zhang, Y.; Shi, S. Hydrophilic Gallic Acid-Imprinted Polymers over Magnetic Mesoporous Silica Microspheres with Excellent Molecular Recognition Ability in Aqueous Fruit Juices. Food Chem. 2015, 49, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luo, L.; Cai, R.; Chen, H. A Sensitive and Selective Molecularly Imprinted Sensor Combined with Magnetic Molecularly Imprinted Solid Phase Extraction for Determination of Dibutyl Phthalate. Biosens. Bioelectron. 2013, 49, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Azodi-Deilami, S.; Abdouss, M.; Asadi, E.; Hassani Najafabadi, A.; Sadeghi, S.; Farzaneh, S.; Asadi, S. Magnetic Molecularly Imprinted Polymer Nanoparticles Coupled with High Performance Liquid Chromatography for Solid-Phase Extraction of Carvedilol in Serum Samples. J. Appl. Polym. Sci. 2014, 131, 1–10. [Google Scholar] [CrossRef]
- Renkecz, T.; Ceolin, G.; Horváth, V. Selective Solid Phase Extraction of Propranolol on Multiwell Membrane Filter Plates Modified with Molecularly Imprinted Polymer. Analyst 2011, 136, 2175–2182. [Google Scholar] [CrossRef]
- Andersson, L.I.; Paprica, A.; Arvidsson, T. A Highly Selective Solid Phase Extraction Sorbent for Preconcentration of Sameridine Made by Molecular Imprinting. Chromatographia 1997, 46, 57–62. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Maelaningsih, F.S.; Apriliandi, F.; Sabarudin, A. Synthesis and Characterisation of a Monolithic Imprinted Column Using a Methacrylic Acid Monomer with Porogen Propanol for Atenolol Analysis. J. Anal. Methods Chem. 2020, 2020, 3027618. [Google Scholar] [CrossRef]
- Zhang, Q.; Fu, Q.; Amut, E.; Fang, Q.; Zeng, A.; Chang, C. Preparation and Evaluation of Propranolol-Imprinted Monolithic Stationary Phase by in Situ Technique and Application in Analysis of Propranolol in Biological Samples. Anal. Lett. 2009, 42, 535–554. [Google Scholar] [CrossRef]
- Annisa, D.; Tasfiyati, A.N.; Sulistyarti, H.; Sabarudin, A. Production of Organic Polymer Based Monolithic Columns for Anion Separation Using High Performance Liquid Chromatography. Nat. B J. Health Environ. Sci. 2015, 3, 008–016. [Google Scholar] [CrossRef][Green Version]
- ALOthman, Z.A. Preparation and Characterization of Alkyl Methacrylate Capillary Monolithic Columns. J. Saudi Chem. Soc. 2012, 16, 271–278. [Google Scholar] [CrossRef]
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, M.M. Promoting Green Engineering through Green Chemistry. Environ. Sci. Technol. 2003, 37, 5349–5353. [Google Scholar] [CrossRef] [PubMed]
| Name of Drug | Doses (mg) | Cmax (ng/mL) | Tmax (h) | Ref |
|---|---|---|---|---|
| Atenolol | 100 | 537.1 + 112.7 | 3.4 + 1.0 | [23] |
| Carvedilol | 8 | 6.93 | 5.98 | [24] |
| 128 | 77.94 | 6.02 | ||
| Bisoprolol | 5 | 31 | 3 | [35] |
| Metoprolol | 80 | 100 | 1 | [36] |
| Labetalol | 200 | 182 ± 57 (fasting state); 180 ± 33 (after food) | 1.42 ± 0.28 (fasting state); 2.08 ± 0.15 (after food) | [37] |
| Oxprenolol | 80 | 22.5 | 1.21 | [38] |
| Propranolol | 40 | 24.9 | 2.1 | [39] |
| Analyte | Sample | Extraction Method | Method | Linearities | LOD and LOQ | Ref |
|---|---|---|---|---|---|---|
