One-Step Route to Fe2O3 and FeSe2 Nanoparticles Loaded on Carbon-Sheet for Lithium Storage
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of the Fe2O3@C Composite (Developed by Us)
2.3. Synthesis of the FeSe2 @C Composite (Developed by Us)
2.4. Materials Characterization
2.5. Electrochemical Measurement
3. Results and Discussions
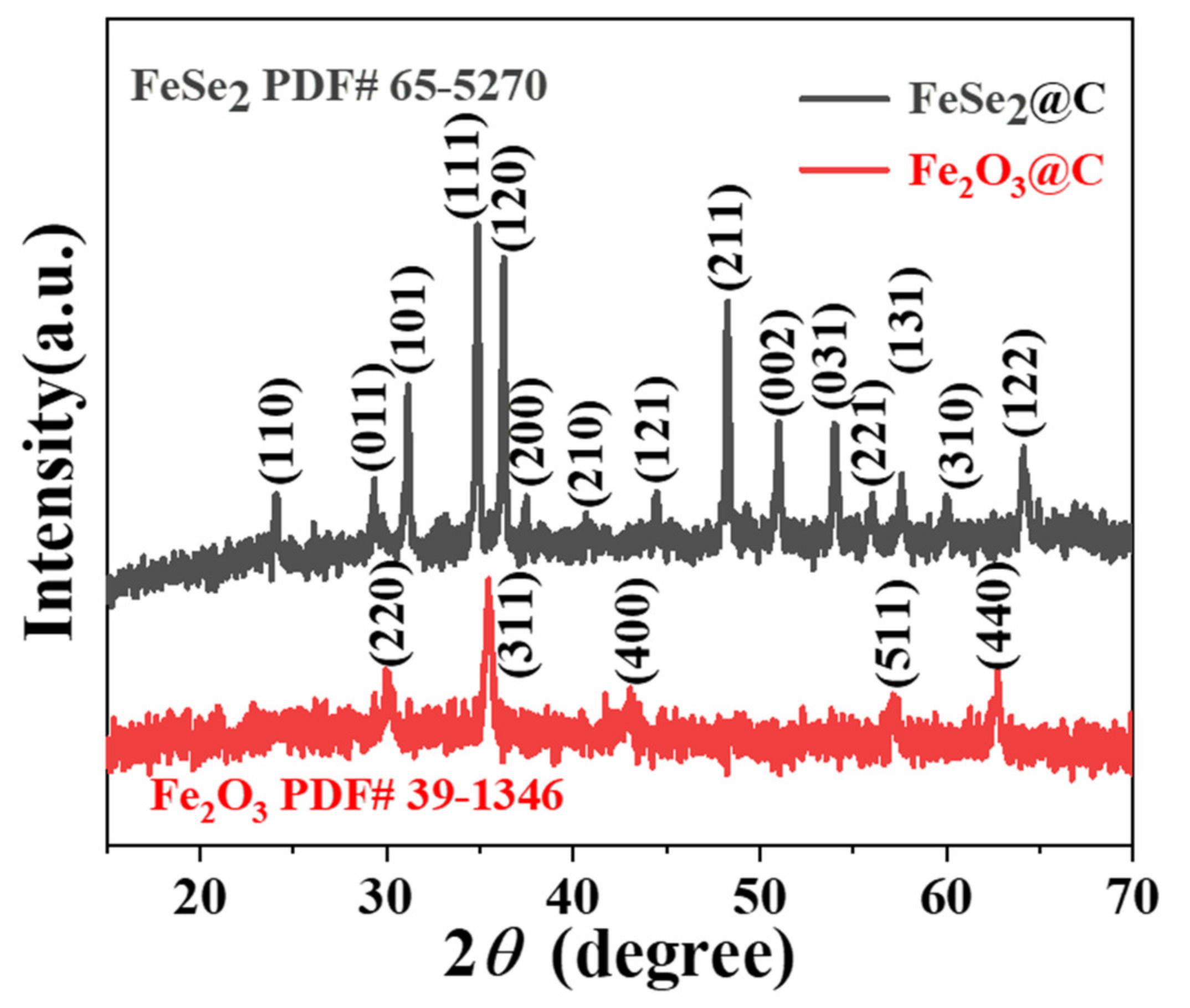
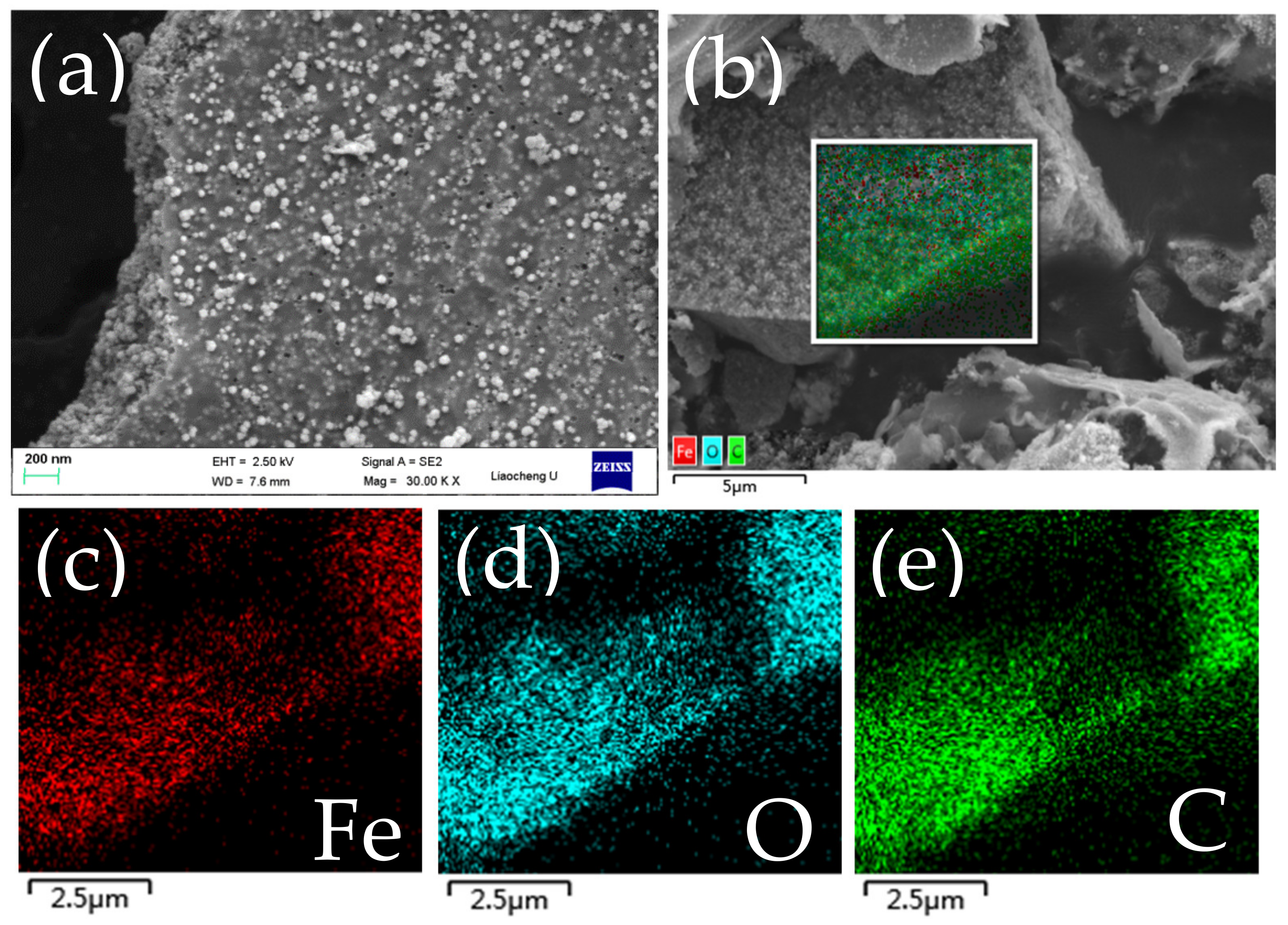
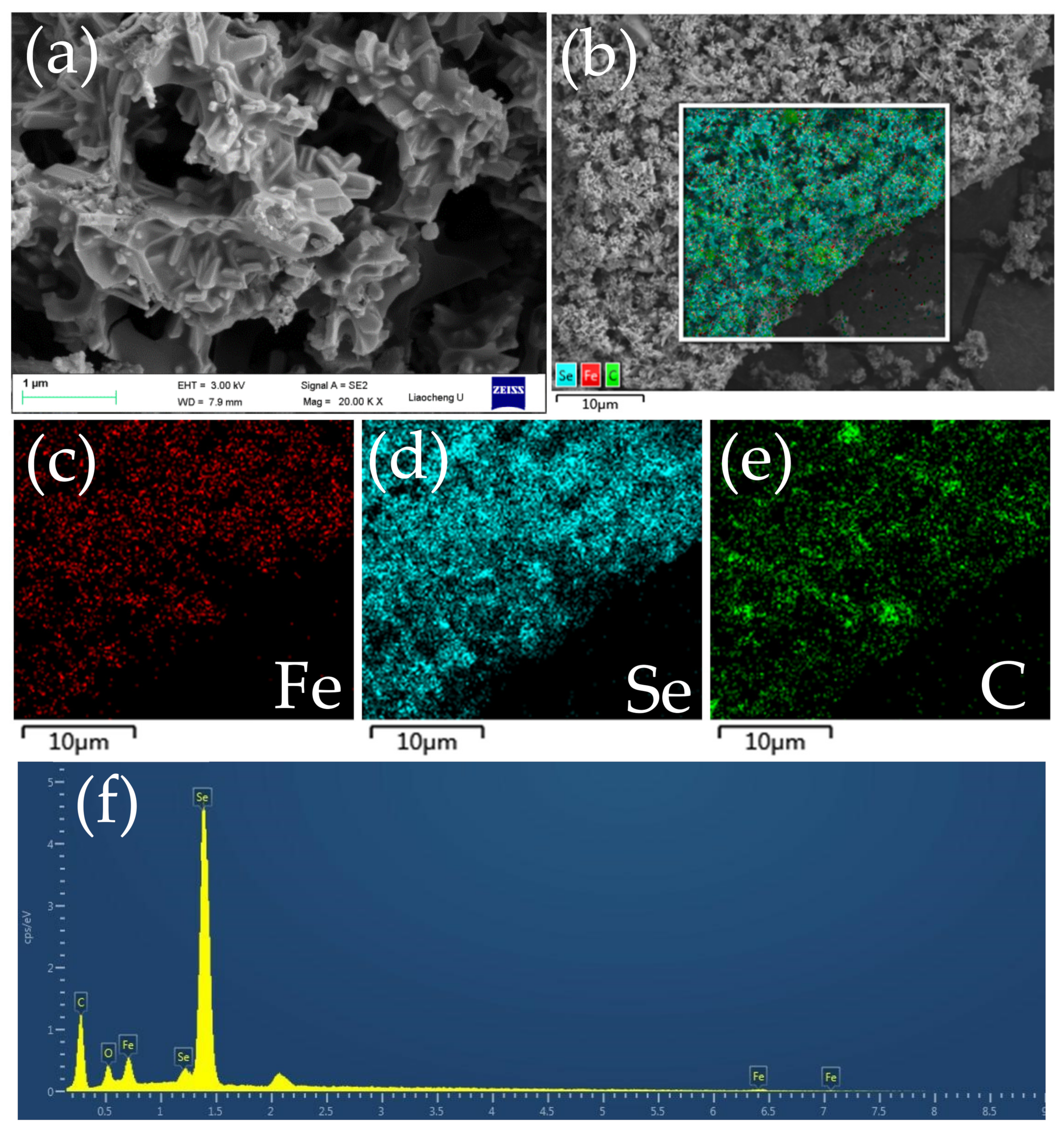
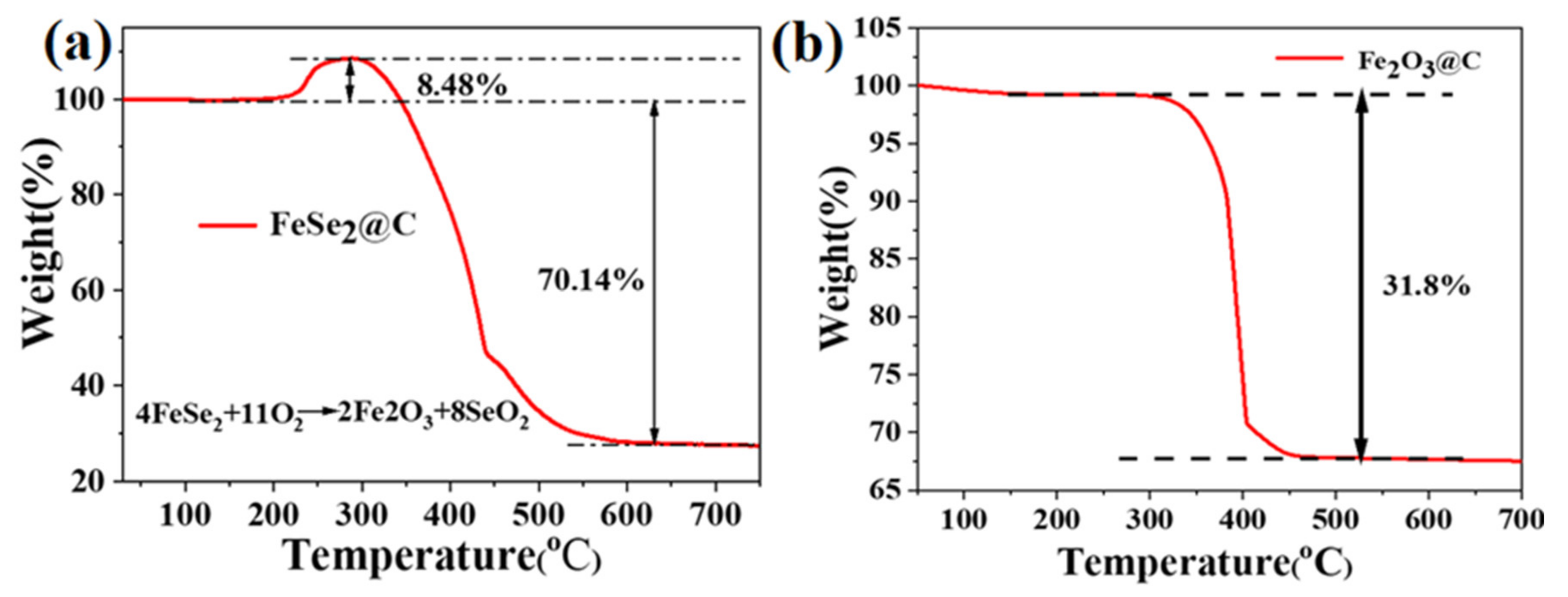
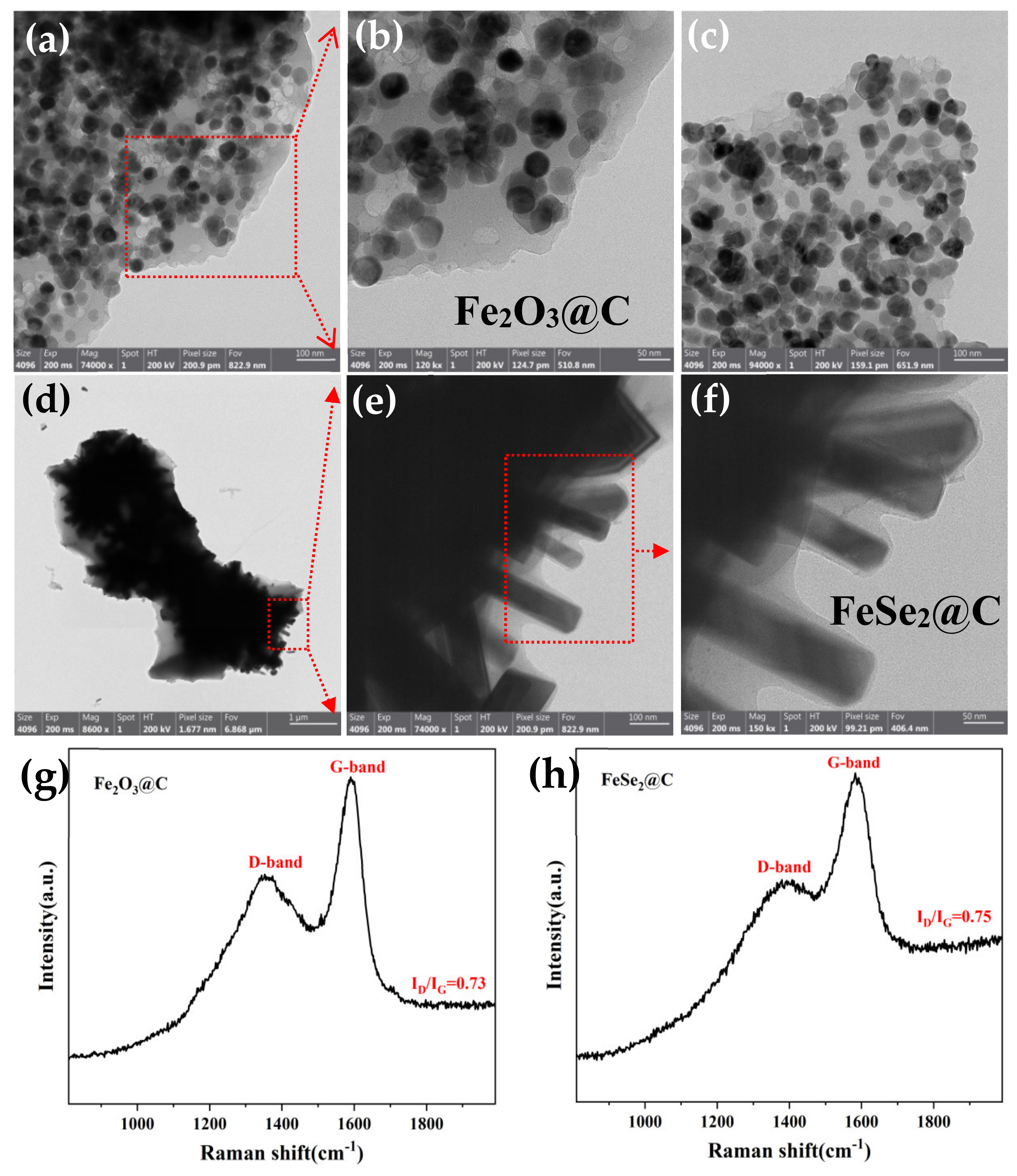
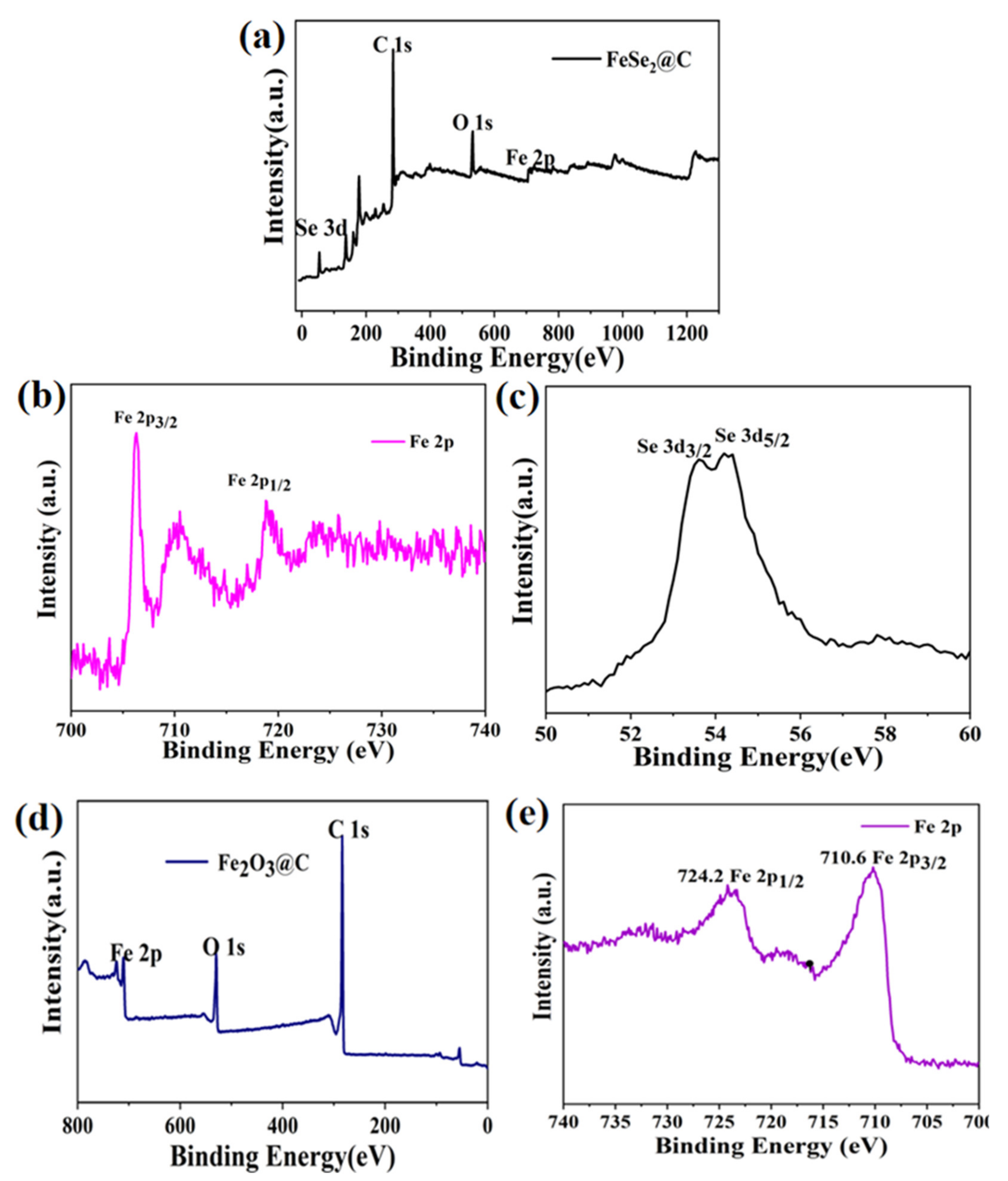
3.1. Composition and Microstructures of the Samples
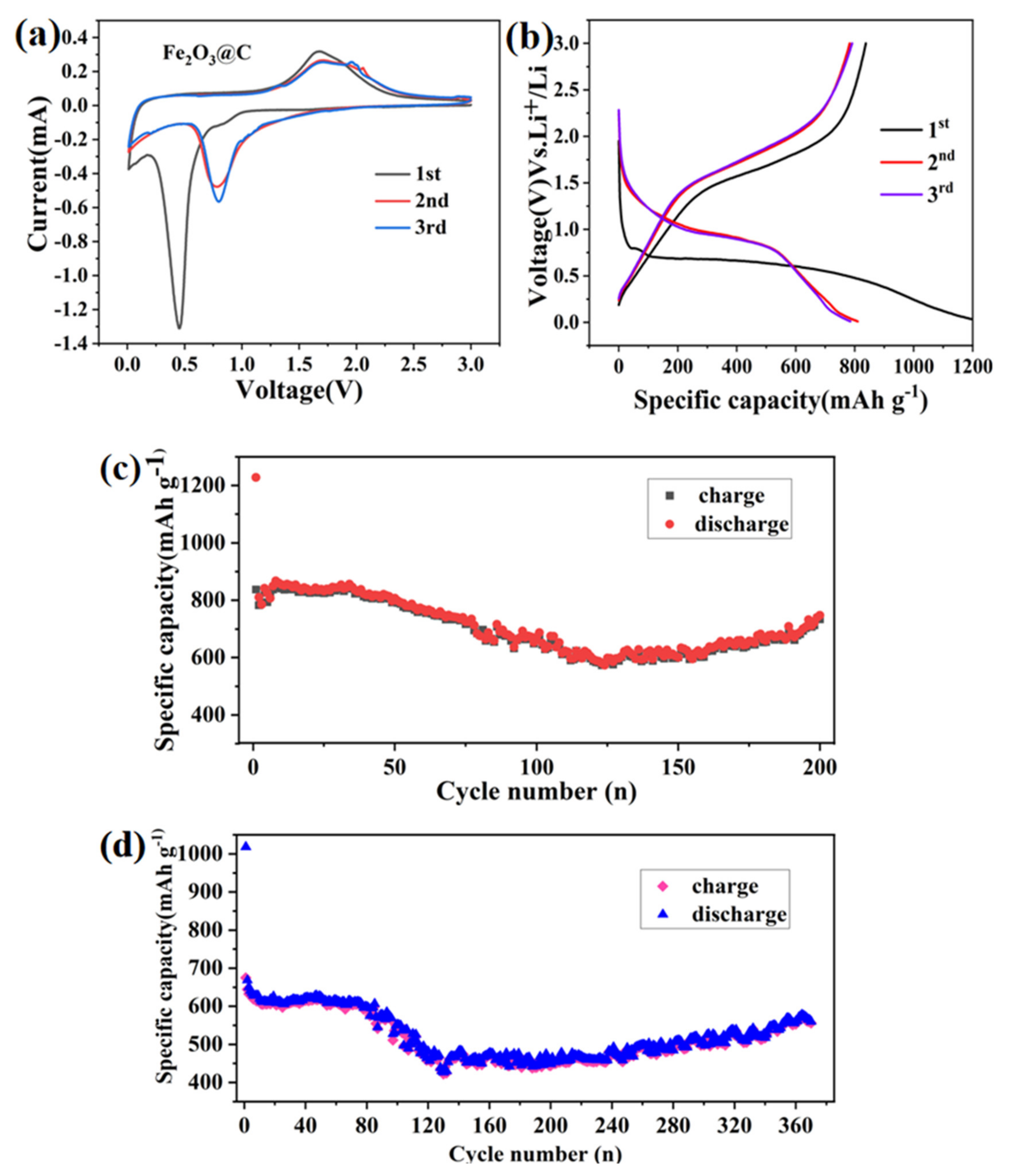
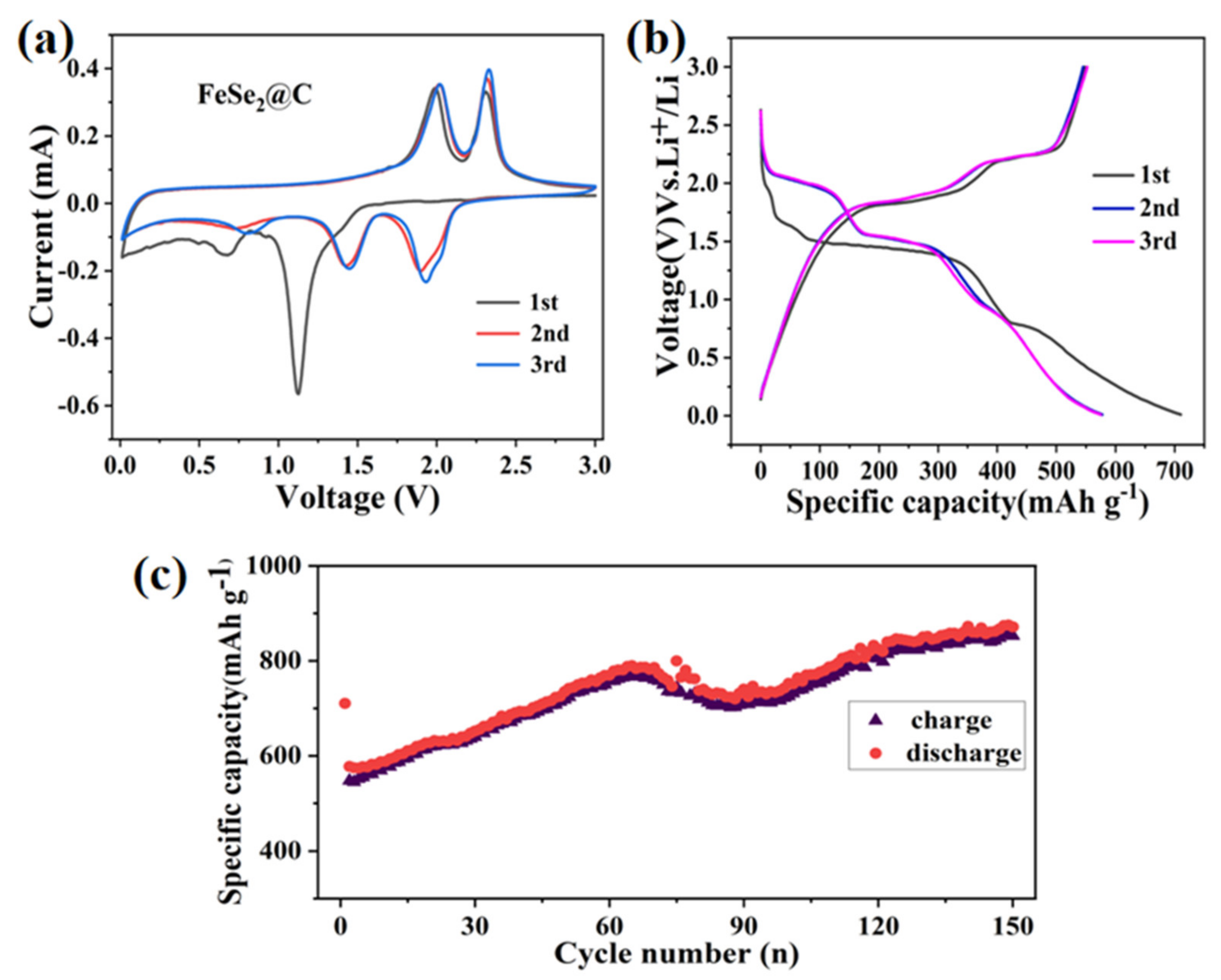
3.2. Electrochemical Property in Half-Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Feng, J.; Ru, J.; Feng, Y.; Lian, R.; Yang, J. Constructing