New Cysteine-Containing PEG-Glycerolipid Increases the Bloodstream Circulation Time of Upconverting Nanoparticles
Abstract
1. Introduction
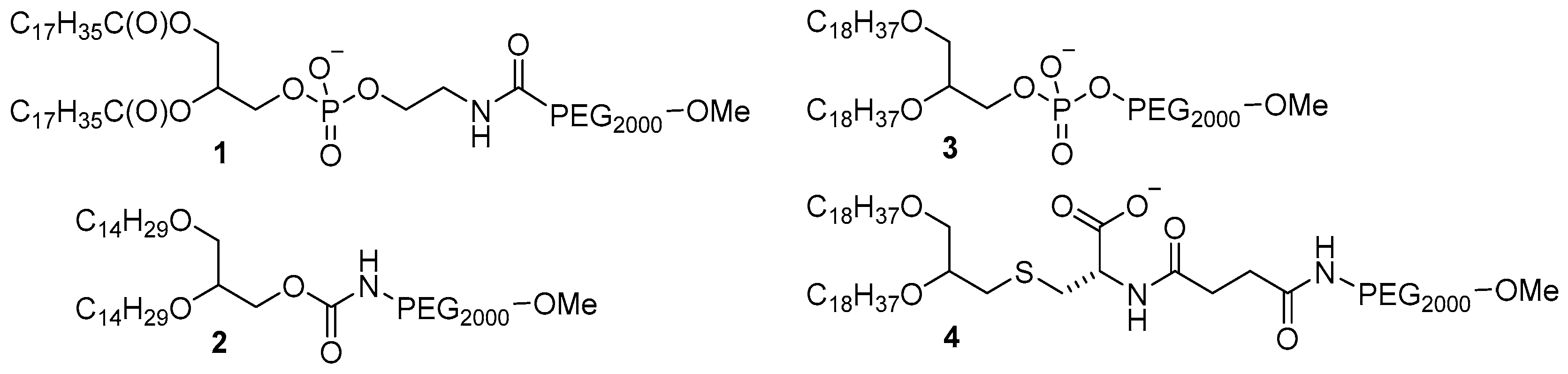
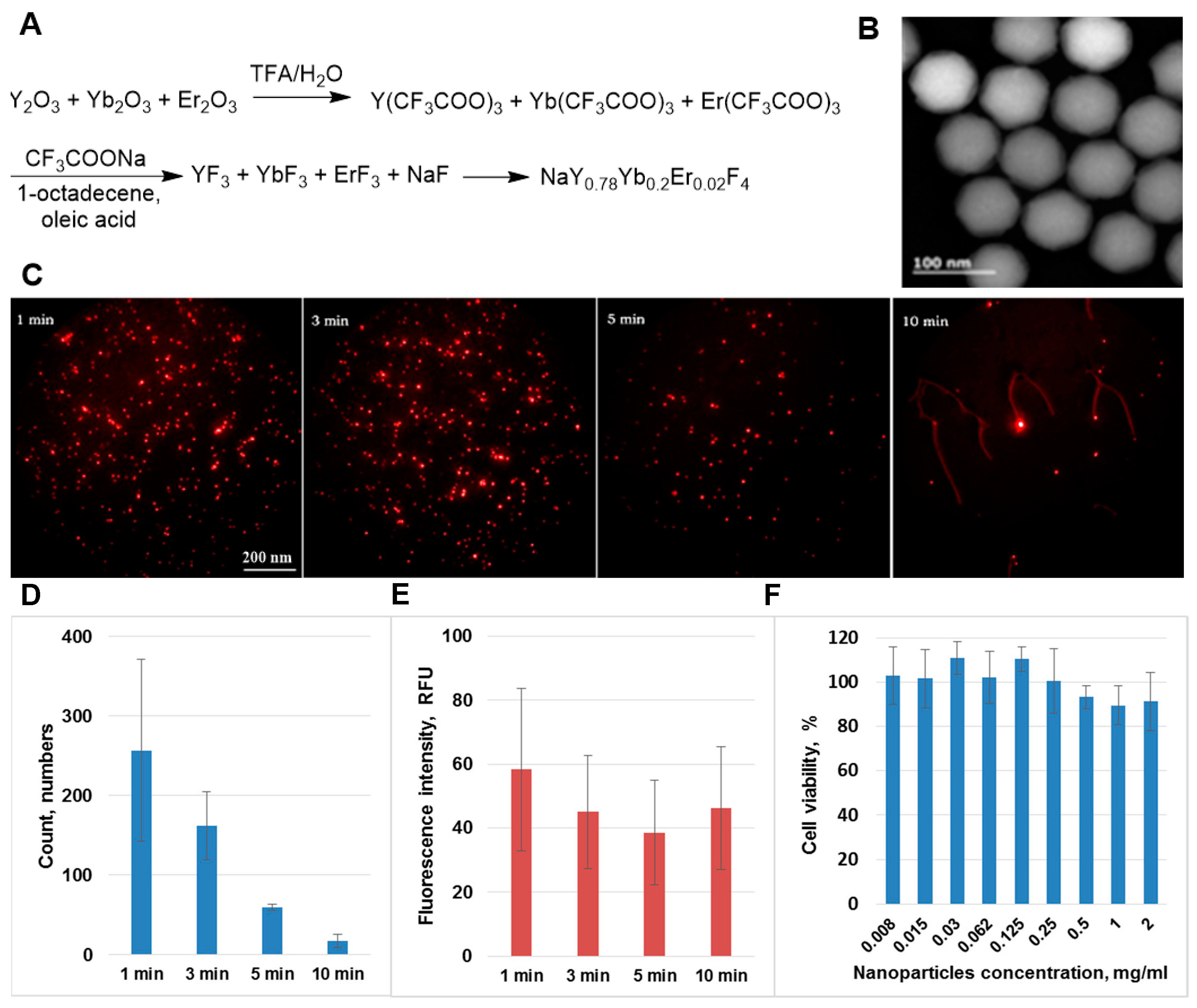
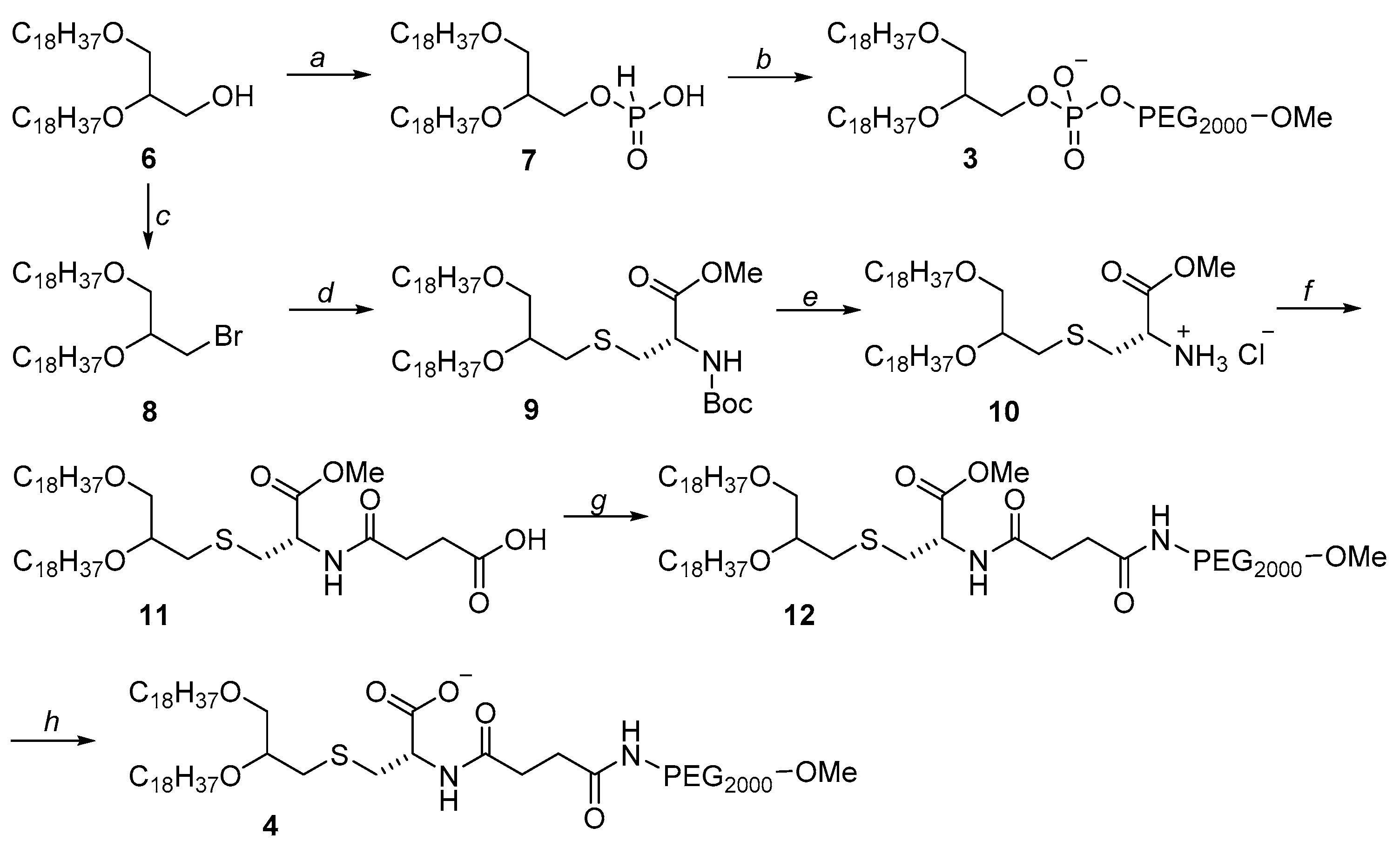
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of PEG-Glycerolipids
3.3. Synthesis of Upconverting Nanoparticles NaYF4: Yb3+, Er3+
3.4. Modification of Upconverting Nanoparticles with the PEG-Glycerolipids 1, 2, 3, 4
3.5. Particle Analysis
3.6. Cytotoxicity of Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Mironov, A.F.; Zhdanova, K.A.; Bragina, N.A. Nanosized vehicles for delivery of photosensitizers in photodynamic diagnosis and therapy of cancer. Russ. Chem. Rev. 2018, 87, 859–881. [Google Scholar] [CrossRef]
- Francés-Soriano, L.; Zakharko, M.A.; González-Béjar, M.; Panchenko, P.A.; Herranz-Pérez, V.; Pritmov, D.A.; Grin, M.A.; Mironov, A.F.; García-Verdugo, J.M.; Fedorova, O.A. Nanohybrid for photodynamic therapy and fluorescence imaging tracking without therapy. Chem. Mater. 2018, 30, 3677–3682. [Google Scholar] [CrossRef]
- Ghosh, S.; Carter, K.A.; Lovell, J.F. Liposomal formulations of photosensitizers. Biomaterials 2019, 218, 119341. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Yaraki, M.T.; Garakani, S.M.; Garakani, S.M.; Ahmadi, S.; Lajevardi, A.; Bagherzadeh, M.; Rabiee, M.; Tayebi, L.; Tahriri, M. Recent advances in porphyrin-based nanocomposites for effective targeted imaging and therapy. Biomaterials 2020, 232, 119707. [Google Scholar] [CrossRef] [PubMed]
