1. Introduction
The degradation of cellulose-based paper is a complex multistimulus process. The raw material used for paper production (type of wood fibers, nonwood fibers, and other components (fillers, additives, pigments)), pulp making (kraft pulping, sulfite, soda, organosolv pulping), sizing (especially aluminum sulfate–rosin mixtures used in the past), initial acidity, and content of lignin and metal ions have the most important influence on paper degradation. During storage of lignocellulosic goods, e.g., books, the degradation process is stimulated by factors such as moisture and acidity; increased temperature; intensity of light; mechanical stress, including folding history and abrasion; and biological contamination [
1,
2]. Storing paper in a dry and dark room, without mechanical stress, is the simplest way to decrease the rate of paper degradation. However, chemical degradation will continue depending on the acidity, moisture content, temperature, presence of redox catalysts, and oxygen concentration [
1,
2]. During storage, the acidity of paper increases due to the increasing concentration of volatile organic acids, such as acetic acid and formic acid, formed by chemical degradation of the material, especially hydrolysis and oxidation of polysaccharides in the paper, particularly cellulose polymer chains. The process can be considered autocatalytic [
3,
4]. The detailed mechanism of acetic acid formation can be found in the recent paper from Potthast et al. [
5]. Other volatile degradation products such as formaldehyde, furfural, and vanillin represent decomposition products of cellulose and lignin [
4,
6,
7]. However, in old books in libraries, many other volatile compounds were identified (aromatics, 2-ethyl-1-hexanol, long-chain aldehydes, alkanes, and esters of fatty acids) [
8]. The wide spectrum of decomposition products can be ascribed to the presence of aluminum and sulfate anions incorporated in the paper in the process of sizing using the alum–rosin procedure [
9]. Although oxidation is not such an acute problem as acid hydrolysis due to alumina, it is usually accelerated by the presence of transition metals in the paper [
10,
11]. Other factors influencing the decomposition are solvents used in printing inks and pigments, very often containing iron species.
The Py-GC/MS method is suitable for the analysis of volatile compounds present in the paper and formed during paper aging or by thermal decomposition of paper. Characterization of degradation products, design, and description of degradation mechanisms of individual paper components can be studied using an analytical pyrolyzer coupled with a gas chromatography–mass spectrometry set-up [
12,
13,
14,
15]. The main pyrolysis products of cellulose, cellobiose, and glucose include levoglucosan and levoglucosenone; furans, e.g., 5-hydroxymethyl furfural and furfural; and small molecular substances such as CO
2, 1-hydroxy-2-propanone, acetaldehyde, acetic acid, and propanoic acid [
15]. The Py-GC/MS equipment allows treating a sample of organic matter by dropping it into a pyrolytic microreactor preheated to temperatures of up to 1000 °C. Volatilized compounds are then transported by the carrier gas to an efficient chromatographic column, where components are separated and identified based on their mass spectra. By applying this technique, chemical structures of decomposition products can be identified with the aim of characterizing the chemical features of the paper. This information also enables the determination of different organic materials such as sizing agents, ink components, waxes, resins, oils, binders, pigments, and dyes in paper [
16,
17,
18]. The chemical composition of the original components of a sample can be reconstructed by detailed interpretation of the molecular profile of thermal degradation products. However, due to a huge number of consecutive and parallel reactions, the interpretation of complex matrices is challenging.
Basic components of lignocellulosic materials start to decompose at different temperatures during heating in inert gas: amorphous hemicellulose at around 250 °C, crystalline cellulose fibers at around 300 °C, and lignin at 350 °C [
19]. All the mentioned materials are obviously present in the studied newsprint papers, and all of them can be chemically changed/degraded during shelf time. It is worth mentioning that a micropyrolytic approach was also successfully applied for the characterization of products from fast micropyrolysis of lignocellulosic materials [
20], where the main focus was on the characterization of bio-oil obtained by pyrolysis.
The aim of this work was to study the processes of chemical degradation of paper during aging by applying the Py-GC/MS method for the determination of formed volatile organic compounds.
2. Results
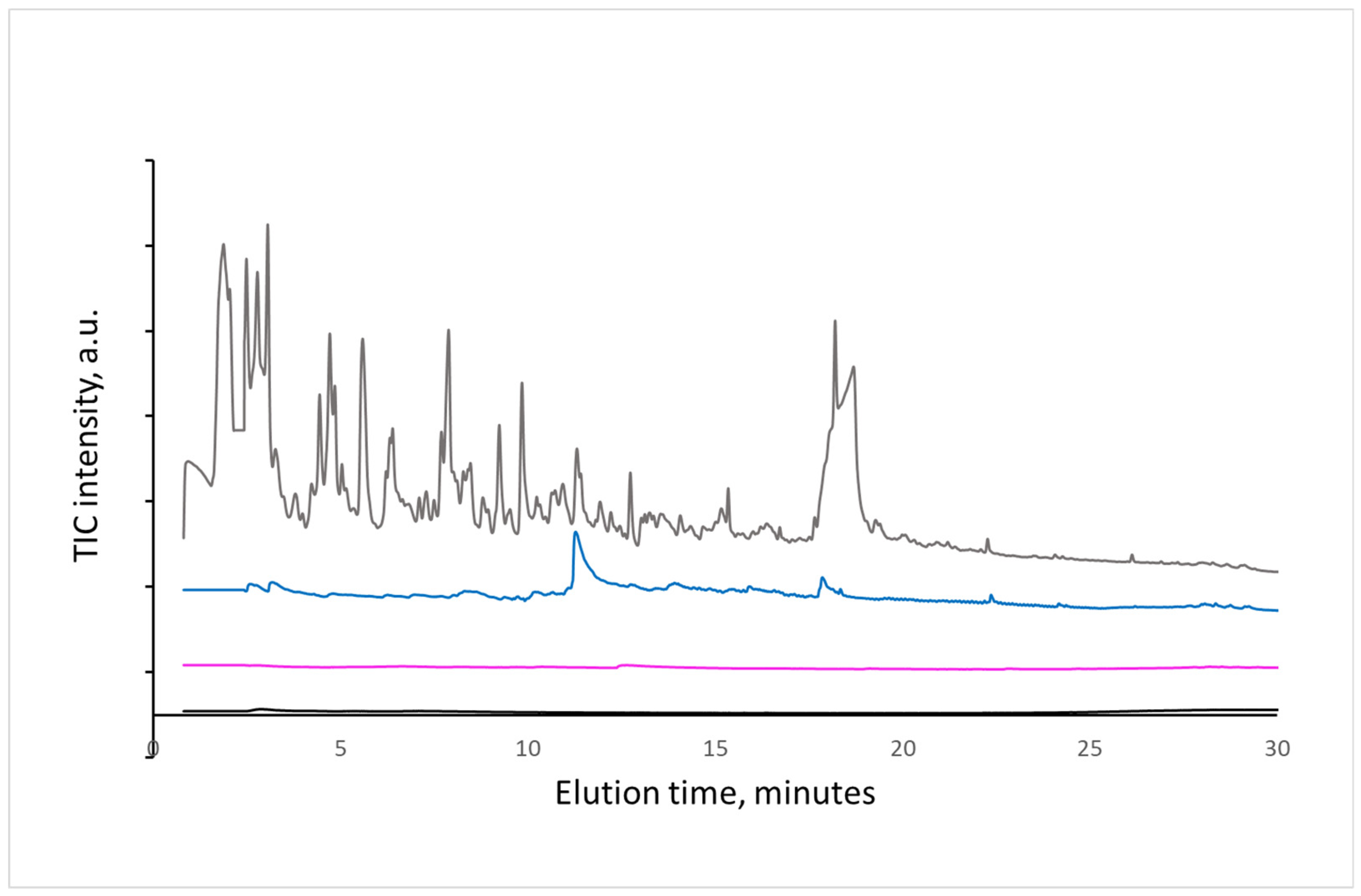
The composition of volatile organic compounds formed during natural and artificial aging of a paper was studied by Py-GC/MS methods. Paper sheets were artificially aged for 0, 2, 15, 20, and 60 days, and some of them were naturally aged in laboratory conditions for 10 years without mechanical stress and light in a closed aluminum foil envelope. Paper samples of 1 mg were dropped into the pyrolyzer preheated to 200, 250, 300, or 500 °C, and evaporated compounds were collected by the splitless method at the beginning of the capillary column for 20 s. After flashing out the injection port and pyrolyzer by opening the split valve, the collected volatile compounds were analyzed by the GC/MS method. When comparing data obtained at 200, 250, and 300 °C (
Figure 1 and
Figure 2), a reasonable evolution of volatile and decomposition products was found at 300 °C. Therefore, we took this temperature as a starting point for elucidation of sample degradation analysis.
In the first series of experiments, volatile compounds released from practically new (unused) newspaper were identified. As it is seen in
Figure 1, the first traces of volatile compounds of unknown origin were recognized at 300 °C. At 500 °C, the sample was decomposed to the expected decomposition products of cellulose and lignin. This result is in agreement with literature data [
19] concerning the stability of the main lignocellulose components.