Heterointerface of Metal Atomic Layer and Amorphous Anode Material for High-Capacity and Fast Lithium Storage. ACS Nano 2019, 13, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, Y.-Z.; Zhang, F.; Li, Q.; Anjum, D.H.; Liang, H.; Liu, Y.; Liu, C.-S.; Alshareef, H.N.; Pang, H. A novel strategy for the synthesis of highly stable ternary SiOx composites for Li-ion-battery anodes. J. Mater. Chem. A 2019, 7, 15969–15974. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, P.; Chen, M.; Jin, H.; Bai, Y.; Li, S.; Ma, F.; Xu, H.; Lian, K. In Situ Synthesis of Multilayer Carbon Matrix Decorated with Copper Particles: Enhancing the Performance of Si as Anode for Li-Ion Batteries. ACS Nano 2019, 13, 3054–3062. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Ge, P.; Hou, H.; Li, S.; Yang, L.; Ji, X. Tailoring Rod-Like FeSe2 Coated with Nitrogen-Doped Carbon for High-Performance Sodium Storage. Adv. Funct. Mater. 2018, 28, 1801765. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.; Ding, Z.; Lee, M.H.; Lim, K.; Yoon, G.; Kang, K. Recent Progress in Electrode Materials for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600943. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Lv, L.; Gu, Y.; Tao, S.; Jiang, X.; Qian, B.; Gao, L. Nano-sized FeSe2 anchored on reduced graphene oxide as a promising anode material for lithium-ion and sodium-ion batteries. J. Mater. Sci. 2019, 54, 4225–4235. [Google Scholar] [CrossRef]
- Bai, J.; Wu, H.; Wang, S.; Zhang, G.; Feng, C. Synthesis and Electrochemical Performances of FeSe2/C as Anode Material for Lithium-ion Batteries. J. Electron. Mater. 2019, 48, 5933–5940. