Fast Melt Cocoa Butter Tablet: Effect of Waxes, Starch, and PEG 6000 on Physical Properties of the Preparation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cocoa Butter Based FMT
2.3. Characterization Tests
2.3.1. Evaluation of Hardness
2.3.2. Evaluation of Weight
2.3.3. Evaluation of Thickness
2.3.4. Friability Test
2.3.5. In Vitro Disintegration Time Test
2.3.6. In Situ Disintegration Time Test
2.3.7. In Vitro Disintegration Time Test in Artificial Saliva
2.3.8. Melting Point Test
2.3.9. Selection of Optimum Base
2.3.10. The Incorporation of Taste-Masked Dapoxetine in Cocoa Butter FMT Formulation
2.4. Palatability Study
2.5. Content Uniformity
2.6. HPLC-UV Assay Method
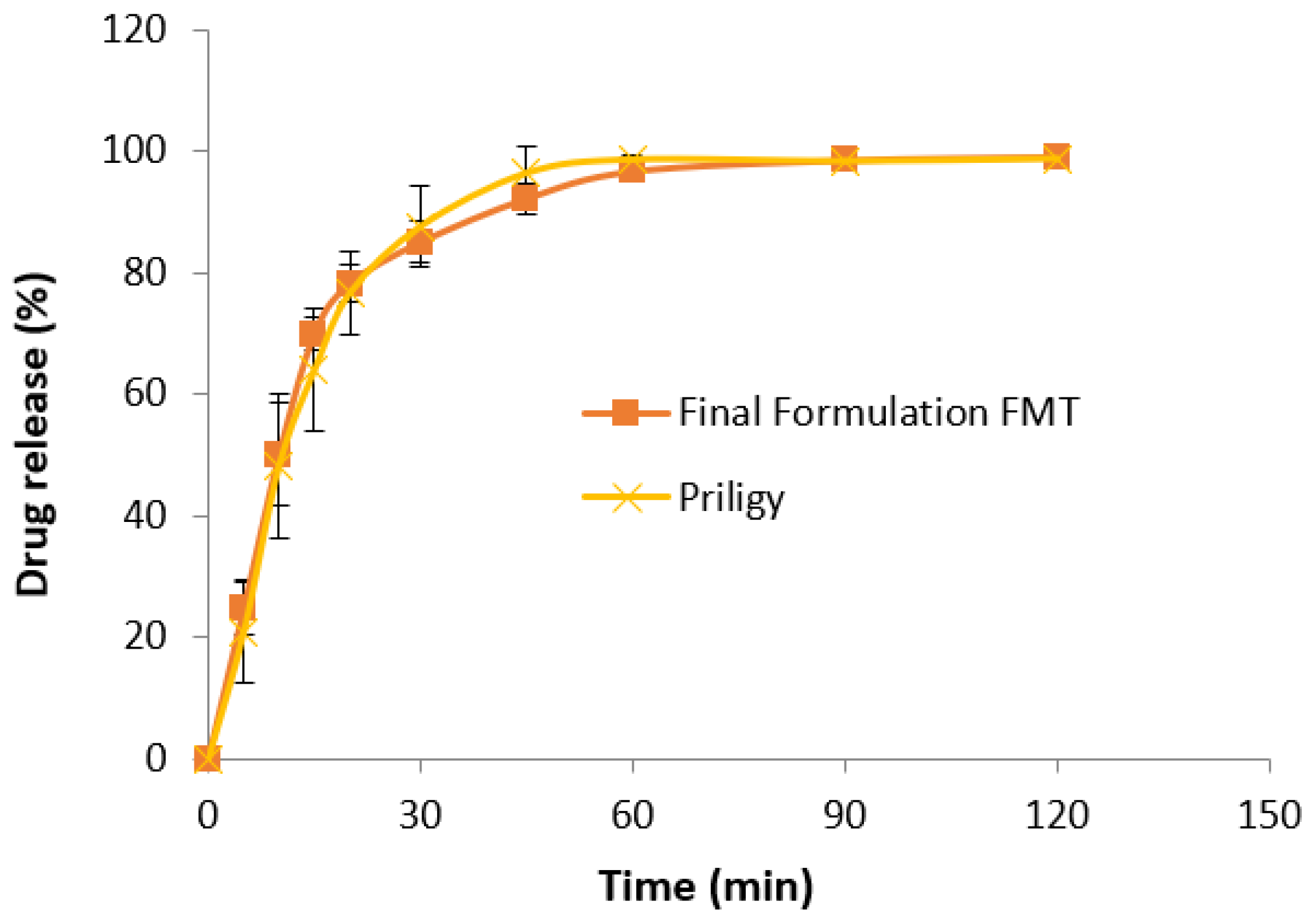
2.7. Drug Release Study
2.8. Stability Study
2.9. Statistical Analysis
3. Results
3.1. Hardness
3.2. Thickness, Weight, and Friability
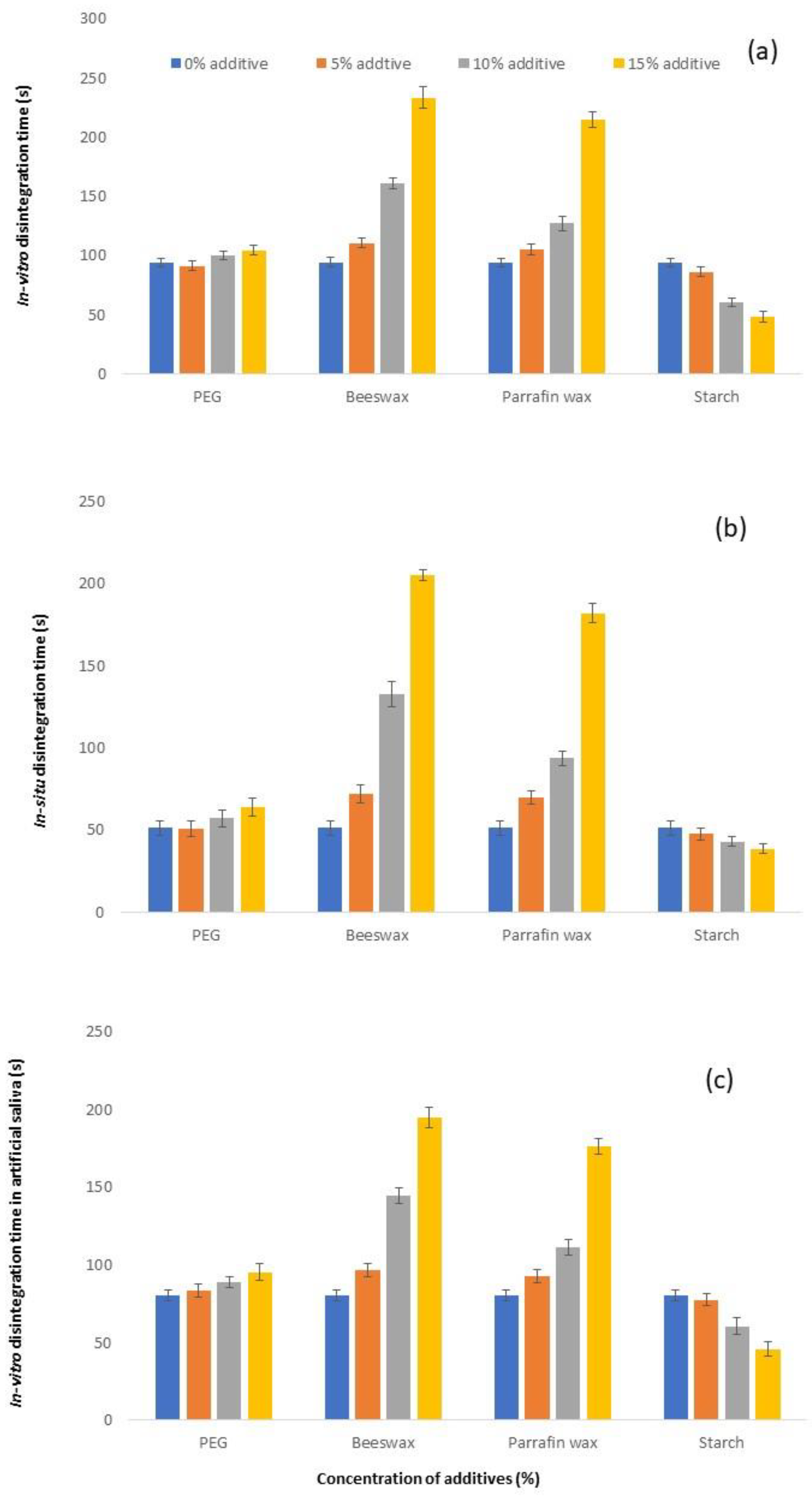
3.3. Disintegration Time
3.4. Selection of Optimum Base
3.5. Palatability Study
3.6. Content Determination
3.7. In Vitro Drug Release Study
3.8. Stability Study
4. Discussion
4.1. Development of FMT Base
4.2. Effect of Additive
4.3. Palatability Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ong, S.G.; Ming, L.C.; Lee, K.S.; Yuen, K.H. Influence of the Encapsulation Efficiency and Size of Liposome on the Oral Bioavailability of Griseofulvin-Loaded Liposomes. Pharmaceutics 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Neoh, C.F.; Long, C.M.; Lim, S.M.; Ramasamy, K.; Shahar, S.; Majeed, A.B.A. Medication use and adherence among multi-ethnic community-dwelling older adults in Malaysia. Geriatr. Gerontol. Int. 2017, 17, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; Nafadi, M.M.; Fatahalla, F.A. Formulation of a fast-dissolving ketoprofen tablet using freeze-drying in blisters technique. Drug Dev. Ind. Pharm. 2006, 32, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Ghosh, B. Fast dissolving drug delivery systems: A review of the literature. Indian J. Pharm. Sci. 2002, 64, 331. [Google Scholar]
- Makino, T.; Yamada, M.; Kikuta, J.i. Fast Dissolving Tablet and Its Production. U.S. Patent No. 5,720,974, 24 February 1998. [Google Scholar]
