Formulation, Characterization and Permeability Studies of Fenugreek (Trigonella foenum-graecum) Containing Self-Emulsifying Drug Delivery System (SEDDS)
Abstract
:1. Introduction
2. Results
2.1. Characterization of Fenugreek Extract
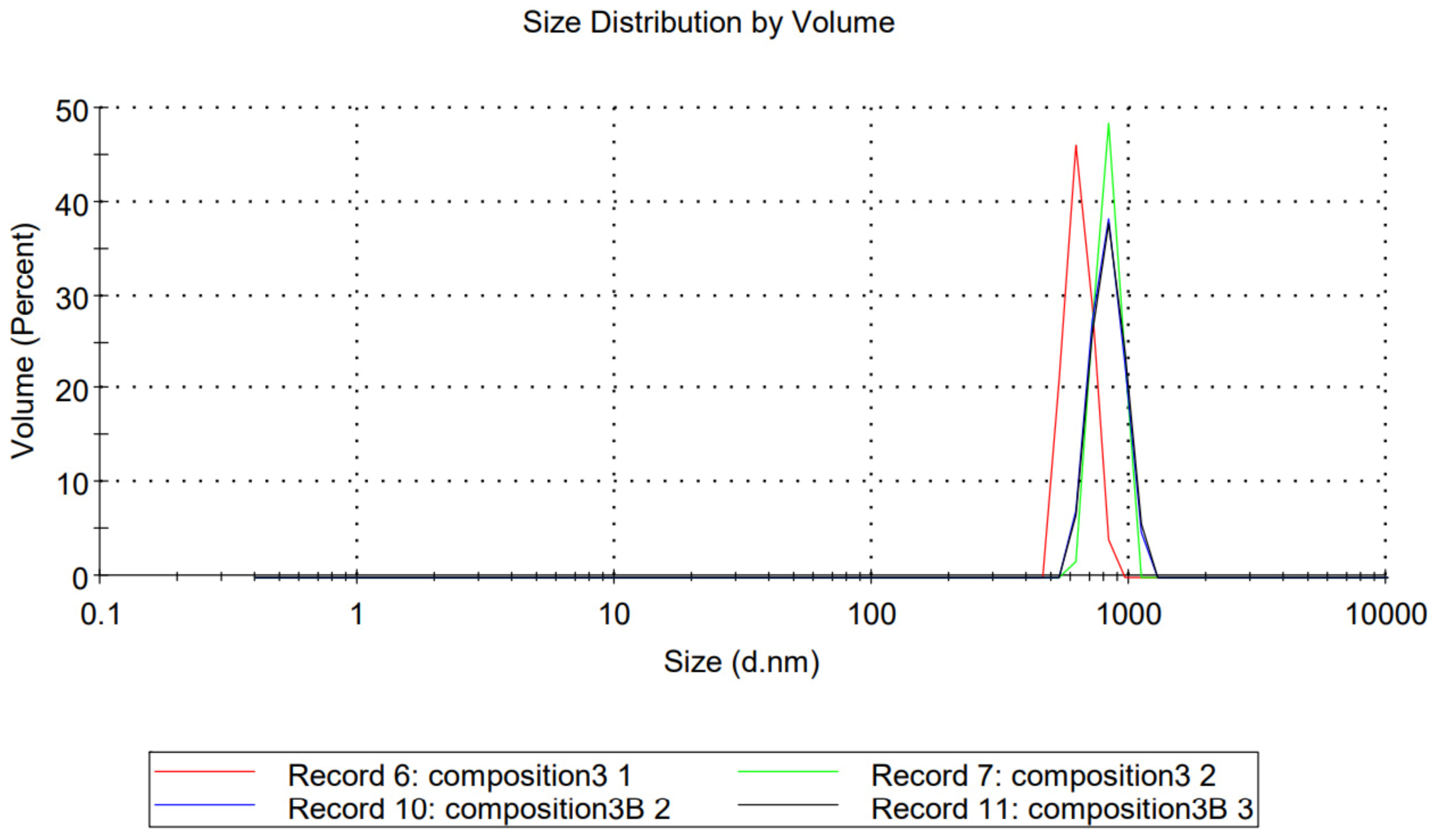
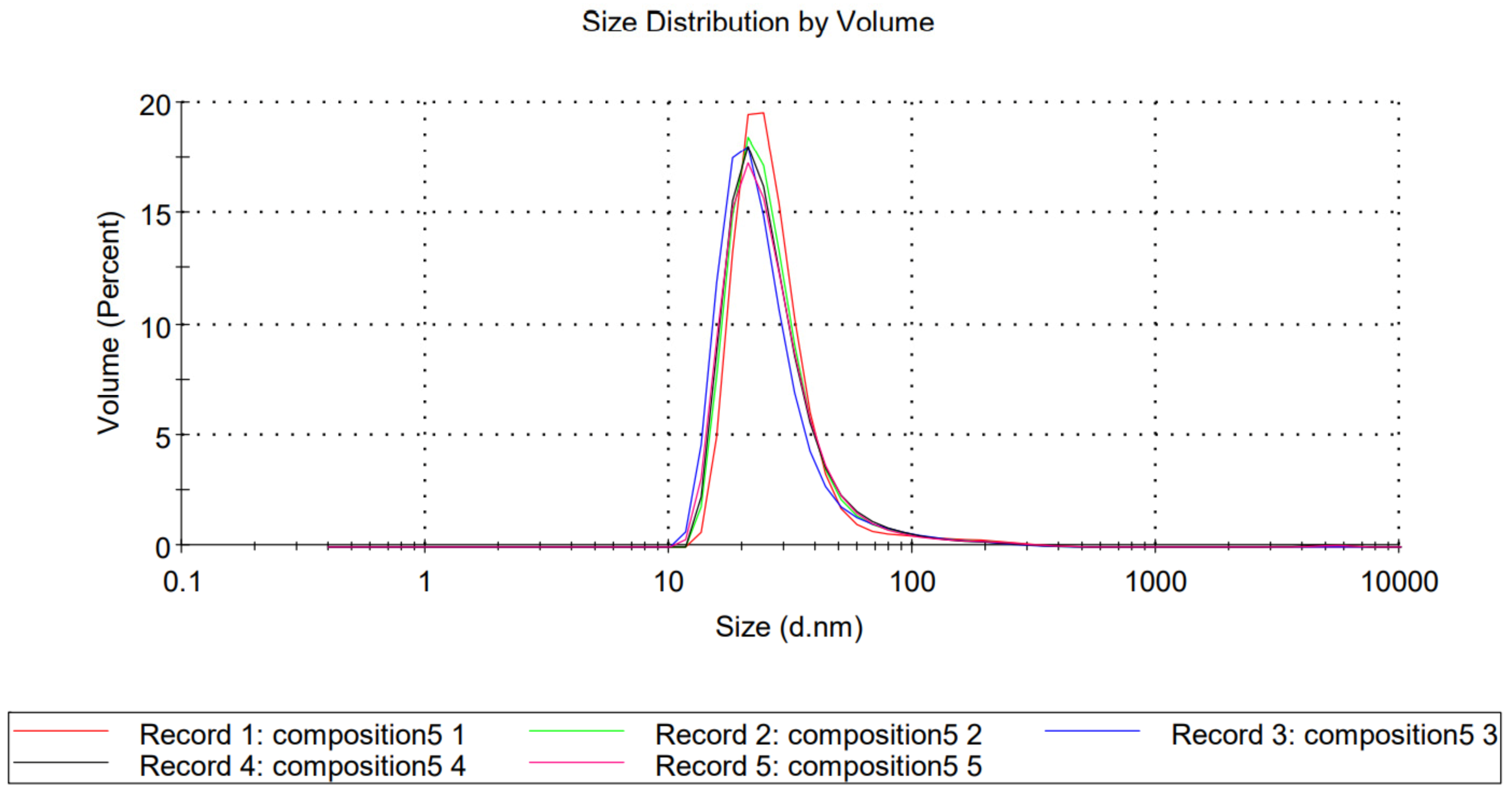
2.2. Dynamic Light Scattering
2.3. Zeta Potential Analysis
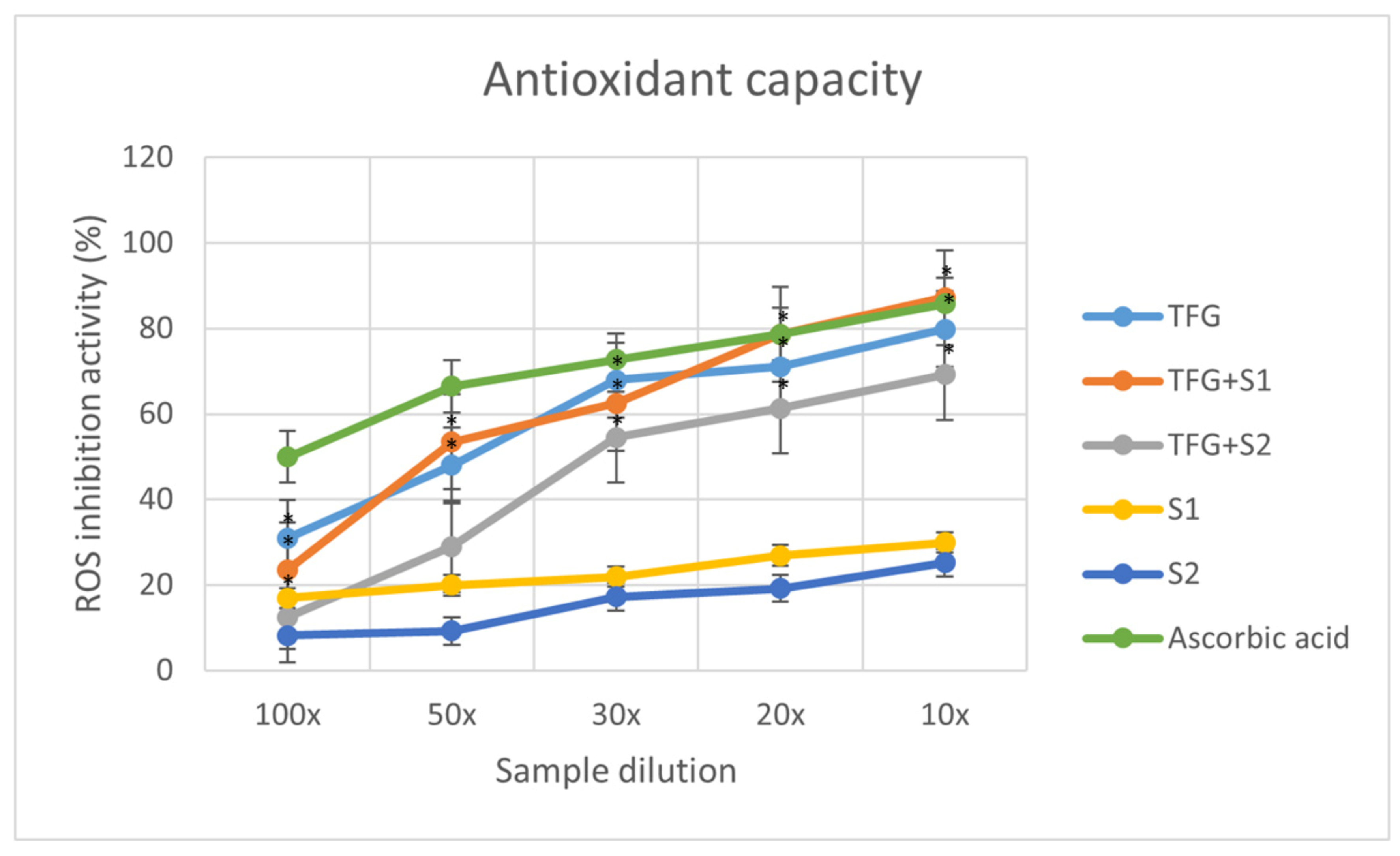
2.4. Antioxidant Capacity
2.5. Superoxide Dismutase (SOD) Assay
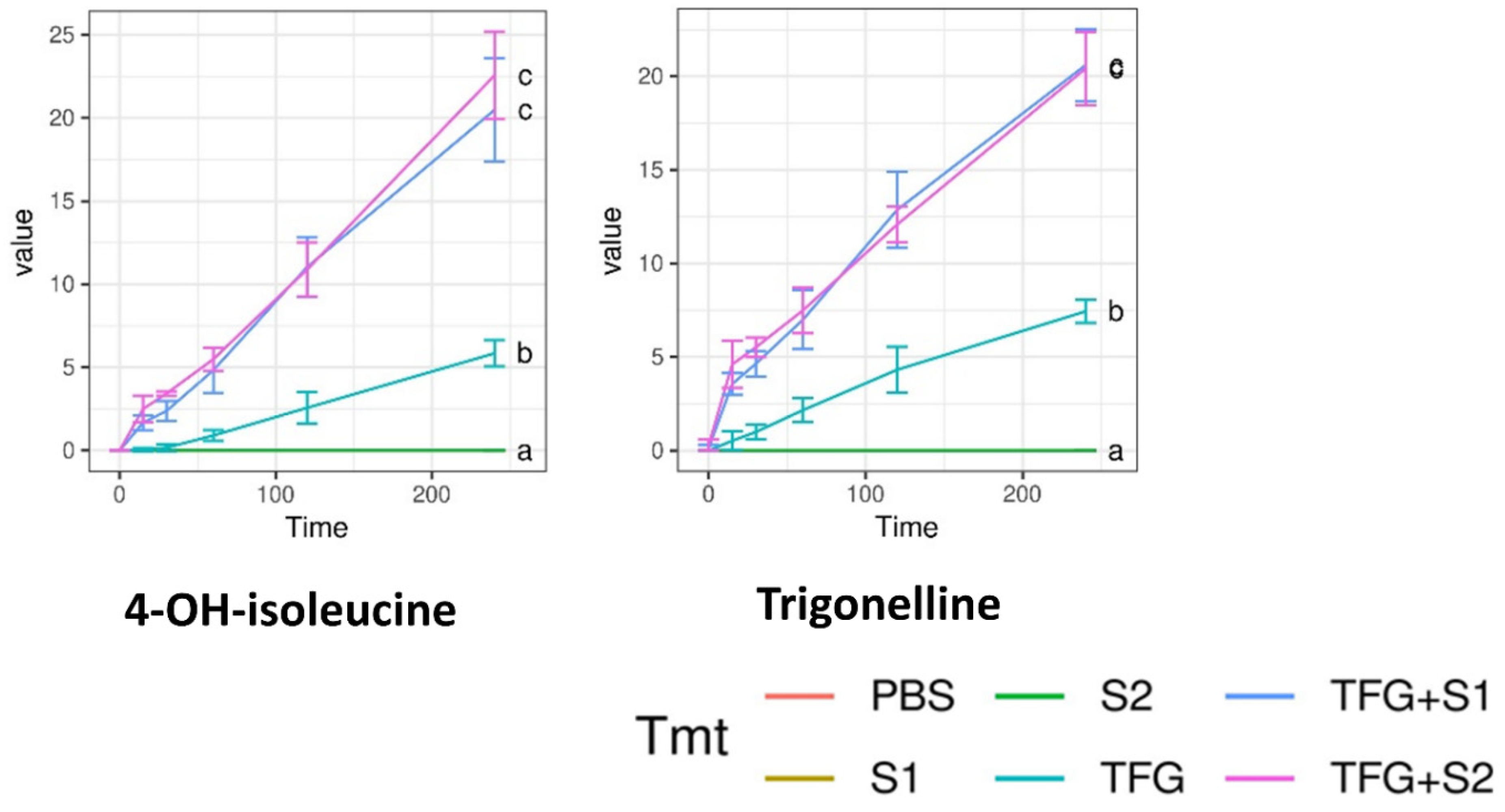
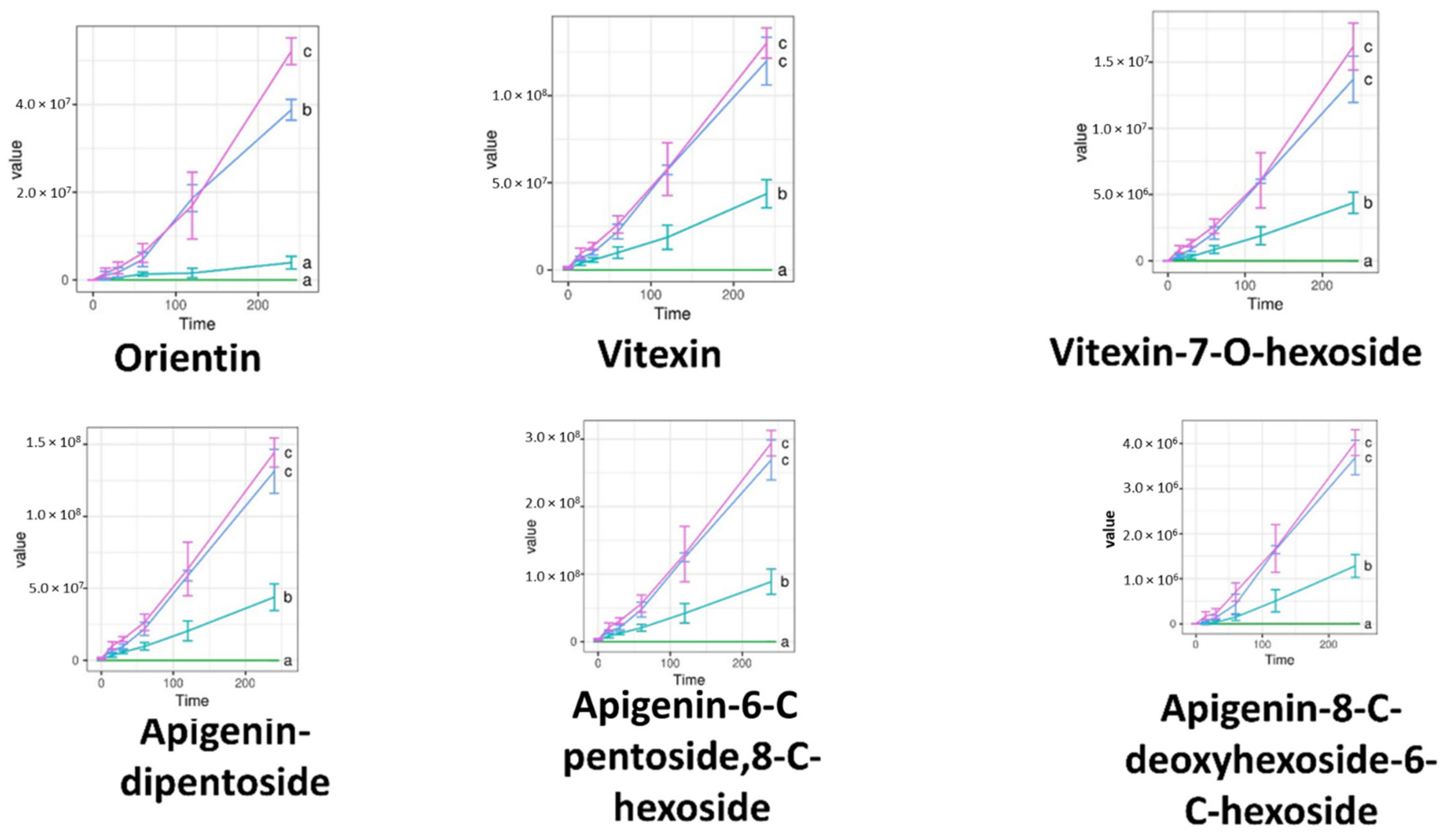
2.6. Permeability Assay
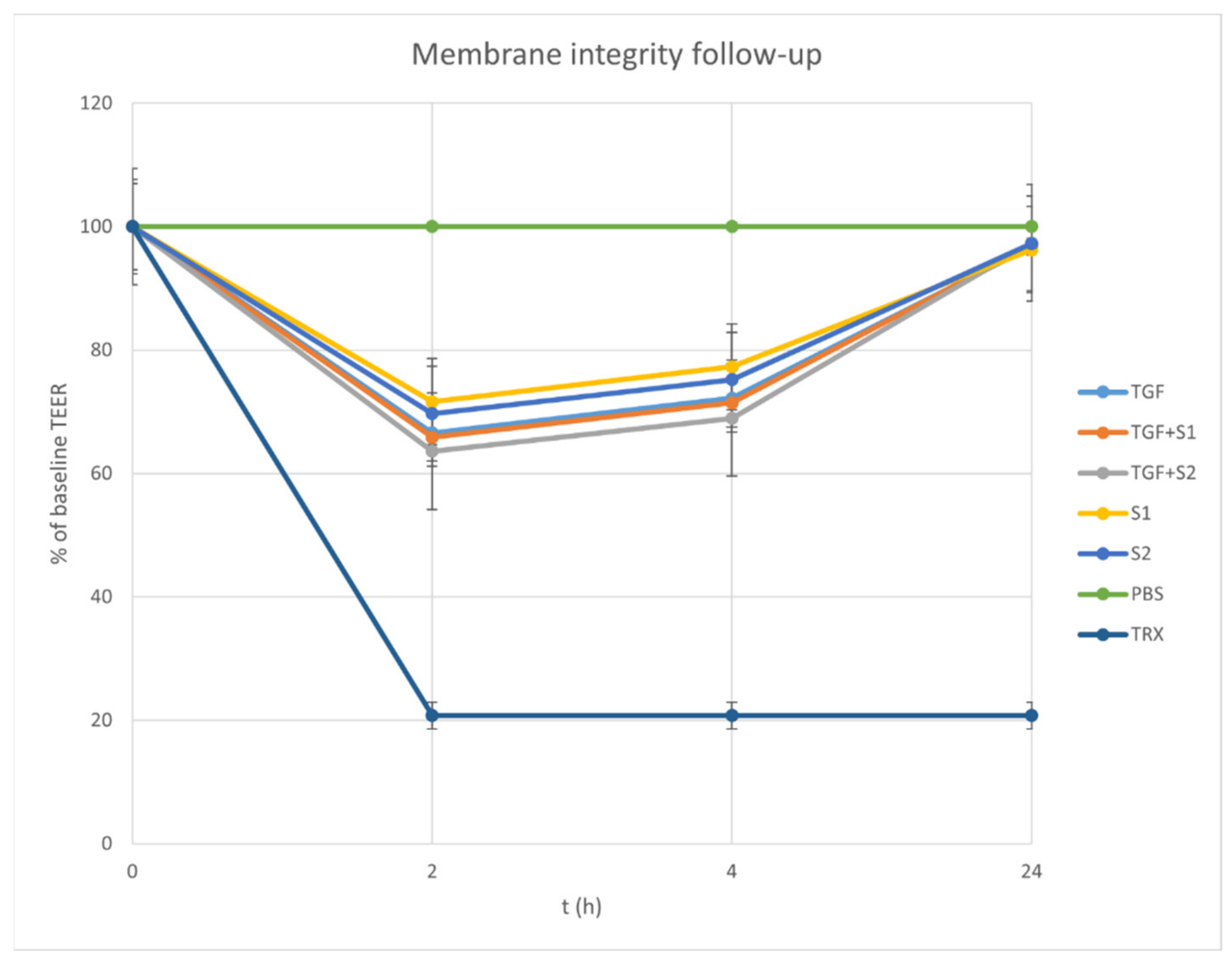
2.7. TEER Follow Up
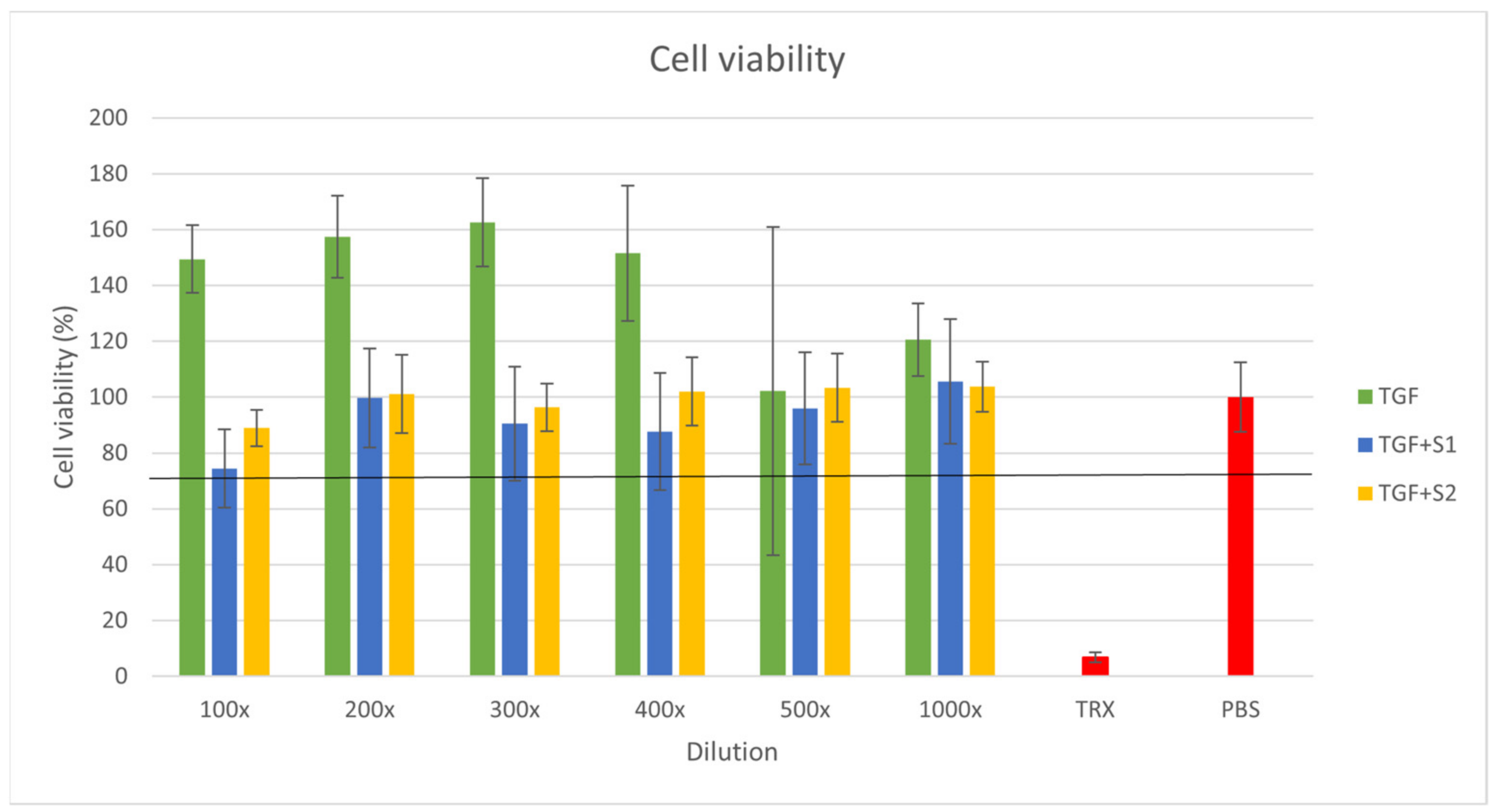
2.8. Citotoxicity Assay
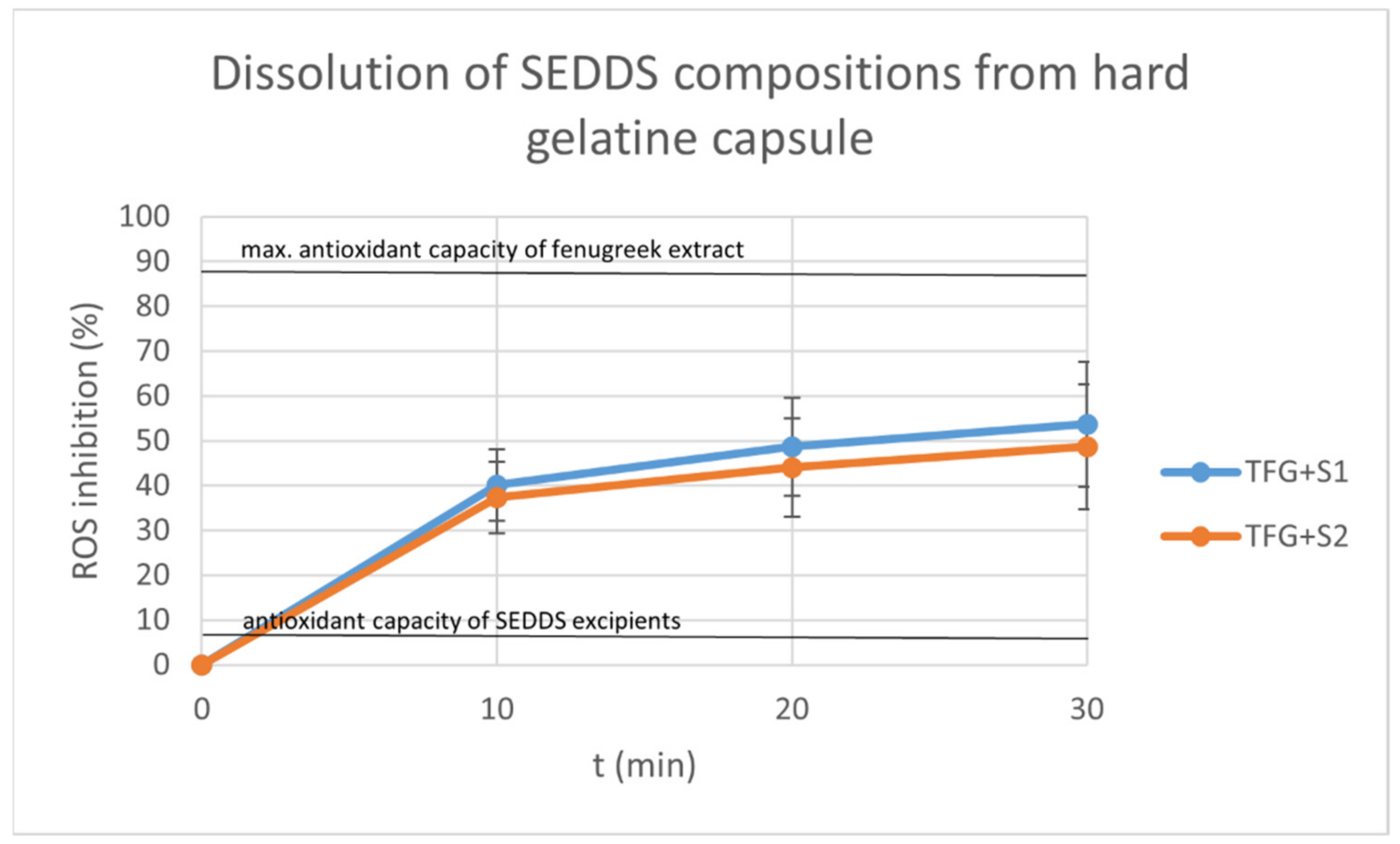
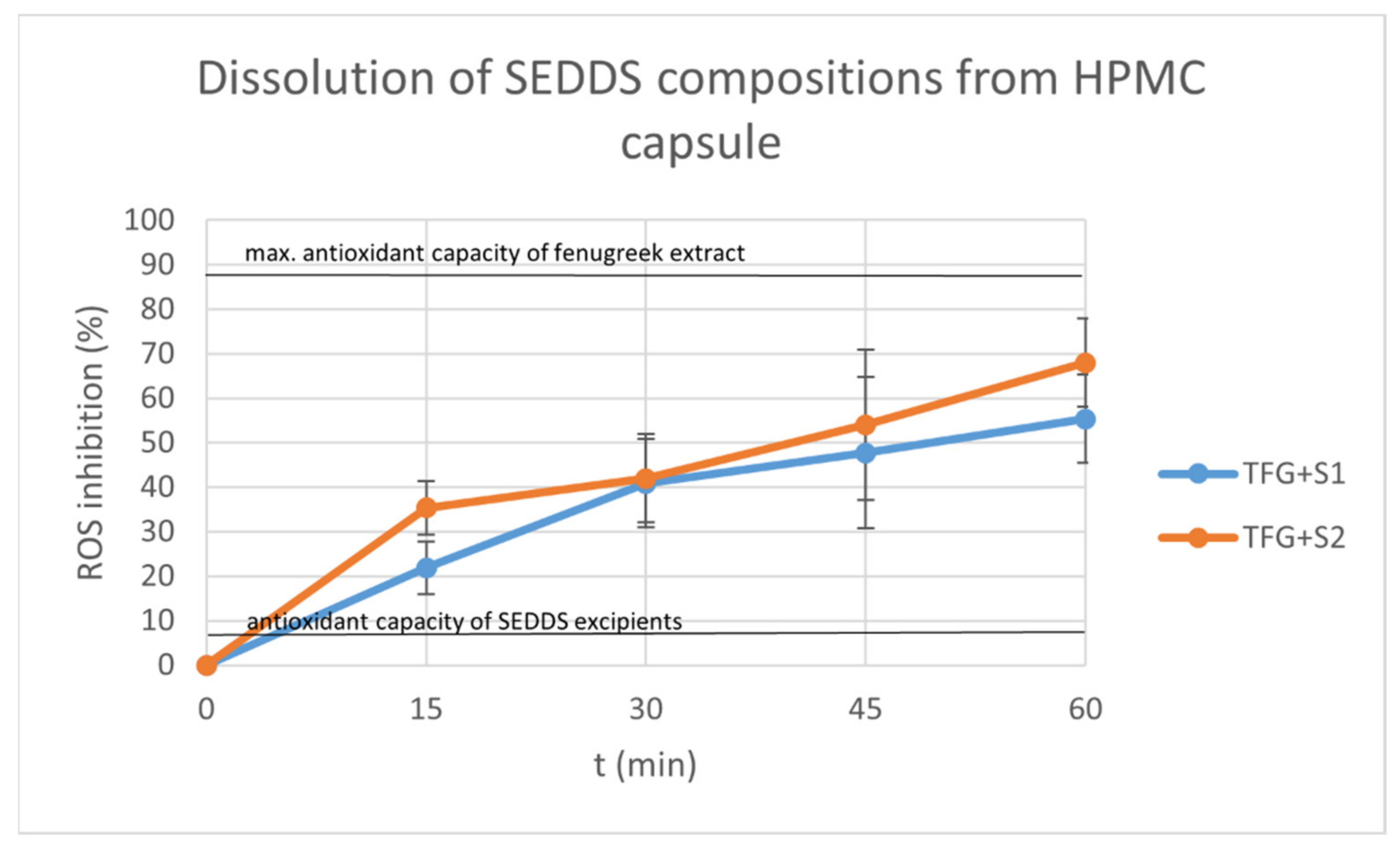
2.9. Dissolution Test
3. Discussion
4. Materials and Methods
4.1. Extraction Process
4.2. Self Emulsifying Drug Delivery Systems
4.3. Caco-2 Cell Line
4.4. Dynamic Light Scattering
4.5. Zeta Potential Analysis
4.6. Antioxidant Capacity
4.7. Superoxide Dismutase (SOD) Assay
4.8. Permeability Assay
4.9. Analytical Method and Metabolomics Quality Control Procedure
4.10. Citotoxicity Assay
4.11. Dissolution Test
4.12. Statystical Analysis and Graph Display
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Snehlata, H.S.; Payal, D.R. Fenugreek (Trigonella foenum-graecum L.): An overview. Int. J. Curr. Pharm. Rev. Res. 2011, 2, 169–187. [Google Scholar]
- Yadav, U.C.S.; Baquer, N.Z. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm. Biol. 2014, 52, 243–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zia, T.; Hasnain, S.N.; Hasan, S.K. Evaluation of the oral hypoglycaemic effect of Trigonella foenum-graecum L. (methi) in normal mice. J. Ethnopharmacol. 2001, 75, 191–195. [Google Scholar] [CrossRef]
- Bin-Hafeez, B.; Haque, R.; Parvez, S.; Pandey, S.; Sayeed, I.; Raisuddin, S. Immunomodulatory effects of fenugreek (Trigonella foenum graecum L.) extract in mice. Int. Immunopharmacol. 2003, 3, 257–265. [Google Scholar] [CrossRef]
- Abhilash, M.B.; Kumar, D.; Deepti, A.; Nair, A.; Greet, V.; An-Katrien, V.; Mieke, V.D.D.; Das Sivadasan, S.; Maliakel, B.; Chakrapani PS, B.; et al. Enhanced absorption ofcurcuminoidsand 3-Acetyl-11-keto-β-boswellic acid from fenugreek galactomannan hydrogel beadlets: A natural approach to the co-delivery of lipophilic phytonutrients. J. Funct. Foods 2021, 79, 104405. [Google Scholar] [CrossRef]