Acetic acid is a typical product of paper aging [
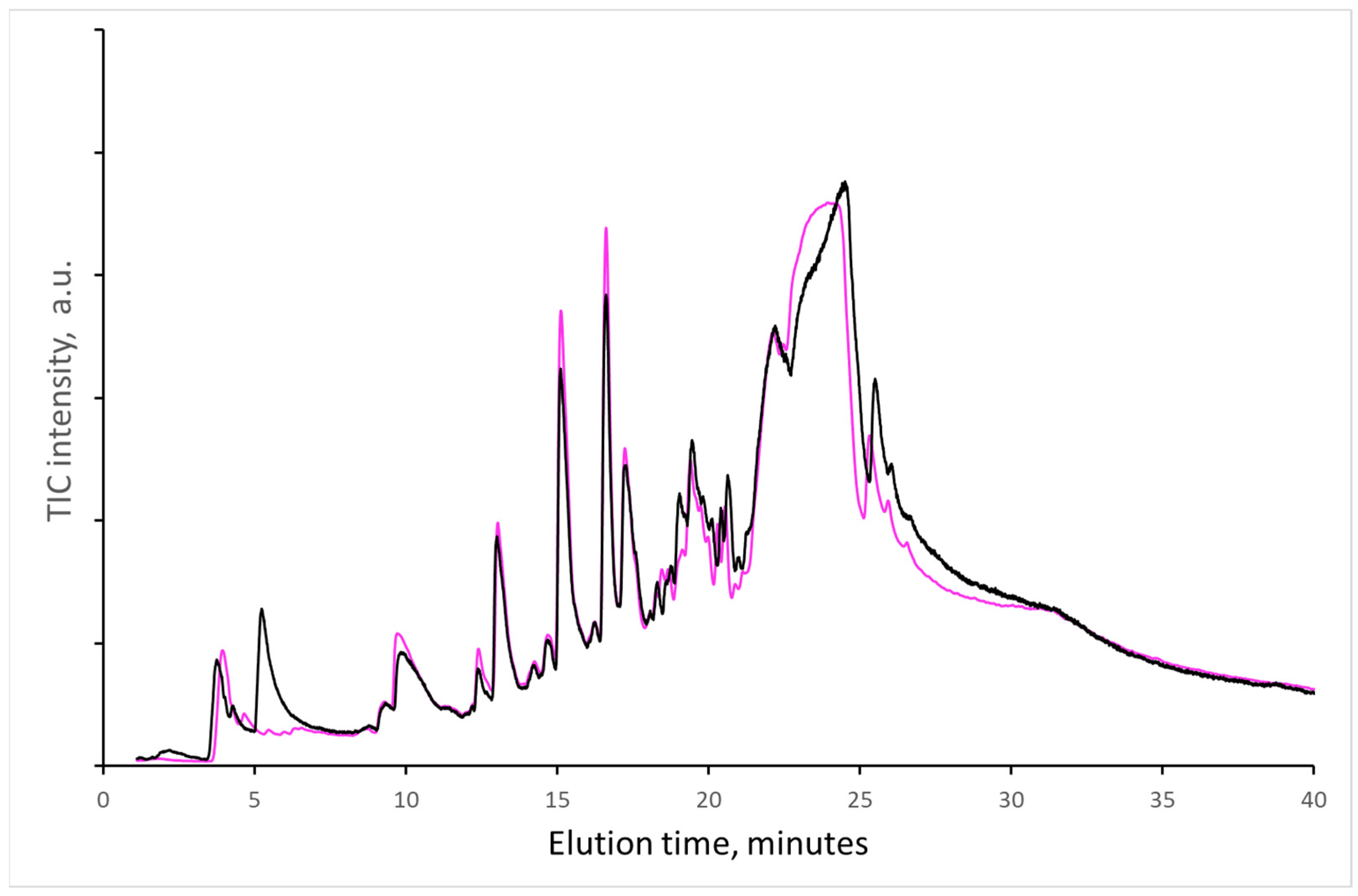
1] and can catalyze the decomposition of paper components. In another experiment, the ability of acetic acid to allow the formation of a detectable amount of volatile compounds within 20 s of sampling time in the Py-GC/MS was verified. A new newspaper sample and old textbook paper (manufactured in 1976) were saturated with acetic acid in its vapors in a closed vessel under laboratory conditions. In the measured pyrograms, no new components were found, except for acetic acid, and the differences in the amount of evaporated compounds are within experimental error (
Figure 3); i.e., acetic acid present in the sample during Py-GC/MS analysis up to 300 °C did not increase or modify the formation of decomposition products of paper components significantly.
No immediate effect of acetic acid on decomposition products indicates a more complicated autocatalytic reaction pathway of cellulose chain decomposition.
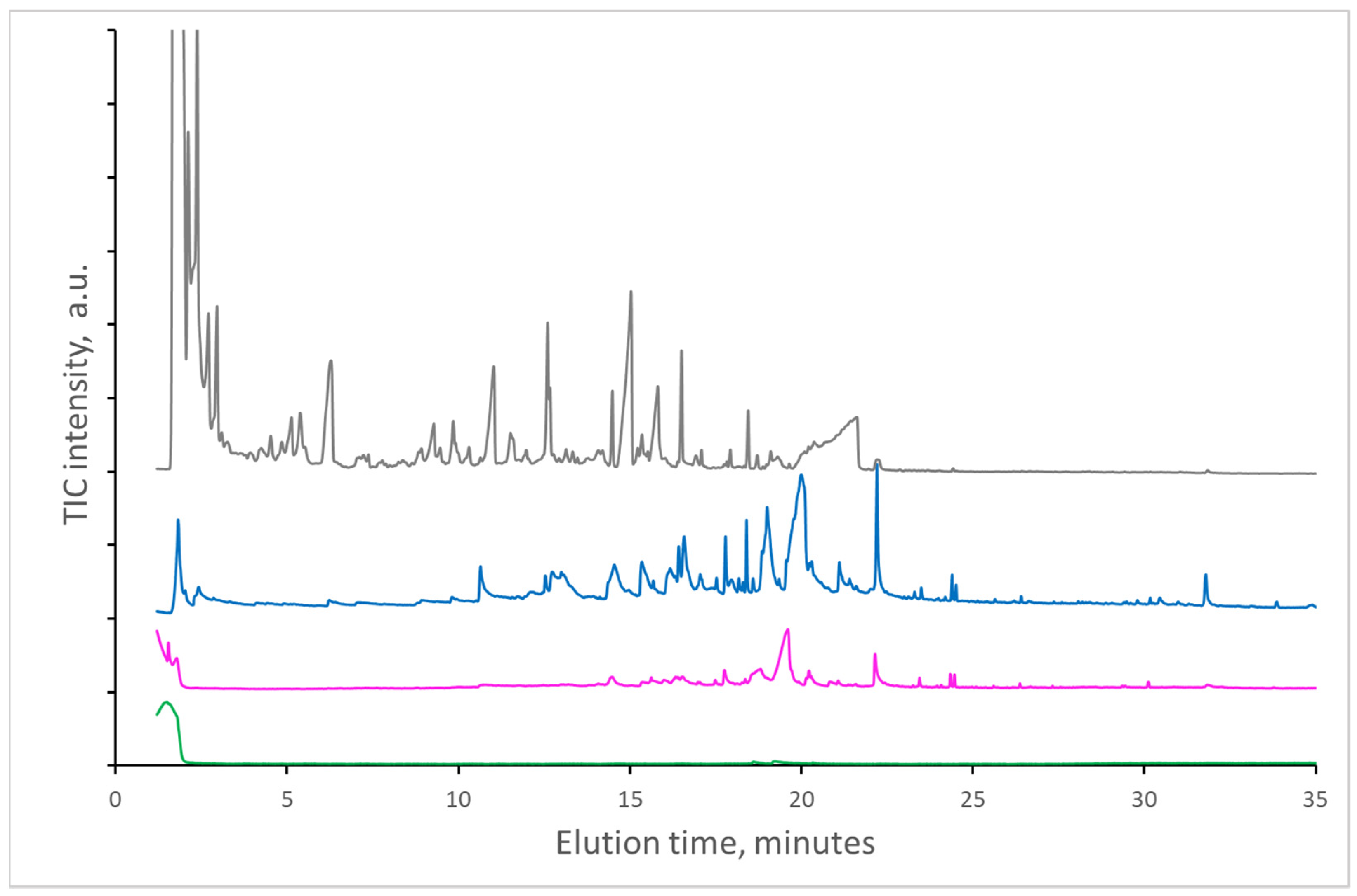
In comparison to new newspaper, a significantly higher amount of volatile compounds was determined in the case of papers after 10-year or longer natural aging (
Figure 2 and
Figure 3) at 250 °C and 300 °C. The identified compounds, which were practically the same at both temperatures, are listed in
Table 1. However, evaporated amounts of compounds at 250 °C are significantly lower. Virtually all compounds determined are formed by the decomposition of cellulose and/or lignin, except for nonanoic acid, palmitic acid, and dibutyl-phthalate, which were probably added during paper manufacturing. In the case of paper from old textbooks, a significantly lower amount of lignin components was found, which indicates a lower content of lignin in the paper used for textbooks.