- Liew, K.B.; Peh, K.K. Investigation on the effect of polymer and starch on the tablet properties of lyophilized orally disintegrating tablet. Arch. Pharm. Res. 2021, 44, 1–10. [Google Scholar] [CrossRef]
- Liew, K.B.; Peh, K.K. Stability indicating HPLC-UV method for determination of dapoxetine HCl in pharmaceutical product. Acta Pol. Pharm. 2014, 71, 393–400. [Google Scholar]
- Fotaki, N.; Long, C.; Tang, K.; Chokshi, H. Dissolution of Amorphous Solid Dispersions: Theory and Practice; Springer: New York, NY, USA, 2014. [Google Scholar]
- Long, C.M. Biopharmaceutical Considerations and In Vitro-In Vivo Correlations (IVIVCs) for Orally Administered Amorphous Formulations; University of Bath: Claverton Down, UK, 2014. [Google Scholar]
- Liew, K.B.; Tan, Y.T.; Peh, K.K. Effect of polymer, plasticizer and filler on orally disintegrating film. Drug Dev. Ind. Pharm. 2014, 40, 110–119. [Google Scholar] [CrossRef]
- Bhasin, R.K.; Bhasin, N.; Ghosh, P.K. Advances in formulation of orally disintegrating dosage forms: A review article. Indo Glob. J. Pharm. Sci. 2011, 1, 328–353. [Google Scholar]
- Baheti, A.; Kumar, L.; Bansal, A.K. Excipients used in lyophilization of small molecules. J. Excip. Food Chem. 2016, 1, 1135. [Google Scholar]
- Lai, F.; Pini, E.; Corrias, F.; Perricci, J.; Manconi, M.; Fadda, A.M.; Sinico, C. Formulation strategy and evaluation of nanocrystal piroxicam orally disintegrating tablets manufacturing by freeze-drying. Int. J. Pharm. 2014, 467, 27–33. [Google Scholar] [CrossRef]
- Venkata, R.R.S.; Sathyanarayana, D.; Manavalan, R.; Sreekanth, J. Comparison of lyophilization and compression technique of risperidone oral disintegrating tablets. Der. Pharm. Chem. 2010, 2, 172–184. [Google Scholar]
- Widjaja, B.; Setyawan, D.; Moechtar, J. Development of piroxicam orally disintegrating tablets by freeze drying method. Int. J. Pharm. Pharm. Sci. 2013, 5, 795–798. [Google Scholar]
- Allen, L.V., Jr.; Wang, B. Process for Making a Particulate Support Matrix for Making a Rapidly Dissolving Tablet. U.S. Patent No. 5,587,180, 24 December 1996. [Google Scholar]
- Allen, L.V., Jr.; Wang, B. Method of Making a Rapidly Dissolving Tablet. U.S. Patent No. 5,635,210, 8 December 1994. [Google Scholar]