- Alqahtani, A.; Raut, B.; Khan, S.; Mohamed, J.M.; Fatease, A.A.; Alqahtani, T.; Alamri, A.; Ahmad, F.; Krishnaraju, V. The Unique Carboxymethyl Fenugreek Gum Gel Loaded Itraconazole Self-Emulsifying Nanovesicles for Topical Onychomycosis Treatment. Polymers 2022, 14, 325. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ubaidullah, M.; Ahmed, J.; Ahamad, T.; Ahmad, T.; Shaikh, S.F.; Naushad, M. Synthesis of NiOx@NPC composite for high-performance supercapacitor via waste PET plastic-derived Ni-MOF. Compos. Part B Eng. 2020, 183, 107655. [Google Scholar] [CrossRef]
- Al-Enizi, A.M.; Ahmed, J.; Ubaidullah, M.; Shaikh, S.F.; Ahamad, T.; Naushad, M.; Zheng, G. Utilization of waste polyethylene terephthalate bottles to develop metal-organic frameworks for energy applications: A clean and feasible approach. J. Clean. Prod. 2020, 248, 119251. [Google Scholar] [CrossRef]
- Ito, Y. (Ed.) Drug delivery systems. In Photochemistry for Biomedical Applications: From Device Fabrication to Diagnosis and Therapy; Springer: Cham, Switzerland, 2018; pp. 231–275. [Google Scholar]
- Gursoy, R.N.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef]
- Tripathi, S.; Kushwah, V.; Thanki, K.; Jain, S. Triple antioxidant SNEDDS formulation with enhanced oral bioavailability: Implication of chemoprevention of breast cancer. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1431–1443. [Google Scholar] [CrossRef]
- Date, A.A.; Desai, N.; Dixit, R.; Nagarsenker, M. Self-nanoemulsifying drug delivery systems: Formulation insights, applications and advances. Nanomedicine 2010, 5, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, N.; Shi, J.; García-Closas, M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat. Rev. Genet. 2016, 17, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Zhang, M.; Pang, Y.; Li, Z.; Zhao, A.; Feng, J. Self-emulsifying drug delivery system and the applications in herbal drugs. Drug Deliv. 2015, 22, 475–486. [Google Scholar] [CrossRef] [Green Version]
- Verma, R. Recent Developments in Self-microemulsifying Drug Delivery System: An Overview. Asian J. Pharm. 2020, 13, 59–72. [Google Scholar]
- Wu, W.; Wang, Y.; Que, L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur. J. Pharm. Biopharm. 2006, 63, 288–294. [Google Scholar] [CrossRef]