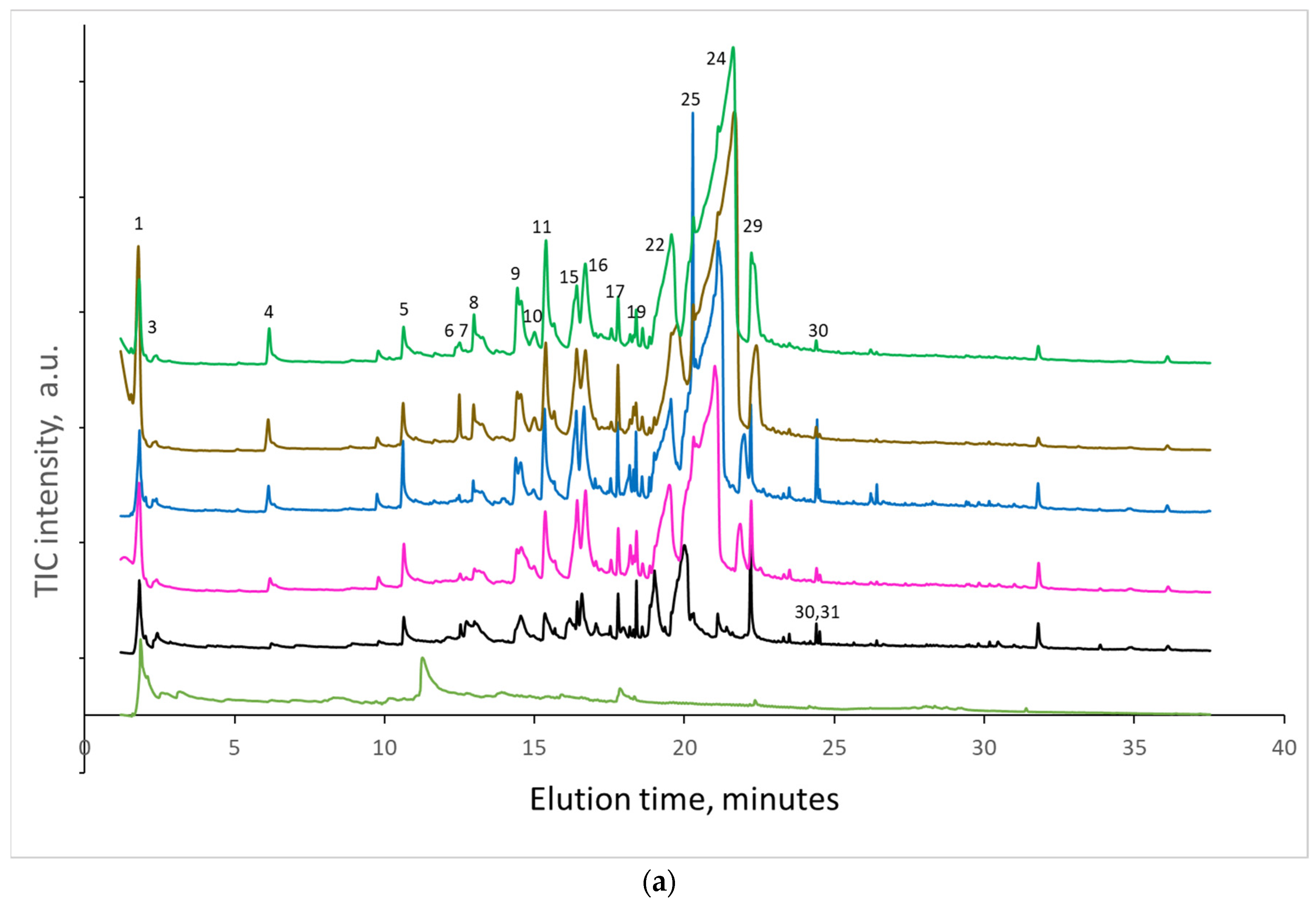
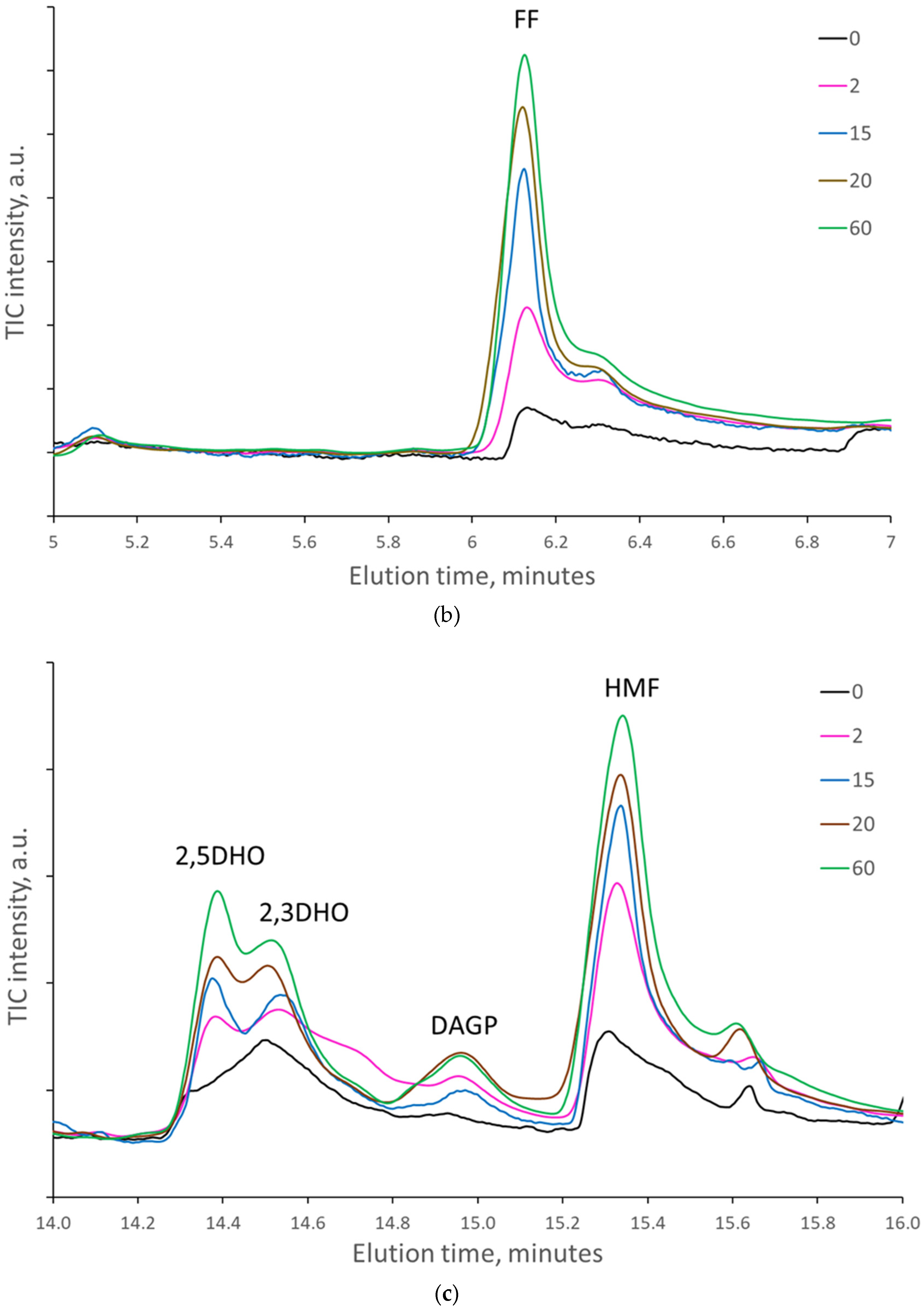
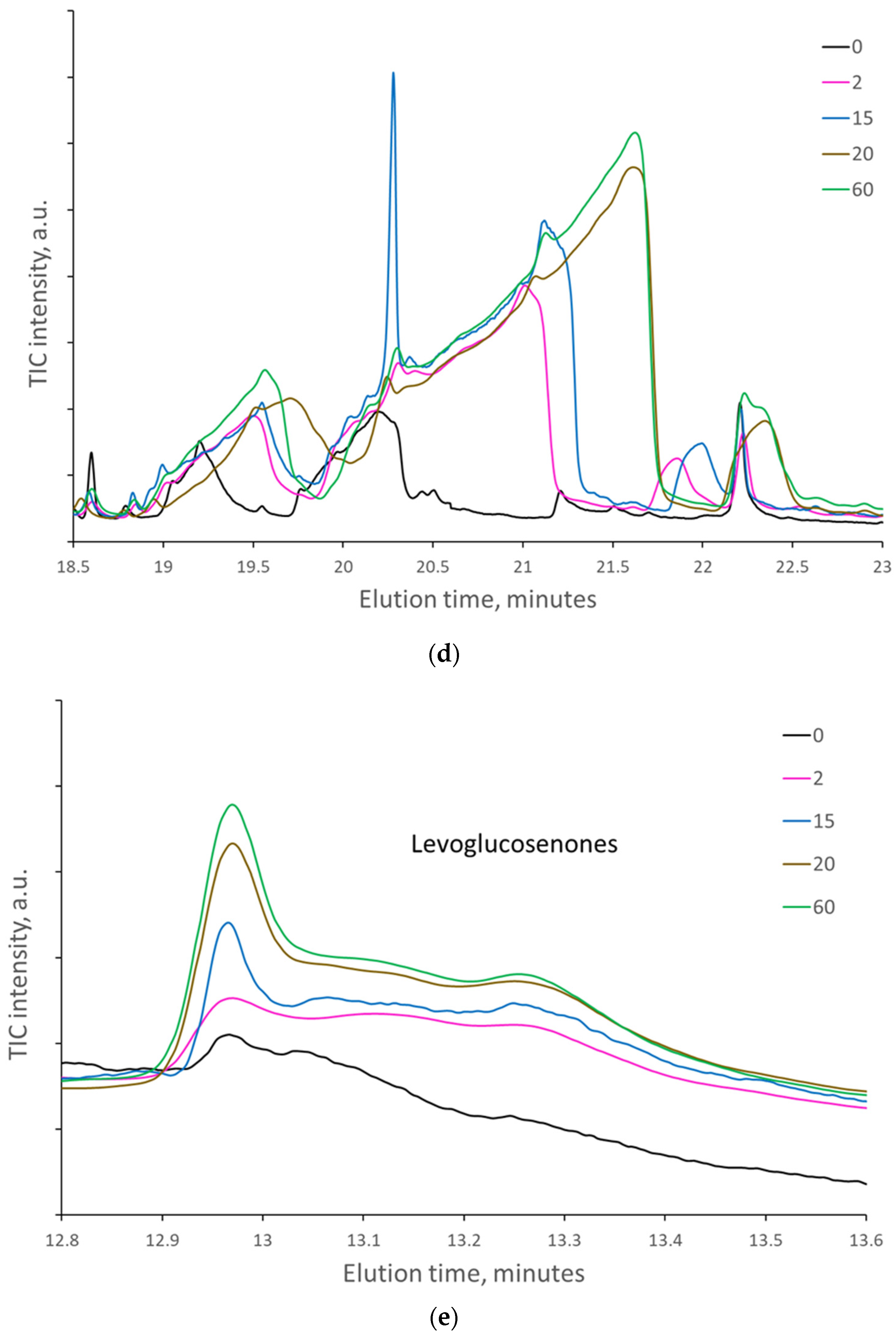
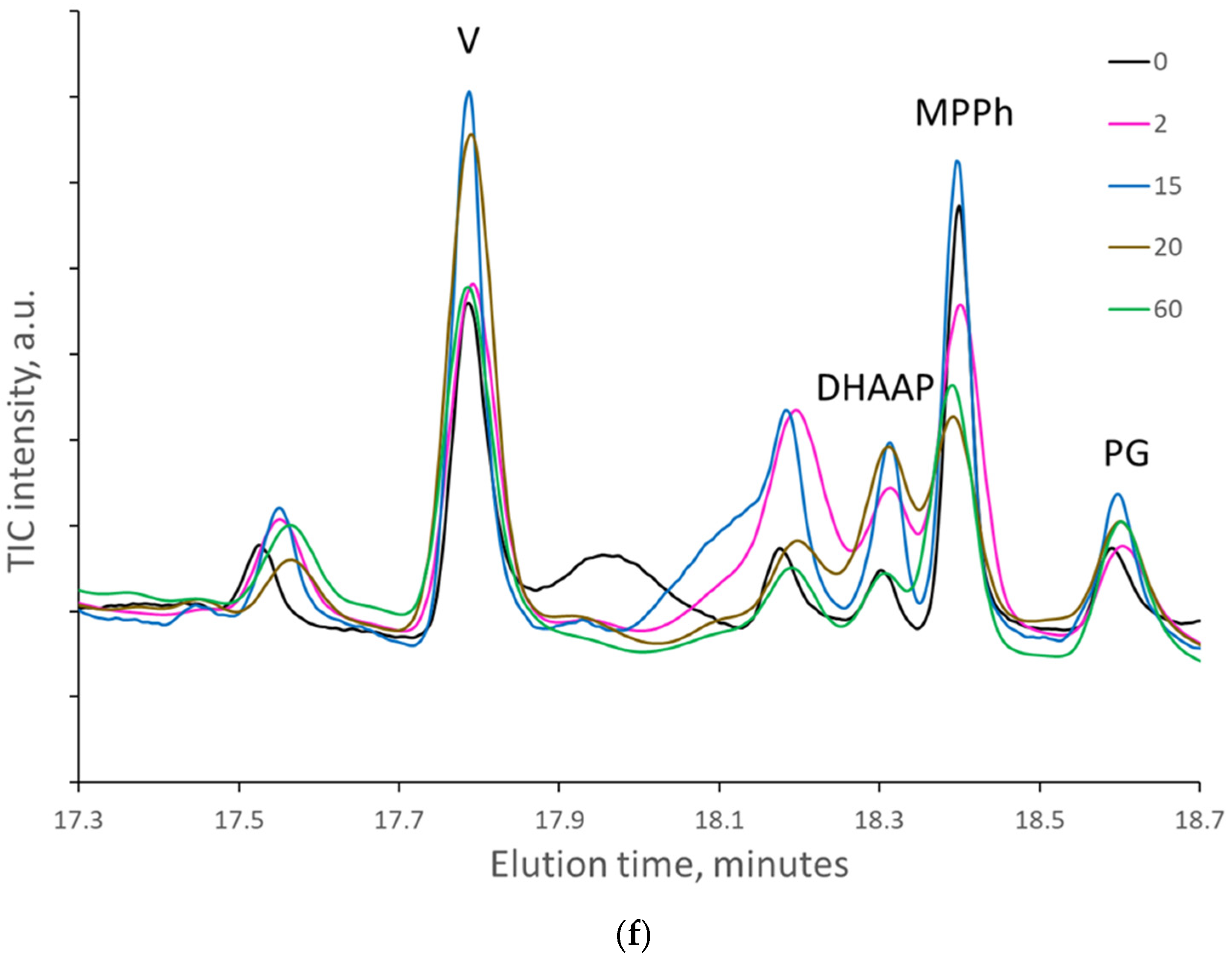
When the paper sheets at the beginning of natural aging were artificially aged, the amount of some cellulose decomposition products regularly increased with the number of days of artificial aging. At the same time, the amount of lignin degradation products varied randomly without a regular increase (
Figure 4a–f).
Before discussing the effect of natural aging on the products of cellulose and lignin decomposition, it should be noted that not all decomposition products are able to elute from the column at the experimental conditions and thus are not detected by the MS detector. Among the lignin degradation products, only compounds containing one aromatic ring were detected. These compounds contain zero, one or two –OH groups; one or two CH3O- groups; and zero or one alkyl group with maximally three carbons. The detected decomposition products of cellulose contain from one to six carbons of one glucose unit of cellulose. No products containing carbons of two or more glucose units were detected. Similarly, the volatility of compounds with four or more –OH groups is too low to allow elution from the used column.
When the samples were pyrolyzed at 500 °C, no correlation was found considering the decomposition product of cellulose or lignin and the time of artificial aging. The area of levoglucosan (LG) and other cellulose decomposition products was approximately the same in the range of experimental error for all times of artificial aging.
However, when the samples were pyrolyzed at 300 °C, the highest increase in the area of decomposition products was detected for levoglucosan (LG), which is formed mainly by hydrolysis or substitution of the last β-glycosidic bonds between glucose units at the end of cellulose macromolecules. Artificial aging for 2 and 5 days increased the area of LG nearly 5-fold, while 20 and 60 days increased the area by almost 8-fold. In papers naturally aged for 45 and 56 years (papers from 1976 and 1965, respectively), the area of LG increased nearly 20-fold compared to those naturally aged for 10 years. The amount of levoglucosenones, formed by dehydration of levoglucosan, was increased by artificial aging up to 2.5 times. The amount of FF and HMF, formed by the dehydration of the glucose unit of cellulose, increased up to 12- and 4-fold, respectively.