- Allen, L.V., Jr.; Wang, B. Rapidly Dissolving Tablets. U.S. Patent No. 5,807,576, 3 February 1994. [Google Scholar]
- Gohel, M.; Patel, M.; Amin, A.; Agrawal, R.; Dave, R.; Bariya, N. Formulation design and optimization of mouth dissolve tablets of nimesulide using vacuum drying technique. AAPs PharmSciTech 2004, 5, 10–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, H.; Rothe, W. Preparation of Porous Tablets. U.S. Patent No. 3,885,026, 20 May 1975. [Google Scholar]
- Koizumi, K.-i.; Watanabe, Y.; Morita, K.; Utoguchi, N.; Matsumoto, M. New method of preparing high-porosity rapidly saliva soluble compressed tablets using mannitol with camphor, a subliming material. Int. J. Pharm. 1997, 152, 127–131. [Google Scholar] [CrossRef]
- Roser, B.J.; Blair, J. Rapidly Soluble Oral Solid Dosage Forms, Methods of Making Same, and Compositions Thereof. U.S. Patent No. 5,762,961, 9 June 1998. [Google Scholar]
- Parakh, S.R.; Gothoskar, A.V. A review of mouth dissolving tablet technologies. Pharm. Technol. 2003, 27, 92–100. [Google Scholar]
- Kuno, Y.; Kojima, M.; Ando, S.; Nakagami, H. Evaluation of rapidly disintegrating tablets manufactured by phase transition of sugar alcohols. J. Control. Release 2005, 105, 16–22. [Google Scholar] [CrossRef]
- Dobetti, L. Fast-melting tablets: Developments and technologies. Pharm. Technol. 2001, 44–50. [Google Scholar]
- Brniak, W.; Jachowicz, R.; Krupa, A.; Skorka, T.; Niwinski, K. Evaluation of co-processed excipients used for direct compression of orally disintegrating tablets (ODT) using novel disintegration apparatus. Pharm. Dev. Technol. 2013, 18, 464–474. [Google Scholar] [CrossRef]
- Liew, K.B.; Tan, Y.T.; Peh, K.K. Taste-masked and affordable donepezil hydrochloride orally disintegrating tablet as promising solution for non-compliance in Alzheimer’s disease patients. Drug Dev. Ind. Pharm. 2015, 41, 583–593. [Google Scholar] [CrossRef]
- Saleem, M.A.; Taher, M.; Sanaullah, S.; Najmuddin, M.; Ali, J.; Humaira, S.; Roshan, S. Formulation and Evaluation of Tramadol hydrochloride Rectal Suppositories. Indian J. Pharm. Sci. 2008, 70, 640–644. [Google Scholar] [CrossRef] [Green Version]