- Chen, Z.Q.; Liu, Y.; Zhao, J.H.; Wang, L.; Feng, N.P. Improved oral bioavailability of poorly water-soluble indirubin by a supersaturatable self-microemulsifying drug delivery system. Int. J. Nanomed. 2012, 7, 1115–1125. [Google Scholar]
- Kalantari, A.; Kósa, D.; Nemes, D.; Ujhelyi, Z.; Fehér, P.; Vecsernyés, M.; Váradi, J.; Fenyvesi, F.; Kuki, Á.; Gonda, S.; et al. Self-Nanoemulsifying Drug Delivery Systems Containing Plantago lanceolata—An Assessment of Their Antioxidant and Antiinflammatory Effects. Molecules 2017, 22, 1773. [Google Scholar] [CrossRef] [Green Version]
- Jyothi, D.; Koland, M.; Priya, S.; James, J. Formulation of Herbal Capsule Containing Trigonella foenum-graecum Seed Extract for the Treatment of Diabetes. J. Young Pharm. 2017, 9, 352–356. [Google Scholar] [CrossRef] [Green Version]
- Jain, M. Process Optimization for Formulating Trigonella foenum-graecum and Gymnema sylvestre Added Vegetable Cereal Mix Using Response Surface Methodology. J. Nutr. Health Food Eng. 2016, 4, 416–428. [Google Scholar] [CrossRef]
- Bire, P.; Trivedi, S.S.; Durugkar, N.; Umekar, M. Formulation and Cytotoxic Characterization of Trigonella foenum Loaded Polymeric Nanoparticles. J. Drug Deliv. Ther. 2020, 10, 187–192. [Google Scholar] [CrossRef]
- Adimoolam, S.; Phonhaxa, S. Trigonella-foenum graecum L. seed mucilage-based mucoadhesive microspheres of diclofenac sodium. J. Anal. Pharm. Res. 2018, 7, 114–119. [Google Scholar] [CrossRef] [Green Version]
- Taofeek, O. Molecular Docking and Admet Analyses of Photochemicals from Nigella sativa (blackseed), Trigonella foenum-graecum (Fenugreek) and Anona muricata (Soursop) on SARS-CoV-2 Target. Sci. Open, 2020; preprint. [Google Scholar]
- Okawara, M.; Tokudome, Y.; Todo, H.; Sugibayashi, K.; Hashimoto, F. Effect of ß-Cyclodextrin derivatives on the diosgenin absorption in caco-2 cell monolayer and rats. Biol. Pharm. Bull. 2014, 37, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Wahlang, B.; Pawar, Y.B.; Bansal, A.K. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur. J. Pharm. Biopharm. 2011, 77, 275–282. [Google Scholar] [CrossRef]
- Piazzini, V.; Rosseti, C.; Bigagli, E.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Prediction of Permeation and Cellular Transport of Silybum marianum Extract Formulated in a Nanoemulsion by Using PAMPA and Caco-2 Cell Models. Planta Med. 2017, 83, 1184–1193. [Google Scholar] [CrossRef]