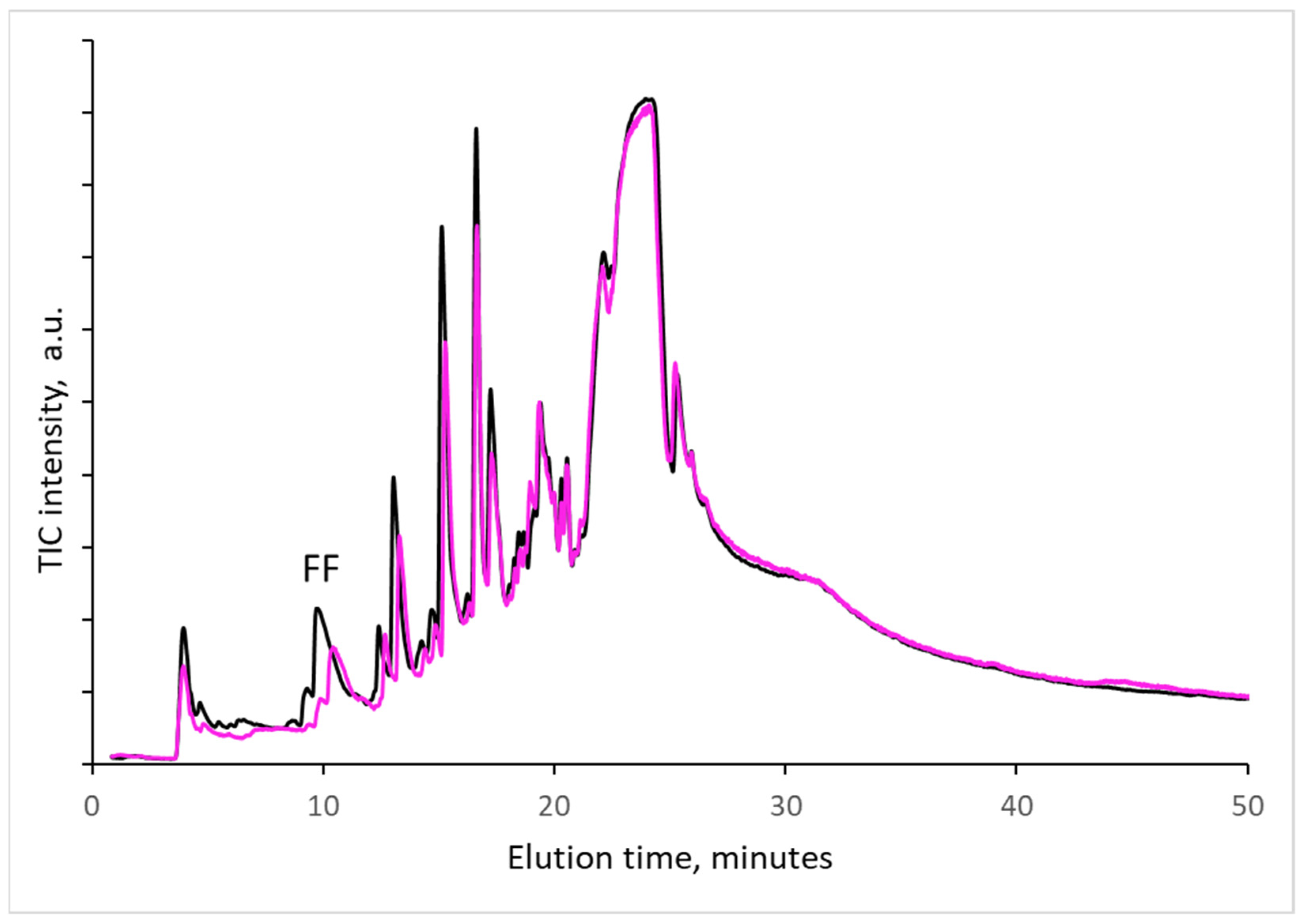
In several experiments, it was determined whether the decomposition products were formed in the microreactor of the pyrolyzer (during 20 s of evaporated product collection from samples at 300 °C) or had already formed during artificial or natural aging; i.e., compounds determined during heating in the pyrolyzer were simply evaporated from samples without cellulose decomposition. As practically all decomposition products can be washed out by water and methanol, samples of papers from old textbooks were carefully washed with water and then with methanol and dried at room temperature. Pyrograms before and after washing are compared in
Figure 5. As can be seen, washing removed a very small to negligible portion of the decomposition product. The amount of furfural, which was also recognized by other authors studying paper aging (e.g., [
1]), is significantly lower. Strilc et al. [
4] confirmed that furaldehyde is a valuable source of information and there is a correlation between furaldehyde emissions and the pH of the cellulosic environment. From our measurement follows that the detected decomposition products are formed mainly during the thermal decomposition of paper at 300 °C. This temperature is at the beginning of cellulose decomposition determined by DTG experiments [
19]. A new paper sheet, typically with long macromolecules of cellulose polymer chains stabilized in cellulose bundles by hydrogen bonds (consequently with a low amount of reducing or non-reducing ends of the molecules) is able to withstand this temperature during the Py-GC/MS analysis. The amount of formed volatile decomposition products during 20 s of pyrolysis is negligible. During natural or artificial aging, macromolecules are split/shortened; thus, significantly more molecular ends are formed and can undergo further decomposition. The original tight network of the hydrogen bonds is reorganized, and glucose units are kept by fewer hydrogen bonds in the fiber. The significantly increased amount of macromolecular ends and decreased amount of hydrogen bonds result in a markedly higher rate of decomposition product formation.
As it is known, the most significant changes during paper aging occur in the structure of cellulose bonds, consequently affecting the properties of microfibrils, macrofibrils, cellulose fibers, and overall mechanical strength. Changes in color due to the creation of chromophores and the generation of colored chemical compounds were also observed in the previous research [
21]. Decomposition of cellulose bonds and the following reactions are catalyzed by acids, which can be residual or newly formed during aging. Of the newly formed acids, the most important is acetic acid [
1].
Based on our results and published data, the following reaction network of cellulose decomposition and the formation of cellulose decomposition compounds is proposed:
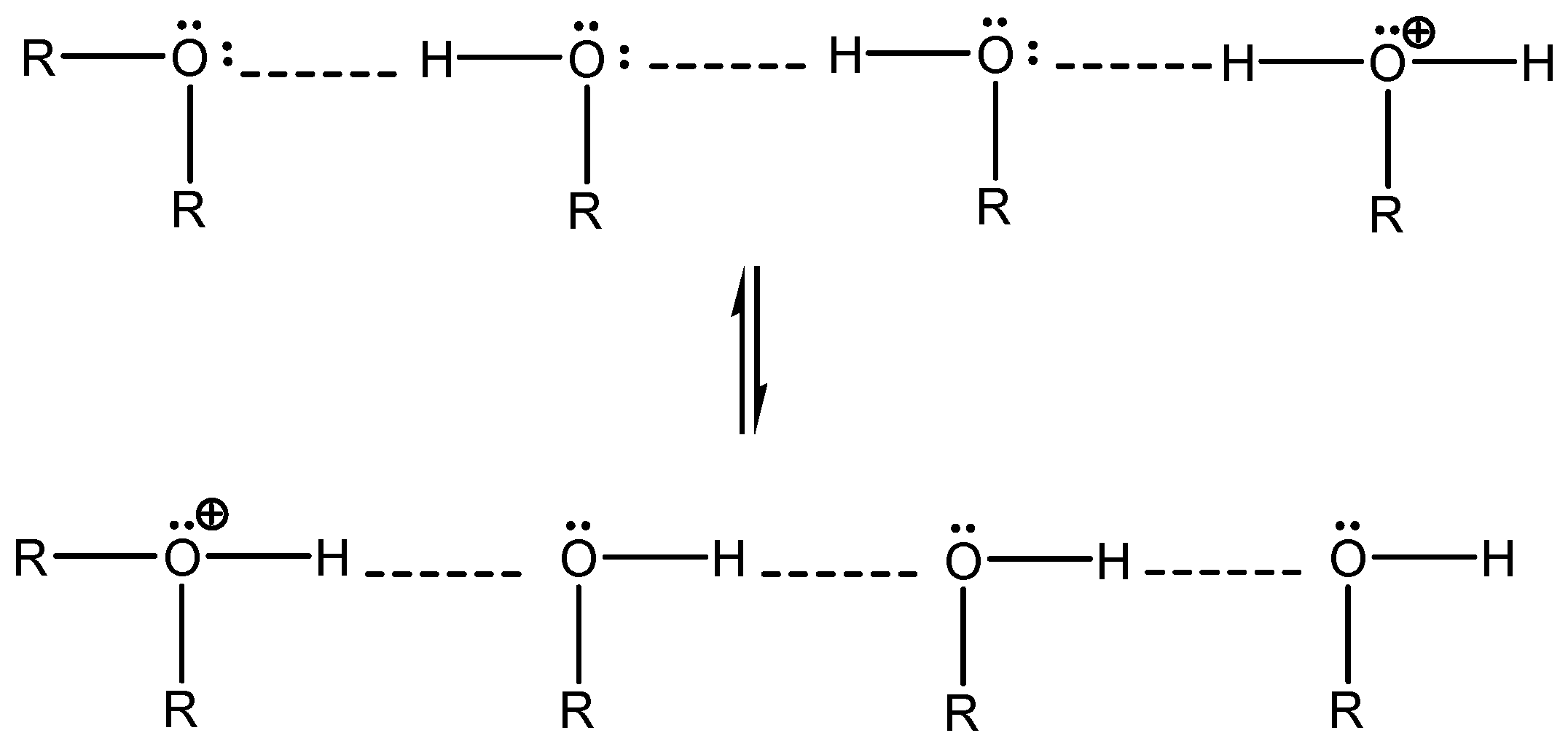
Protons, which are “the real catalyst” in acid-catalyzed reactions, can migrate very efficiently both along the hydrogen bond network and from one cellulosic chain to another (
Scheme 1).