- Kasparaviciene, G.; Savickas, A.; Kalveniene, Z.; Velziene, S.; Kubiliene, L.; Bernatoniene, J. Evaluation of beeswax influence on physical properties of lipstick using instrumental and sensory methods. Evid.-Based Complement. Altern. Med. 2016, 2016, 3816460. [Google Scholar] [CrossRef] [PubMed]
- Belniak, P.; Swiader, K.; Szumilo, M.; Hyla, A.; Poleszak, E. Comparison of physicochemical properties of suppositories containing starch hydrolysates. Saudi Pharm. J. 2017, 25, 365–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowe, R.C.; Sheskey, P.; Quinn, M. Handbook of Pharmaceutical Excipients; Libros Digitales-Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Rahman, B.M.; Wahed, M.I.I.; Khondkar, P.; Ahmed, M.; Islam, R.; Barman, R.K.; Islam, M. Effect of starch 1500 as a binder and disintegrant in lamivudine tablets prepared by high shear wet granulation. Pak. J. Pharm. Sci. 2008, 21, 455–459. [Google Scholar] [PubMed]
- Herbert, A.L.; Lean, L.; Josoph, B.S. Pharmaceutical Dosage Forms, Tablets, 2nd ed.; Marcel Decker: New York, NY, USA, 1989. [Google Scholar]
- Patel, N.R.; Hopponent, R.E. Mechanism of action of starch as a disintegrating agent in aspirin tablets. J. Pharm. Sci. 1966, 55, 1065–1068. [Google Scholar] [CrossRef]
- Drew, M.C. Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytol. 1975, 75, 479–490. [Google Scholar] [CrossRef]
- Gontard, N.; Duchez, C.; Cuq, J.L.; Guilbert, S. Edible composite films of wheat gluten and lipids: Water vapour permeability and other physical properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- Chiou, W.L.; Riegelman, S. Preparation and dissolution characteristics of several fast-release solid dispersions of griseofulvin. J. Pharm. Sci. 1969, 58, 1505–1510. [Google Scholar] [CrossRef]
- Duke, J.A. CRC Handbook of Medicinal Herbs; CRC Press Inc.: Boca Raton, FL, USA, 1985; 677p. [Google Scholar]
- Scientific Committee on Food. Opinion of the Scientific Committee on Food on Glycyrrhizinic Acid and Its Ammonium Salt; European Commission Heath and Consumer Protection Directorate General: Brussels, Belgium, 2003. [Google Scholar]
- Brown, D. Orally disintegrating tablets-taste over speed. Drug Deliv. Technol. 2003, 3, 58–61. [Google Scholar]