- Qiang, Z.; Ye, Z.; Hauck, C.; Murphy, P.A.; McCoy, J.A.; Widrlechner, M.P.; Reddy, M.B.; Hendrich, S. Permeability of rosmarinic acid in Prunella vulgaris and ursolic acid in Salvia officinalis extracts across Caco-2 cell monolayers. J. Ethnopharmacol. 2011, 137, 1107–1112. [Google Scholar] [CrossRef] [Green Version]
- Piazzini, V.; Monteforte, E.; Luceri, C.; Bigagli, E.; Bilia, A.R.; Bergonzi, M.C. Nanoemulsion for improving solubility and permeability of vitex agnus-castus extract: Formulation and in vitro evaluation using PAMPA and Caco-2 approaches. Drug Deliv. 2017, 24, 380–390. [Google Scholar] [CrossRef] [Green Version]
- Flynn, T.J.; Vohra, S.N. Simultaneous determination of intestinal permeability and potential drug interactions of complex mixtures using Caco-2 cells and high-resolution mass spectrometry: Studies with Rauwolfia serpentina extract. Chem. Biol. Interact. 2018, 290, 37–43. [Google Scholar] [CrossRef]
- Sha, X.; Yan, G.; Wu, Y.; Li, J.; Fang, X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur. J. Pharm. Sci. 2005, 24, 477–486. [Google Scholar] [CrossRef]
- Patel, M.H.; Sawant, K.K. Self microemulsifying drug delivery system of lurasidone hydrochloride for enhanced oral bioavailability by lymphatic targeting: In vitro, Caco-2 cell line and in vivo evaluation. Eur. J. Pharm. Sci. 2019, 138, 105027. [Google Scholar] [CrossRef] [PubMed]
- Sangsen, Y.; Wiwattanawongsa, K.; Likhitwitayawuid, K.; Sritularak, B.; Wiwattanapatapee, R. Comparisons between a self-microemulsifying system and lipid nanoparticles of oxyresveratrol on the physicochemical properties and Caco-2 cell permeability. Eur. J. Lipid Sci. Technol. 2017, 119, 1600053. [Google Scholar] [CrossRef]
- Liu, J.; Werner, U.; Funke, M.; Besenius, M.; Saaby, L.; Fanø, M.; Mu, H.; Müllertz, A. SEDDS for intestinal absorption of insulin: Application of Caco-2 and Caco-2/HT29 co-culture monolayers and intra-jejunal instillation in rats. Int. J. Pharm. 2019, 560, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Rasheed, D.M.; Kropf, M.; Heiss, A.G. Metabolite profiling in Trigonella seeds via UPLC-MS and GC-MS analyzed using multivariate data analyses. Anal. Bioanal. Chem. 2016, 408, 8065–8078. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.-P.; Zhao, Y.; Pang, X.; Yu, H.-S.; Xiong, C.-Q.; Zhang, J.; Gao, Y.; Yu, K.; Liu, C.; Ma, B.-P. Characterization and identification of steroidal saponins from the seeds of Trigonella foenum-graecum by ultra high-performance liquid chromatography and hybrid time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2013, 74, 257–267. [Google Scholar] [CrossRef]