| Atenolol | Plasma | LLE | HPLC with fluorescence detector | 10–1000 ng/mL | NM | [25] |
| Atenolol | Plasma | LLE | HPLC with fluorescence detector | 10–1000 ng/mL | NM | [23] |
| Atenolol | Urine | LLE | HPLC with fluorescence detector | 5–150 ng/mL | 1.5 ng/mL; 5.0 ng/mL | [26] |
| Propranolol | Plasma | Protein precipitation | HPLC with DAD detector | 20–280 ng/mL | NM | [28] |
| Metoprolol | Serum | Protein precipitation | HPLC-MS/MS | 5–250 ng/mL | NM | [27] |
| Bisoprolol | 1–250 ng/mL | |||||
| Metoprolol | Urine and plasma | Continuous flow membrane microextraction | HPLC | 5–700 µg/mL | 1.0 ng/mL (LOD) | [30] |
| Propranolol | 3–1000 µg/mL | 0.5 ng/mL (LOD) | ||||
| Carvedilol | Urine, plasma, and tablet | Ionic liquid microextraction | Spectrofluorometer | 0–250 μg/L | 1.7 μg/L (LOD) | [29] |
| Celiprolol | Plasma | SPE | HPLC with fluorescence detector | 1–1000 ng/L | NM | [32] |
| Carvedilol | Serum | Stir bar sorptive extraction | HPLC with UV detector | 1.0–120.0 ng mL | 0.3 and 1.0 ng/mL | [31] |
| 23 compounds of β-Blockers | Animal food | SPE coupled with a clean-up step using methanol | HPLC coupled with linear ion trap mass spectrometry | 5–200 μg/L | NM | [22] |
| Bisoprolol | Wastewater treatment plants | SPE | Liquid chromatography coupled with mass spectrometry (LC-MS/MS) | 1–100 ng/mL | 0.34–7.37 ng/L (LOQ) | [33] |
| Nadolol | ||||||
| Betaxolol | ||||||
| Atenolol | ||||||
| Propranolol | ||||||
| Pindolol | ||||||
| Atenolol | Urine and plasma | AALLME using floating organic droplet solidification | UV-Vis spectrophotometry | 0.30–6.00 μg/mL | 0.30 μg/mL (LOQ) | [34] |
| Propranolol | 0.30–1.40 μg/mL | 0.26 μg/mL (LOQ) | ||||
| Carvedilol | 0.30–2.00 μg/mL | 0.30 μg/mL (LOQ) | ||||
| Timolol | Plasma | Cation-exchange SPE | Ion-pairing UPLC | 5–300 ng/mL | 1.7 ng/mL (LLOD); 5.0 ng/mL (LLOQ) | [40] |
| Metoprolol | Urine | A salting-out assisted liquid–liquid extraction (SALLE) | Hydrophilic interaction liquid chromatography-ultraviolet detection (HILIC-UV) | 0.2–8.0 µg/mL | NM | [41] |
| Propranolol | 0.1–4.0 µg/mL | |||||
| Carvedilol | 0.1–4.0 µg/mL | |||||
| 5-hydroxy carvedilol | 0.2–8.0 µg/mL | |||||
| O-desmethyl carvedilol | 0.1–4.0 µg/mL | |||||
| α-hydroxy metoprolol | 0.2–8.0 µg/mL | |||||
| O-desmethyl metoprolol | 0.2–8.0 µg/mL | |||||
| 5-hydroxy propranolol | 0.1–4.0 µg/mL | |||||
| Atenolol, metoprolol, esmolol, pindolol, and arotinolol | River water, influent wastewater (IWW), and effluent wastewater (EWW) | Magnetic solid phase extraction (MSPE) | Chiral LC-MS/MS | 5–500 ng/mL | 0.50–1.45 ng/L, 1.63–3.75 ng/L | [42] |
| 21 β-blockers and 6 metabolites | Milk powder | Extracted using acetonitrile and purified with SPE | HPLC coupled with quadrupole orbitrap high-resolution mass spectrometry (HPLC-Q-Orbitrap HRMS) | 0.5–500 µg/kg | 0.2–1.5 µg/kg (LOD), 0.5–5.0 µg/kg (LOQ) | [43] |
| Atenolol | Human bone | SPE | Gas chromatography–mass spectrometry | 0.1–150 ng/mg | 0.1 ng/mL (LOD) | [44] |
| Bisoprolol | 0–15 ng/mg | 0.3 ng/mL (LOD) | ||||