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, C.; Li, C.M. Lychee-like FeS2@FeSe2 core–shell microspheres anode in sodium ion batteries for large capacity and ultralong cycle life. J. Mater. Chem. 2017, A5, 19195–19202. [Google Scholar] [CrossRef]
- Zhu, D.L. Preparation of Porous Fe2O3 Nanorods for Lithium Ions Batteries with Excellent Electrochemical Performance. Nat. Sci. 2019, 36, 79–84. [Google Scholar]
- Hassan, M.F.; Guo, Z.; Chen, Z.; Liu, H.K. α-Fe2O3 as an anode material with capacity rise and high rate capability for lithium-ion batteries. Mater. Res. Bull. 2011, 46, 858–864. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, Z.; Fu, J.; Xia, K.; Lu, J.; Tang, H.; Ye, Z. FeSe2/carbon nanotube hybrid lithium-ion battery for harvesting energy from triboelectric nanogenerators. Chem. Commun. 2019, 55, 10960–10963. [Google Scholar] [CrossRef]
- Khan, Z.; Singh, P.; Ansari, S.A.; Manippady, S.R.; Jaiswal, A.; Saxena, M. VO2 Nanostructures for Batteries and Supercapacitors: A Review. Small 2021, 17, 2006651. [Google Scholar] [CrossRef]
- Wang, T.; Guo, W.; Wang, G.; Wang, H.; Bai, J.; Wang, B. Highly dispersed FeSe2 nanoparticles in porous carbon nanofibers as advanced anodes for sodium and potassium ion batteries. J. Alloys Compd. 2020, 834, 155265. [Google Scholar] [CrossRef]
- Jiao, R.; Zhao, L.; Zhou, S.; Zhai, Y.; Wei, D.; Zeng, S.; Zhang, X. Effects of Carbon Content and Current Density on the Li+ Storage Performance for MnO@C Nanocomposite Derived from Mn-Based Complexes. Nanomaterials 2020, 10, 1629. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, S.K.; Kang, Y.C. A Salt-Templated Strategy toward Hollow Iron Selenides Graphitic Carbon Composite Microspheres with Interconnected Multi cavities as High-Performance Anode Materials for Sodium-Ion Batteries. Small 2019, 15, 1803043. [Google Scholar] [CrossRef]