- Król-Kogus, B.; Głód, D.; Krauze-Baranowska, M. Qualitative and quantitative HPLC-ELSD-ESI-MS analysis of steroidal saponins in fenugreek seed. Acta Pharm. 2020, 70, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Jiang, W.; Liu, Z.; Wang, J.; Fu, T.; Wang, Y. Analysis and identification of chemical constituents of fenugreek by UPLC-IT-MSnand UPLC-Q-TOF-MS. Chem. Res. Chin. Univ. 2017, 33, 721–730. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. 3—Methods for characterization of nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 43–58. ISBN 978-0-08-100557-6. [Google Scholar]
- Avalos-Soriano, A.; De la Cruz-Cordero, R.; Rosado, J.L.; Garcia-Gasca, T. 4-Hydroxyisoleucine from Fenugreek (Trigonella foenum-graecum): Effects on Insulin Resistance Associated with Obesity. Molecules 2016, 21, 1596. [Google Scholar] [CrossRef] [Green Version]
- Fuller, S.; Stephens, J.M. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: Mechanisms of actions and potential effects on metabolic syndrome. Adv. Nutr. 2015, 6, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Eddouks, M.; Lemhadri, A.; Hebi, M.; El Hidani, A.; Zeggwagh, N.A.; El Bouhali, B.; Hajji, L.; Burcelin, R. Capparis spinosa L. aqueous extract evokes antidiabetic effect in streptozotocin-induced diabetic mice. Avicenna J. Phytomed. 2017, 7, 191–198. [Google Scholar]
- Trommelen, J.; Tomé, D.; van Loon, L.J.C. Gut amino acid absorption in humans: Concepts and relevance for postprandial metabolism. Clin. Nutr. Open Sci. 2021, 36, 43–55. [Google Scholar] [CrossRef]
- Renukuntla, J.; Vadlapudi, A.D.; Patel, A.; Boddu, S.H.S.; Mitra, A.K. Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 2013, 447, 75–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Chan, L.; Zhou, S. Trigonelline: A plant alkaloid with therapeutic potential for diabetes and central nervous system disease. Curr. Med. Chem. 2012, 19, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Islam, M.S. Spice-Derived Bioactive Ingredients: Potential Agents or Food Adjuvant in the Management of Diabetes Mellitus. Front. Pharmacol. 