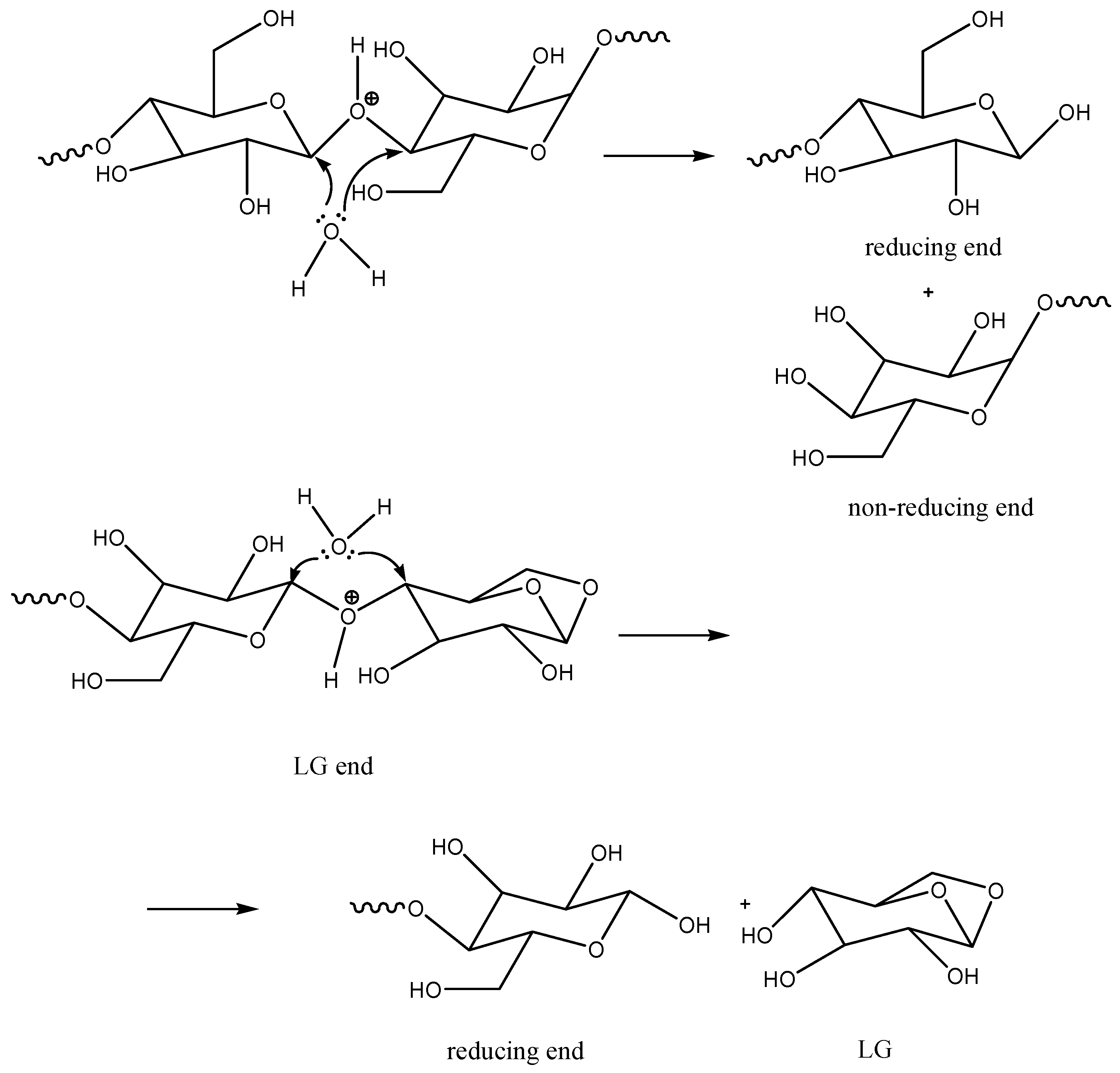
Then, protons on the oxygen of β(1→4)-glycosidic bonds (further also etheric O) can decrease the density of electrons on both carbons connected with this etheric oxygen atom (O) and catalyze hydrolysis or substitution of etheric O at ambient temperature. The content of water in common paper is about 3–10 wt.%, which is theoretically enough to hydrolyze all β(1→4)-glycosidic bonds in cellulose. Hydrolysis of any β(1→4)-glycosidic bond provides one reducing and one non-reducing end of the cellulose macromolecule (
Scheme 2). In the inner macromolecules of the polymer cellulose chain, where the presence of water is less probable, protons on etheric O can catalyze the substitution of this oxygen by oxygen of the hydroxymethyl group (C6 in glucose). The formation of the levoglucosanic end of the macromolecule can be observed (
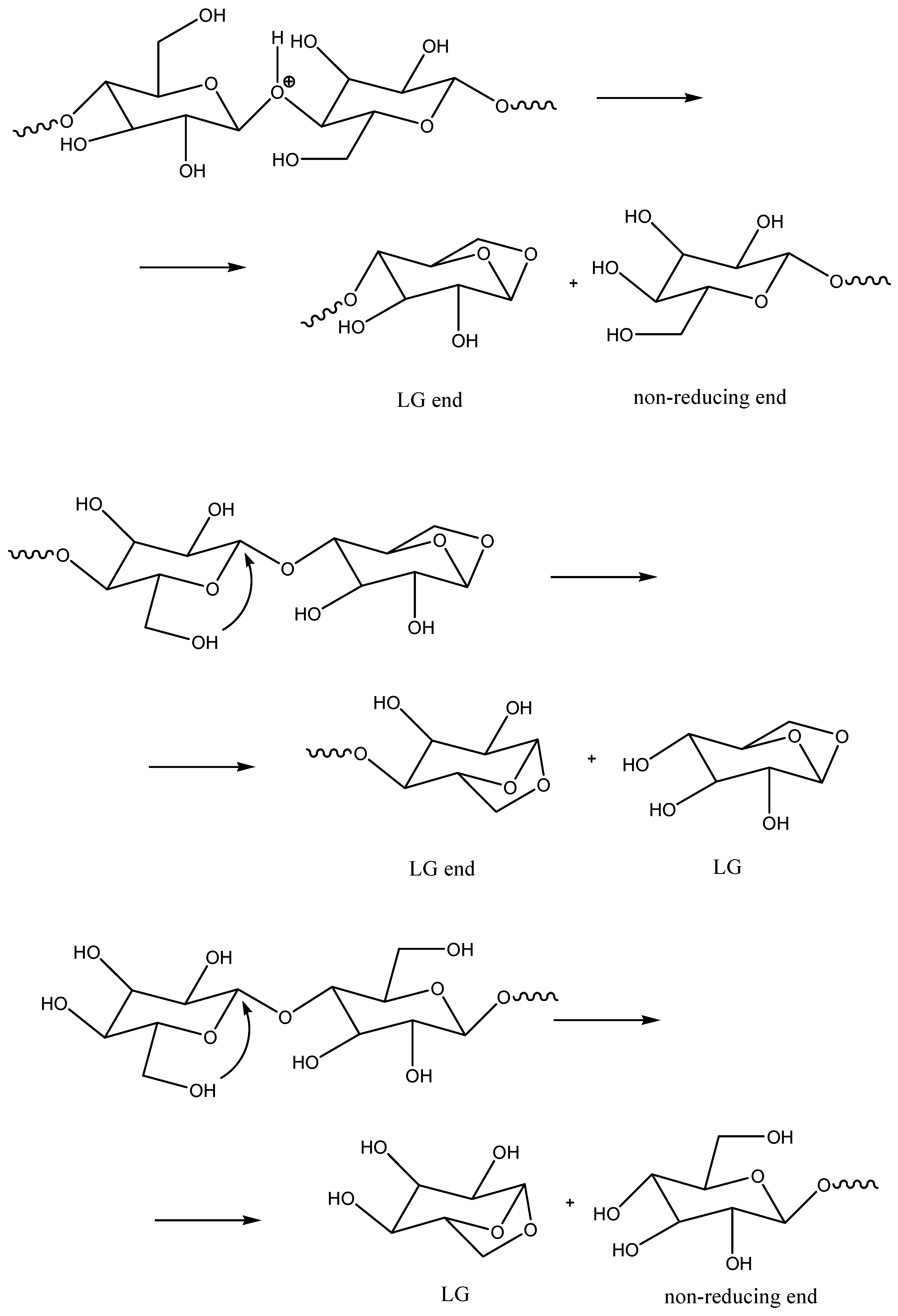
Scheme 3).
These reactions are supported mainly by mechanical stress at lower temperatures, and at 300 °C, they are also supported by the thermal motion of atoms in the molecules. The substitution reaction can be catalyzed by protons not only on the surface of cellulose fibers, but also in internal macromolecules, where water molecules are not present, but protons can enter through a network of hydrogen bonds (
Scheme 1 and
Scheme 3).
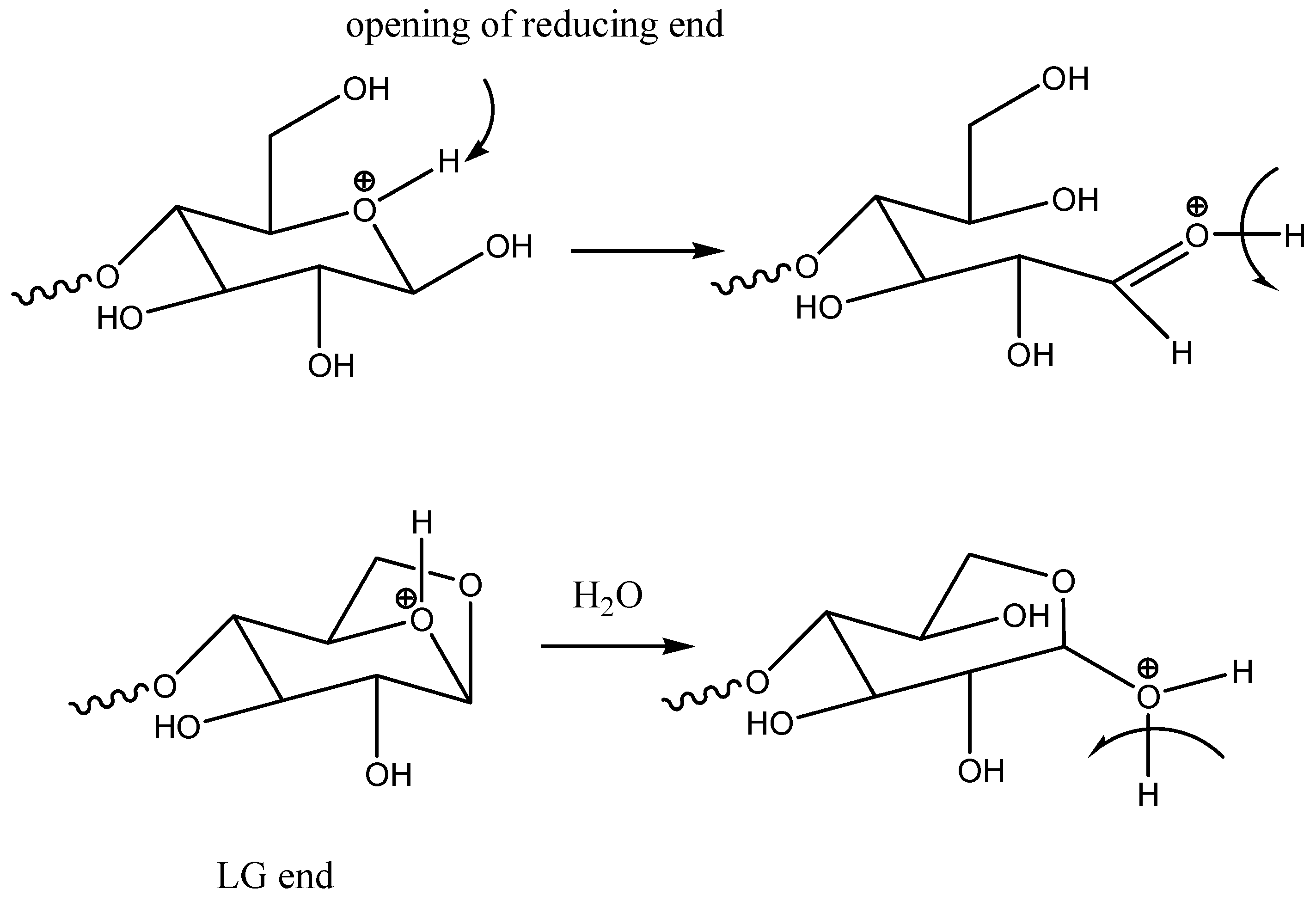
Further hydrolysis or substitution on the levoglucosanic end produces levoglucosan, similarly to the substitution on the non-reducing end of the macromolecule (
Scheme 3). Hydrolysis or substitution on the reducing end produces glucose, similarly to the hydrolysis on the non-reducing end of the macromolecule. Proton-catalyzed cleavage of β(1→4)-glycosidic bonds can take months and years of natural aging at ambient temperatures or days of artificial aging at nearly 100 °C. When a higher temperature is applied (e.g., 300 °C as in our Py-GC/MS experiments), these reactions proceed at a second timescale. Levoglucosan and its derivatives formed by dehydration or isomerization are the main decomposition products of cellulose, as determined by the Py-GC/MS method applied in this work.