- Maniruzzaman, M.; Boateng, J.S.; Chowdhry, B.Z.; Snowden, M.J.; Douroumis, D. A review on the taste masking of bitter APIs: Hot-melt extrusion (HME) evaluation. Drug Dev. Ind. Pharm. 2014, 40, 145–156. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, L.; Liu, G.; He, D.; Yin, H.; Wang, H.; Wu, J.; Liao, H.; Zhang, J. Novel taste-masked orally disintegrating tablets for a highly soluble drug with an extremely bitter taste: Design rationale and evaluation. Drug Dev. Ind. Pharm. 2013, 39, 1364–1371. [Google Scholar] [CrossRef]
- Velmurugan, S.; Vinushitha, S. Oral disintegrating tablets: An overview. Int. J. Chem. Pharm. Sci. 2010, 1, 1–12. [Google Scholar]

| Ingredient | Formulation (mg) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | C12 | C13 | |
| Cocoa butter | 500 | 475 | 450 | 425 | 475 | 450 | 425 | 475 | 450 | 425 | 475 | 450 | 425 |
| PEG 6000 | - | 25 | 50 | 75 | - | - | - | - | - | - | - | - | - |
| Beeswax | - | - | - | - | 25 | 50 | 75 | - | - | - | - | - | - |
| Paraffin wax | - | - | - | - | - | - | - | 25 | 50 | 75 | - | - | - |
| Corn starch | - | - | - | - | - | - | - | - | - | - | 25 | 50 | 75 |
| Total | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 |
| Additive (%) | 0 | 5 | 10 | 15 | 5 | 10 | 15 | 5 | 10 | 15 | 5 | 10 | 15 |
| Formulation | Hardness (kg) |
|---|---|
| C1 (0% additive) | 0.59 ± 0.04 |
| C2 (5% PEG6000) | 0.61 ± 0.04 |
| C3 (10% PEG6000) | 0.71 ± 0.04 |
| C4 (15% PEG6000) | 0.78 ± 0.06 |
| C5 (5% Beeswax) | 0.59 ± 0.03 |
| C6 (10% Beeswax) | 0.64 ± 0.05 |
| C7 (15% Beeswax) | 0.68 ± 0.02 |
| C8 (5% Paraffin Wax) | 0.58 ± 0.04 |
| C9 (10% Paraffin Wax) | 0.55 ± 0.03 |
| C10 (15% Paraffin Wax) | 0.52 ± 0.05 |
| C11 (5% Corn Starch) | 0.77 ± 0.03 |
| C12 (10% Corn Starch) | 0.91 ± 0.04 |
| C13 (15% Corn Starch) | 1.62 ± 0.07 |
| ANOVA | p < 0.05 |
| Post-hoc test (pair-wise comparison) Additives | |
| PEG 6000 | C1 and C3 * C1 and C4 * C2 and C3 * C2 and C4 * |
| Beeswax | C1 and C7 * C5 and C7 * |
| Paraffin wax | C1 and C10 * |
| Corn Starch | C1 and C11 * C1 and C12 * C1 and C13 * C11 and C12 * C11 and C13 * C12 and C13 * |