2018, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Chakraborty, P.; Mukherjee, B.; De Feo, V. Plant-Based Antidiabetic Nanoformulations: The Emerging Paradigm for Effective Therapy. Int. J. Mol. Sci. 2020, 21, 2217. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Alshehri, S.M.; Shakeel, F.; Ibrahim, M.A.; Elzayat, E.M.; Altamimi, M.; Mohsin, K.; Almeanazel, O.T.; Alkholief, M.; Alshetaili, A.; Alsulays, B.; et al. Dissolution and bioavailability improvement of bioactive apigenin using solid dispersions prepared by different techniques. Saudi Pharm. J. 2019, 27, 264–273. [Google Scholar] [CrossRef]
- Abcha, I.; Souilem, S.; Neves, M.A.; Wang, Z.; Nefatti, M.; Isoda, H.; Nakajima, M. Ethyl oleate food-grade O/W emulsions loaded with apigenin: Insights to their formulation characteristics and physico-chemical stability. Food Res. Int. 2019, 116, 953–962. [Google Scholar] [CrossRef]
- Aungst, B.J. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J. Pharm. Sci. 1993, 82, 979–987. [Google Scholar] [CrossRef]
- Boyd, B.J.; Bergström, C.A.S.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Préat, V.; et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Omri, A. Formulation strategies to improve the bioavailability of poorly absorbed drugs with special emphasis on self-emulsifying systems. ISRN Pharm. 2013, 2013, 848043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baghel, P.; Roy, A.; Verma, S.; Satapathy, T.; Bahadur, S. Amelioration of lipophilic compounds in regards to bioavailability as self-emulsifying drug delivery system (SEDDS). Future J. Pharm. Sci. 2020, 6, 21. [Google Scholar] [CrossRef]
- Singh, B.; Beg, S.; Khurana, R.K.; Sandhu, P.S.; Kaur, R.; Katare, O.P. Recent advances in self-emulsifying drug delivery systems (SEDDS). Crit. Rev. Ther. Drug Carr. Syst. 2014, 31, 121–185. [Google Scholar] [CrossRef] [PubMed]
- Assi, R.A.; Abdulbaqi, I.M.; Ming, T.S.; Yee, C.S.; Wahab, H.A.; Asif, S.M.; Darwis, Y. Liquid and solid self-emulsifying drug delivery systems (Sedds) as carriers for the oral delivery of azithromycin: Optimization, in vitro characterization and stability assessment. Pharmaceutics 2020, 12, 1052. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szekalska, M.; Amelian, A.; Szymańska, E.; Winnicka, K. Development and evaluation of liquid and solid self-emulsifying drug delivery systems for atorvastatin. Molecules 2015, 20, 21010–21022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yan, W.; Tian, Y.; Xue, H.; Tang, J.; Zhang, L. Self-microemulsifying drug delivery system of phillygenin: Formulation development, characterization and pharmacokinetic evaluation. Pharmaceutics 2020, 12, 130. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; He, L.; Yue, S.; Huang, Q.; Zhang, Y.; Yang, J. Characterization and evaluation of a self-microemulsifying drug delivery system containing tectorigenin, an isoflavone with low aqueous solubility and poor permeability. Drug Deliv. 2017, 24, 632–640. [Google Scholar] [CrossRef] [Green Version]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł.; Duchnik, W.; Klimowicz, A. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives. AMB Express 2020, 10, 187. [Google Scholar] [CrossRef]
- Shoemaker, M.; Cohen, I.; Campbell, M. Reduction of MTT by aqueous herbal extracts in the absence of cells. J. Ethnopharmacol. 2004, 93, 381–384. [Google Scholar] [CrossRef]
- Bernhard, D.; Schwaiger, W.; Crazzolara, R.; Tinhofer, I.; Kofler, R.; Csordas, A. Enhanced MTT-reducing activity under growth inhibition by resveratrol in CEM-C7H2 lymphocytic leukemia cells. Cancer Lett. 2003, 195, 193–199. [Google Scholar] [CrossRef]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef]
- Ujhelyi, Z.; Kalantari, A.; Vecsernyés, M.; Róka, E.; Fenyvesi, F.; Póka, R.; Kozma, B.; Bácskay, I. The enhanced inhibitory effect of different antitumor agents in self-microemulsifying drug delivery systems on human cervical cancer HeLa cells. Molecules 2015, 20, 13226–13239. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Formulation | Zeta Potential | Standard Deviation |
|---|---|---|
| TFG + S1 | −71.3 mV | 11.8 mV |
| TFG + S2 | −38.5 mV | 7.47 mV |
| ANOVA Followed by Tukey’s Multiple Comparison Test Result | Level of Significance |
|---|---|
| TFG vs. TFG + S1, 10× dilution | **** |
| TFG vs. TFG + S2, 100× dilution | *** |
| TFG + S1 vs. TFG + S2, 100× dilution | **** |
| TFG vs. TFG + S1, 200× dilution | ** |
| TFG vs. TFG + S2, 200× dilution | ns |
| TFG + S1 vs. TFG + S2, 200× dilution | ns |
| TFG vs. TFG + S1, 300× dilution | ** |
| TFG vs. TFG + S2, 300× dilution | ns |
| TFG + S1 vs. TFG + S2, 300× dilution | ns |