- Park, G.D.; Cho, J.S.; Lee, J.K.; Kang, Y.C. Na-ion Storage Performances of FeSex and Fe2O3 Hollow Nanoparticles-Decorated Reduced Graphene Oxide Balls prepared by Nanoscale Kirkendall Diffusion Process. Sci. Rep. 2016, 6, 22432–22442. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Feng, J.; Lv, F.; Zhou, J.; Lin, C.; Wang, K.; Zhang, Y.; Yang, Y.; Wang, W.; Li, J.; et al. Metallic Graphene-Like VSe2 Ultrathin Nanosheets: Superior Potassium-Ion Storage and Their Working Mechanism. Adv. Mater. 2018, 30, e1800036. [Google Scholar] [CrossRef]
- Fan, H.; Yu, H.; Zhang, Y.; Guo, J.; Wang, Z.; Wang, H.; Xu, J. 1D to 3D hierarchical iron selenide hollow nanotubes assembled from FeSe2@C core-shell nanorods for advanced sodium ion batteries. Energy Storage Mater. 2018, 10, 48–55. [Google Scholar] [CrossRef]
- Wu, M.; Chen, J.; Wang, C.; Wang, F.; Yi, B.; Su, W.; Wei, Z.; Liu, S. Facile Synthesis of Fe2O3 Nanobelts/CNTs Composites as High-performance Anode for Lithium-ion Battery. Electrochim. Acta 2014, 132, 533–537. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, J.-K.; Kang, Y.C. Graphitic Carbon-Coated FeSe2 Hollow Nanosphere-Decorated Reduced Graphene Oxide Hybrid Nanofibers as an Efficient Anode Material for Sodium Ion Batteries. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, Y.; Zhou, J.; Xu, K.; Lu, Y.; Zhang, K.; Zhu, L.; Qian, Y. Synchronous synthesis of Kirkendall effect induced hollow FeSe2/C nanospheres as anodes for high performance sodium ion batteries. Chem. Commun. 2018, 54, 5704–5707. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, D.; Xu, L.; Wang, Z.; Jiang, X.; Liu, X.; Ma, Y.; Wang, J. One-Step Route to Fe2O3 and FeSe2 Nanoparticles Loaded on Carbon-Sheet for Lithium Storage. Molecules 2022, 27, 2875. https://doi.org/10.3390/molecules27092875
Wei D, Xu L, Wang Z, Jiang X, Liu X, Ma Y, Wang J. One-Step Route to Fe2O3 and FeSe2 Nanoparticles Loaded on Carbon-Sheet for Lithium Storage. Molecules. 2022; 27(9):2875. https://doi.org/10.3390/molecules27092875
Chicago/Turabian StyleWei, Denghu, Leilei Xu, Zhiqi Wang, Xiaojie Jiang, Xiaxia Liu, Yuxue Ma, and Jie Wang. 2022. "One-Step Route to Fe2O3 and FeSe2 Nanoparticles Loaded on Carbon-Sheet for Lithium Storage" Molecules 27, no. 9: 2875. https://doi.org/10.3390/molecules27092875
APA StyleWei, D., Xu, L., Wang, Z., Jiang, X., Liu, X., Ma, Y., & Wang, J. (2022). One-Step Route to Fe2O3 and FeSe2 Nanoparticles Loaded on Carbon-Sheet for Lithium Storage. Molecules, 27(9), 2875. https://doi.org/10.3390/molecules27092875