| Atenolol | Rabbit plasma | SPE | Derivatization with hydrazonoyl chloride compound (UOSA54), determined using liquid chromatography–tandem mass spectrometry (LC-MS) | 0.2–20.0 ng/mL | 0.08 ng/mL, 0.20 ng/mL | [45] |
| Metoprolol | ||||||
| Bisoprolol | 0.2–18.0 ng/mL | 0.05 ng/mL, 0.20 ng/mL | ||||
| Propranolol | 0.1–15.0 ng/mL | 0.03 ng/mL, 0.10 ng/mL | ||||
| Betaxolol | 0.2–25.0 ng/mL | 0.06 ng/mL, 0.25 ng/mL | ||||
| Metoprolol | Water | Vortex-assisted liquid–liquid microextraction based on in situ formation of a novel hydrophobic natural deep eutectic solvent (NADES-VA-LLME) | HPLC | 1–100 μg/L | 0.2 μg/L, 0.6 μg/L | [46] |
| Metoprolol | Plasma and urine | Magnetic dispersive micro-solid phase extraction | HPLC | 5–10,000 ng/mL | 0.8 ng/mL; 5 ng/mL | [47] |
| Atenolol | 50–5000 ng/mL | 10 ng/mL; 50 ng/mL | ||||
| Propranolol | 10–5000 ng/mL | 2 ng/mL; 10 ng/mL | ||||
| Atenolol | Plasma | LLME using a hydrophobic deep eutectic solvent | Gas chromatography-mass spectrometry (GC-MS) | 0.064–5000 ng/mL | 0.195 ng/mL, 0.645 ng/mL | [48] |
| Propranolol | 0.043–5000 ng/mL | 0.130 ng/mL, 0.435 ng/mL | ||||
| Metoprolol | 0.069–5000 ng/mL | 0.205 ng/mL, 0.692 ng/mL |
| Beta-Blocker Drug | Synthesis Method |
|---|---|
| Atenolol | Bulk polymerization |
| Precipitation polymerization | |
| Carvedilol | Bulk polymerization |
| Surface imprinted polymerization: magnetic molecularly imprinted polymer (MMIP) | |
| Pindolol | Bulk polymerization |
| Sotalol | Bulk polymerization |
| Surface imprinted polymerization: multiwalled carbon nanotubes based magnetic molecularly imprinted polymer (MWCNT-MMIP) | |
| Propranolol | Precipitation polymerization |
| In situ polymerization: thin layer MIPs in multiwell membrane filter plates | |
| In situ polymerization: graphene oxide (GO)/MIP coated stir bar sorbent | |
| Monolithic imprinted polymerization | |
| Oxprenolol | Precipitation polymerization |
| Template | Monomer | Cross-Linker | Porogenic Solvent | Initiator | Q (mg/g) | IF | % Recovery | Application | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Atenolol | Methacrylic acid | EGDMA | Butanol | Benzoyl peroxide | 7.804 | 2.87 ± 0.2 | NM | NM | [64] |
| Atenolol | Methacrylic acid | EGDMA | Propanol | Benzoyl peroxide | 0.1043 | 2.872 | 66.54% | Extraction of atenolol in serum sample | [62] |
| Butanol | 7.804 | 2.868 | 32.22% | ||||||
| Atenolol | Acrylic acid | EGDMA | Dichloro- ethane | Benzoyl peroxide | 3.77 | 4.18 | 74.5–75.1% | Selective removal of atenolol in a human urine sample | [65] |
| Carvedilol | Methacrylic acid | EGDMA | Chloroform | 4,4′-Azobis(4-cyanovaleric acid) | NM | NM | Around 100% | Used as adsorbent of PT-MIP-MS to extract carvedilol enantiomer in human urine | [66] |
| Pindolol | Itaconic acid | EGDMA | Acetonitrile | AIBN | 125.76 * | 2.27 | NM | NM | [67] |
| 4-vinyl pyridine | 9.93 * | 1.89 | |||||||
| Acrylonitrile | 56.732 * | 1.12 | |||||||
| Sotalol | Acrylamide | EGDMA | Dimethylformamide | AIBN | 20.08 | NM | 97.4–102.5 | Used as SPE sorbent for extraction of sotalol in urine sample | [68] |