| TFG vs. TFG + S1, 400× dilution | ** |
| TFG vs. TFG + S2, 400× dilution | ns |
| TFG + S1 vs. TFG + S2, 400× dilution | ns |
| TFG vs. TFG + S1, 500× dilution | ns |
| TFG vs. TFG + S2, 500× dilution | ns |
| TFG + S1 vs. TFG + S2, 500× dilution | ns |
| TFG vs. TFG + S1, 1000× dilution | ns |
| TFG vs. TFG + S2, 1000× dilution | ns |
| TFG | S1 | TFG + S1 | S2 | TFG + S2 | |
|---|---|---|---|---|---|
| Fenugreek extract | 6 mL | - | 6 mL | - | 6 mL |
| PBS | 3 mL | 6 mL | - | 6 mL | - |
| isopropyl myristate | - | 1 mL | 1 mL | 0.25 mL | 0.25 mL |
| Transcutol HP | - | 1 mL | 1 mL | 1.5 mL | 1.5 mL |
| Labrasol | - | 1 mL | 1 mL | 0.5 mL | 0.5 mL |
| Kolliphor RH40 | - | - | - | 0.5 mL | 0.5 mL |
| Capryol 90 | - | - | - | 0.25 mL | 0.25 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinka, D.; Doma, E.; Szendi, N.; Páll, J.; Kósa, D.; Pető, Á.; Fehér, P.; Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; et al. Formulation, Characterization and Permeability Studies of Fenugreek (Trigonella foenum-graecum) Containing Self-Emulsifying Drug Delivery System (SEDDS). Molecules 2022, 27, 2846. https://doi.org/10.3390/molecules27092846
Sinka D, Doma E, Szendi N, Páll J, Kósa D, Pető Á, Fehér P, Ujhelyi Z, Fenyvesi F, Váradi J, et al. Formulation, Characterization and Permeability Studies of Fenugreek (Trigonella foenum-graecum) Containing Self-Emulsifying Drug Delivery System (SEDDS). Molecules. 2022; 27(9):2846. https://doi.org/10.3390/molecules27092846
Chicago/Turabian StyleSinka, Dávid, Enikő Doma, Nóra Szendi, Jázmin Páll, Dóra Kósa, Ágota Pető, Pálma Fehér, Zoltán Ujhelyi, Ferenc Fenyvesi, Judit Váradi, and et al. 2022. "Formulation, Characterization and Permeability Studies of Fenugreek (Trigonella foenum-graecum) Containing Self-Emulsifying Drug Delivery System (SEDDS)" Molecules 27, no. 9: 2846. https://doi.org/10.3390/molecules27092846
APA StyleSinka, D., Doma, E., Szendi, N., Páll, J., Kósa, D., Pető, Á., Fehér, P., Ujhelyi, Z., Fenyvesi, F., Váradi, J., Vecsernyés, M., Szűcs, Z., Gonda, S., Cziáky, Z., Kiss-Szikszai, A., Vasas, G., & Bácskay, I. (2022). Formulation, Characterization and Permeability Studies of Fenugreek (Trigonella foenum-graecum) Containing Self-Emulsifying Drug Delivery System (SEDDS). Molecules, 27(9), 2846. https://doi.org/10.3390/molecules27092846