| 15% Additive | C4 and C7 * C4 and C10 * C4 and C13 * C7 and C10 * C7 and C13 * C10 and C13 * |
| Formulation | In Vitro Disintegration Time (s) | In Situ Disintegration Time (s) | In Vitro Disintegration Time in Artificial Saliva (s) |
|---|---|---|---|
| C1 (0% additive) | 94.17 ± 3.19 | 51.17 ± 4.45 | 80.50 ± 3.73 |
| C2 (5% PEG6000) | 91.17 ± 4.07 | 50.83 ± 4.62 | 83.67 ± 4.23 |
| C3 (10% PEG6000) | 100.17 ± 3.60 | 57.00 ± 4.98 | 88.67 ± 3.56 |
| C4 (15% PEG6000) | 104.67 ± 3.78 | 64.17 ± 5.46 | 95.33 ± 5.28 |
| C5 (5% Beeswax) | 110.83 ± 4.36 | 71.83 ± 5.49 | 97.00 ± 4.29 |
| C6 (10% Beeswax) | 161.17 ± 4.36 | 132.67 ± 7.39 | 144.50 ± 5.24 |
| C7 (15% Beeswax) | 233.67 ± 9.42 | 205.17 ± 3.19 | 194.83 ± 6.88 |
| C8 (5% Paraffin Wax) | 105.50 ± 4.28 | 70.00 ± 4.00 | 92.50 ± 4.23 |
| C9 (10% Paraffin Wax) | 127.17 ± 5.64 | 93.83 ± 4.45 | 111.50 ± 4.85 |
| C10 (15% Paraffin Wax) | 215.33 ± 6.50 | 182.00 ± 5.83 | 176.33 ± 5.24 |
| C11 (5% Corn Starch) | 86.50 ± 4.37 | 47.50 ± 3.39 | 77.67 ± 3.88 |
| C12 (10% Corn Starch) | 60.67 ± 3.72 | 42.83 ± 2.79 | 60.50 ± 5.36 |
| C13 (15% Corn Starch) | 48.50 ± 4.97 | 38.50 ± 2.95 | 46.00 ± 4.52 |
| ANOVA | p < 0.05 | p < 0.05 | p < 0.05 |
| Additives Post-hoc test (pair-wise comparison) | |||
| PEG6000 | C1 and C4 (p < 0.05) C2 and C4 * | C1 and C4 * C2 and C4 * | C1 and C4 * C2 and C4 * |
| Beeswax | C1 and C5 * C1 and C6 * C1 and C7 * C5 and C6 * C5 and C7 * C6 and C7 * | C1 and C5 * C1 and C6 * C1 and C7 * C5 and C6 * C5 and C7 * C6 and C7 * | C1 and C5 * C1 and C6 * C1 and C7 * C5 and C6 * C5 and C7 * C6 and C7 * |
| Paraffin Wax | C1 and C8 * C1 and C9 * C1 and C10 * C8 and C9 * C8 and C10 * C9 and C10 * | C1 and C8 * C1 and C9 * C1 and C10 * C8 and C9 * C8 and C10 * C9 and C10 * | C1 and C8 * C1 and C9 * C1 and C10 * C8 and C9 * C8 and C10 * C9 and C10 * |
| Corn Starch | C1 and C12 * C1 and C13 * C11 and C12 * C11 and C13 * C12 and C13 * | C1 and C12 * C1 and C13 * C11 and C12 * C11 and C13 * C12 and C13 * | C1 and C12 * C1 and C13 * C11 and C12 * C11 and C13 * C12 and C13 * |
| 15% Additive | C4 and C7 * C4 and C10 * C4 and C13 * C7 and C10 * C7 and C13 * C10 and C13 * | C4 and C7 * C4 and C10 * C4 and C13 * C7 and C10 * C7 and C13 * C10 and C13 * | C4 and C7 * C4 and C10 * C4 and C13 * C7 and C10 * C7 and C13 * C10 and C13 * |