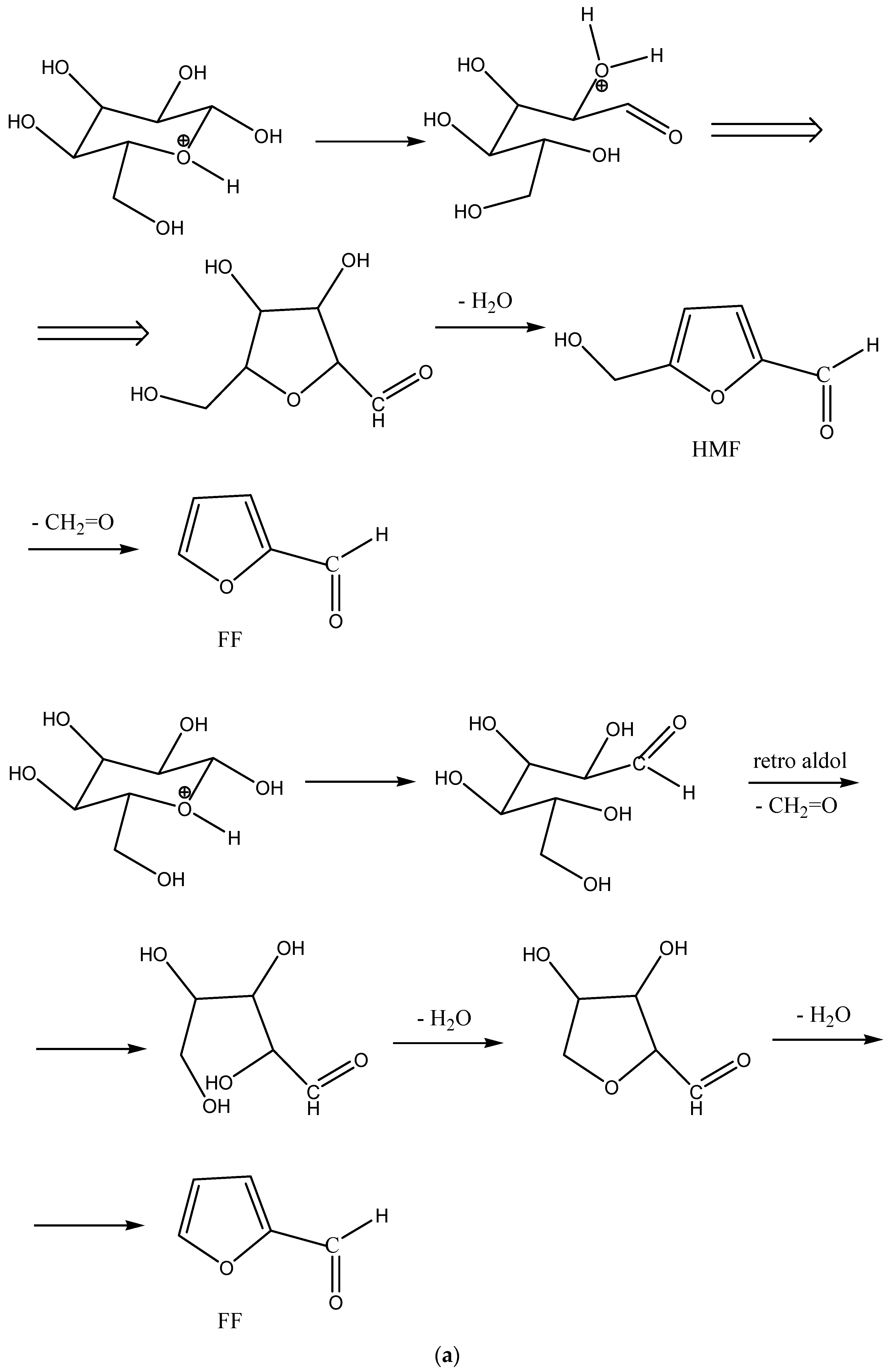
A proton on the acetalic oxygen in the glucopyranose ring can catalyze the opening of this ring and the formation of a reactive aldehydic end of the molecule (
Scheme 4) or glucose, which can undergo further retro-aldol condensation reactions (
Scheme 4 and
Scheme 5).
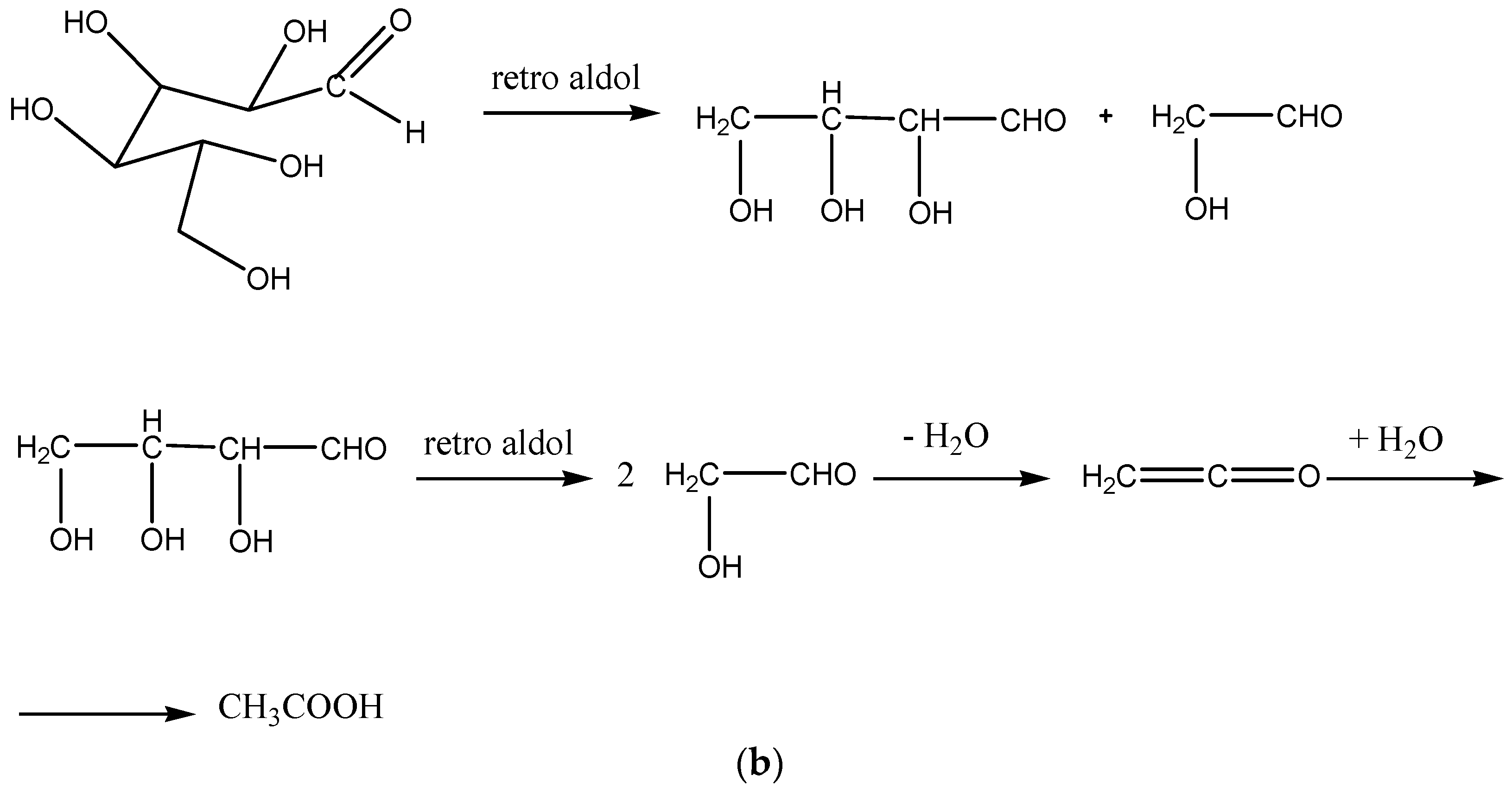
Products of deeper cellulose decomposition, which can be formed from glucose units or from LG by isomerization and dehydration reactions, are summarized in
Scheme 5 and
Scheme 6. All final compounds from
Scheme 5 and
Scheme 6 were found in the pyrograms of decomposition products. In
Scheme 5a, possible mechanisms of HMF and FF formation are presented.