| Template | Monomer | Cross-Linker | Initiator | Porogenic Solvent | Q (mg/g) | IF | % Recovery | Application | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Atenolol | Itaconic acid | EGDMA | Benzoyl peroxide | Methanol: acetonitrile | 4.250 | 11.02 (sample spiked with atenolol) 23.43 (sample spiked with mixed β-blocker) | 93.65 ± 1.29% | Extraction of atenolol in serum sample | [72] |
| Itaconic acid | Methanol | 0.269 | NM | NM | |||||
| Atenolol | Methacrylic acid | EGDMA | Benzoyl peroxide | Propanol | 0.0804 | 11.721 | 74.64% | Extraction of atenolol in serum sample | [62] |
| Butanol | 2.950 | 4.160 | 10.86% | ||||||
| Atenolol | Methacrylic acid | EGDMA | Benzoyl peroxide | Butanol | 2.950 | 4.16 ± 2.1 | NM | NM | [64] |
| Atenolol | Methyl methacrylate | EGDMA | Benzoyl peroxide | Butanol | 2.166 | 5.967 | NM | NM | [83] |
| Oxprenolol | Methacrylic acid | EGDMA | AIBN | Acetonitrile | 82.6 | NM | NM | Online MIP-SPE couple liquid chromatography and spectrometry conditions | [80] |
| (R,S) Propranolol | 4,4′-Azobis(4-cyanovaleric) acid (functionalized initiator) | Trimethylolpropane trimethacrylate (TRIM) | Acetonitrile | 25.51 | NM | NM | Not mentioned in article, may be used to separate the chiral molecule in pharmaceutical product or others | [53] | |
| (S)-Propranolol | 2.03 | ||||||||
| Template | Method | M | C | P | I | Q (mg/g) | IF | Ref |
|---|---|---|---|---|---|---|---|---|
| Atenolol | Bulk | Methacrylic acid | EGDMA | Butanol | Benzoyl peroxide | 7.804 | 2.87 ± 0.2 | [64] |
| Precipitation | 2.950 | 4.16 ± 2.1 | ||||||
| Atenolol | Bulk | Methacrylic acid | EGDMA | Butanol | Benzoyl peroxide | 7.804 | 2.868 | [62] |
| Precipitation | 2.950 | 4.160 | ||||||
| Atenolol | Bulk | Methacrylic acid | EGDMA | Propanol | Benzoyl peroxide | 0.1043 | 2.872 | |
| Precipitation | 0.0804 | 11.721 |
| Polymerization Methods | Advantages | Disadvantages |
|---|---|---|
| Bulk polymerization |
|
|
| Precipitation polymerization |
|
|
| Surface imprinted polymerization |
|
|
| In situ polymerization |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanah, A.N.; Susanti, I.; Mutakin, M. An Update on the Use of Molecularly Imprinted Polymers in Beta-Blocker Drug Analysis as a Selective Separation Method in Biological and Environmental Analysis. Molecules 2022, 27, 2880. https://doi.org/10.3390/molecules27092880
Hasanah AN, Susanti I, Mutakin M. An Update on the Use of Molecularly Imprinted Polymers in Beta-Blocker Drug Analysis as a Selective Separation Method in Biological and Environmental Analysis. Molecules. 2022; 27(9):2880. https://doi.org/10.3390/molecules27092880
Chicago/Turabian StyleHasanah, Aliya Nur, Ike Susanti, and Mutakin Mutakin. 2022. "An Update on the Use of Molecularly Imprinted Polymers in Beta-Blocker Drug Analysis as a Selective Separation Method in Biological and Environmental Analysis" Molecules 27, no. 9: 2880. https://doi.org/10.3390/molecules27092880
APA StyleHasanah, A. N., Susanti, I., & Mutakin, M. (2022). An Update on the Use of Molecularly Imprinted Polymers in Beta-Blocker Drug Analysis as a Selective Separation Method in Biological and Environmental Analysis. Molecules, 27(9), 2880. https://doi.org/10.3390/molecules27092880