| Ingredient | Weight (mg/tablet) |
|---|---|
| Kyron T-134/Dapoxetine (0.75:0.25) | 120 |
| Cocoa butter | 425 |
| Corn starch | 75 |
| Ammonium glycyrrhizinate | 30 |
| Parameter | Time (Month) | ANOVA | |||
|---|---|---|---|---|---|
| (A) 0 | (B) 3 | (C) 6 | (D) 12 | ||
| Hardness * (kg) | 2.93 ± 0.22 | 2.96 ± 0.24 | 2.96 ± 0.23 | 2.98 ± 0.22 | p = 0.975 |
| Weight * (mg) | 651.20 ± 2.30 | 651.50 ± 1.43 | 651.60 ± 2.07 | 651.70 ± 1.89 | p = 0.946 |
| Thickness * (mm) | 10.10 ± 0.06 | 10.16 ± 0.06 | 10.15 ± 0.05 | 10.12 ± 0.05 | p = 0.060 |
| Friability * (%) | 0.35 | 0.36 | 0.34 | 0.35 | - |
| In vitro disintegration time ** (s) | 151.67 ± 6.98 | 153.00 ± 5.33 | 152.67 ± 3.98 | 154.83 ± 6.08 | p = 0.809 |
| In situ disintegration time ** (s) | 94.33 ± 3.98 | 97.00 ± 3.69 | 94.67 ± 5.16 | 96.17 ± 3.97 | p = 0.668 |
| In vitro disintegration time in artificial saliva ** (s) | 123.83 ± 4.40 | 124.67 ± 3.50 | 123.83 ± 3.82 | 124.83 ± 3.76 | p = 0.951 |
| Drug content * (%) | 100.56 ± 1.04 | 100.11 ± 1.22 | 100.98 ± 0.98 | 99.89 ± 0.89 | p = 0.478 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liew, K.B.; Ming, L.C.; Goh, B.-H.; Peh, K.K. Fast Melt Cocoa Butter Tablet: Effect of Waxes, Starch, and PEG 6000 on Physical Properties of the Preparation. Molecules 2022, 27, 3128. https://doi.org/10.3390/molecules27103128
Liew KB, Ming LC, Goh B-H, Peh KK. Fast Melt Cocoa Butter Tablet: Effect of Waxes, Starch, and PEG 6000 on Physical Properties of the Preparation. Molecules. 2022; 27(10):3128. https://doi.org/10.3390/molecules27103128
Chicago/Turabian StyleLiew, Kai Bin, Long Chiau Ming, Bey-Hing Goh, and Kok Khiang Peh. 2022. "Fast Melt Cocoa Butter Tablet: Effect of Waxes, Starch, and PEG 6000 on Physical Properties of the Preparation" Molecules 27, no. 10: 3128. https://doi.org/10.3390/molecules27103128
APA StyleLiew, K. B., Ming, L. C., Goh, B.-H., & Peh, K. K. (2022). Fast Melt Cocoa Butter Tablet: Effect of Waxes, Starch, and PEG 6000 on Physical Properties of the Preparation. Molecules, 27(10), 3128. https://doi.org/10.3390/molecules27103128