Scheme 5b shows the proposed retro-aldol condensation and the formation of hydroxyacetaldehyde and consecutively of acetic acid.
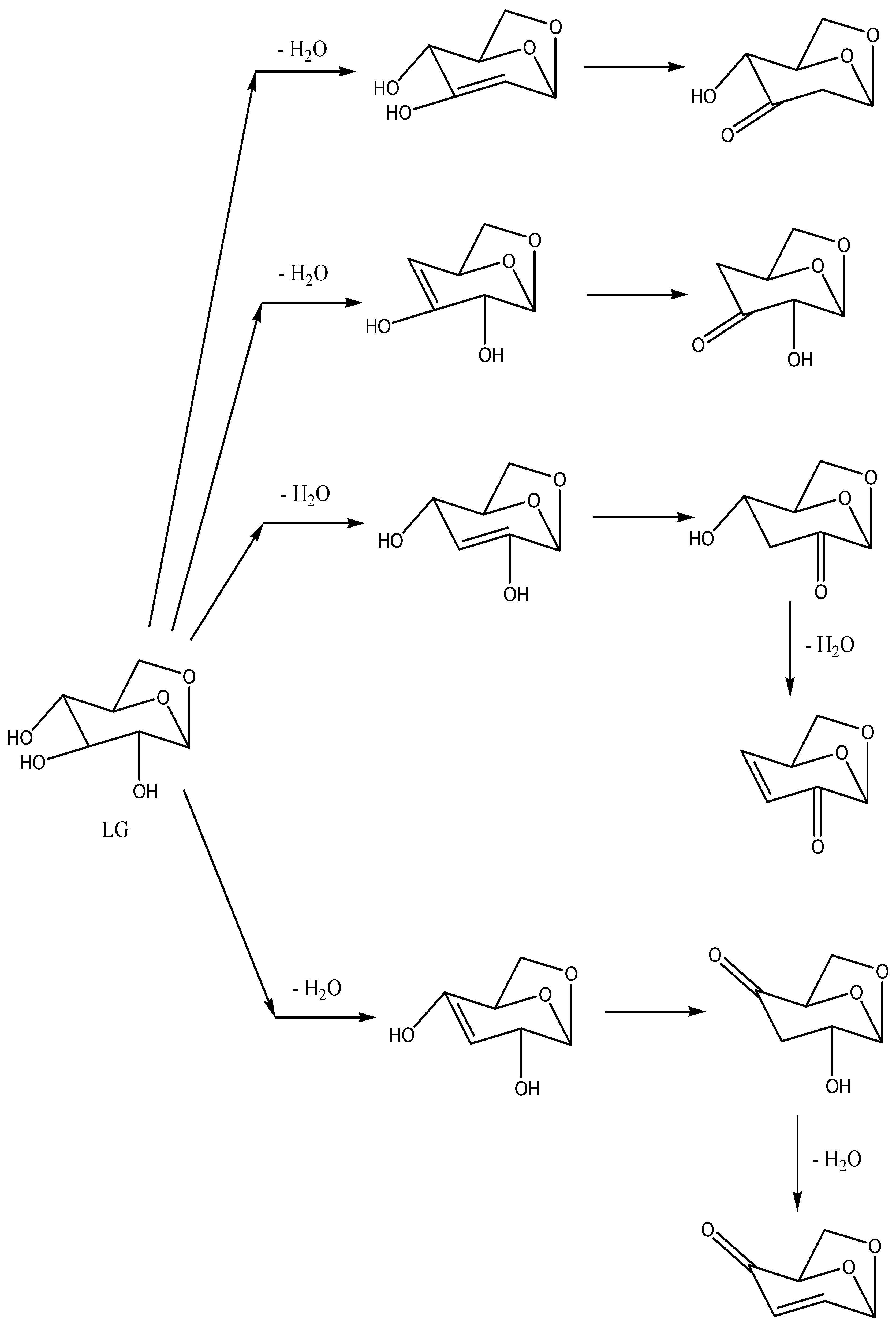
Levoglucosans (LG in
Scheme 6) have three –OH groups with several possible spatial orientations, which results in low volatility (reflected in long elution time and a wide peak in the pyrogram). By decreasing the number of –OH groups and molecular weight, elution times of their dehydration products (progressively: levoglucosene, levoglucosanone, and levoglucosenone) are shortened (
Table 1). All the cellulose decomposition products with more –OH groups and several possible spatial orientations are eluted in the wider peak.
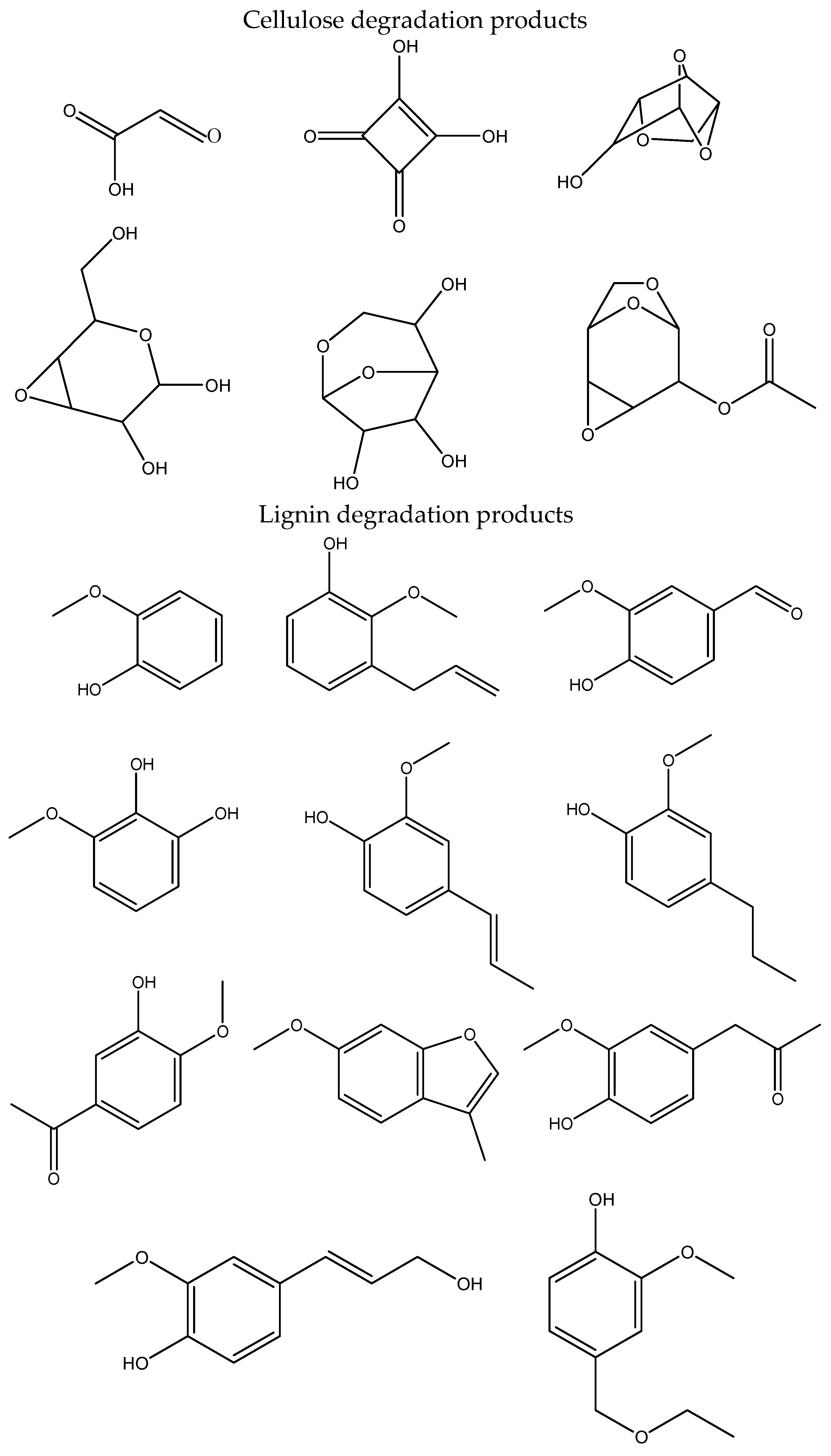
Some decomposition products of cellulose and lignin, identified from mass spectrum libraries, are summarized in
Scheme 7.