- Luiz, M.T.; Di Filippo, L.D.; Tofani, L.B.; de Araújo, J.T.C.; Dutra, J.A.P.; Marchetti, J.M.; Chorilli, M. Highlights in targeted nanoparticles as a delivery strategy for glioma treatment. Int. J. Pharm. 2021, 604, 120758. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. In Site-Specific Protein Labeling; Gautier, A., Hinner, M.J., Eds.; Humana Press: New York, NY, USA, 2015; pp. 29–53. ISBN 978-1-4939-2271-0. [Google Scholar]
- Rana, A.; Bhatnagar, S. Advancements in folate receptor targeting for anti-cancer therapy: A small molecule-drug conjugate approach. Bioorg. Chem. 2021, 112, 104946. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Gai, S.; Li, C.; Yang, P.; Lin, J. Recent progress in rare earth micro/nanocrystals: Soft chemical synthesis, luminescent properties, and biomedical applications. Chem. Rev. 2014, 114, 2343–2389. [Google Scholar] [CrossRef]
- Woodle, M.C.; Lasic, D.D. Sterically stabilized liposomes. BBA-Rev. Biomembr. 1992, 1113, 171–199. [Google Scholar] [CrossRef]
- Panda, S.; Bhol, C.S.; Bhutia, S.K.; Mohapatra, S. PEG-PEI-modified gated N-doped mesoporous carbon nanospheres for pH/NIR light-triggered drug release and cancer phototherapy. J. Mater. Chem. B 2021, 9, 3666–3676. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Deng, X.; Jia, F.; Cui, X.; Lu, J.; Pan, Z.; Wu, Y. Colloidally stabilized DSPE-PEG-Glucose/Calcium phosphate hybrid nanocomposites for enhanced photodynamic cancer therapy via complementary mitochondrial Ca2+ overload and autophagy inhibition. Appl. Mater. Interfaces 2021, 13, 39112–39125. [Google Scholar] [CrossRef]
- Shmendel, E.V.; Timakova, A.A.; Maslov, M.A.; Morozova, N.G.; Chupin, V.V. Synthesis of mannose-containing neoglycolipids as a component of targeted delivery system for transfer of nucleic acids into antigen-presenting cells. Russ. Chem. Bull. 2012, 61, 1497–1501. [Google Scholar] [CrossRef]
- Shmendel, E.V.; Maslov, M.A.; Morozova, N.G.; Serebrennikova, G.A. Synthesis of neoglycolipids for the development of non-viral gene delivery systems. Russ. Chem. Bull. 2010, 59, 2281–2289. [Google Scholar] [CrossRef]
- Sutter, M.; Da Silva, E.; Duguet, N.; Raoul, Y.; Métay, E.; Lemaire, M. Glycerol ether synthesis: A bench test for green chemistry concepts and technologies. Chem. Rev. 2015, 115, 8609–8651. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, W.; Das, G.K.; Yang Tan, T.T. Engineering lanthanide-based materials for nanomedicine. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 71–96. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Han, G. Lanthanide-doped upconversion nanoparticles for imaging-guided drug delivery and therapy. In Advances in Nanotheranostics I; Dai, Z., Ed.; Springer: Berlin, Germany, 2016; pp. 139–164. ISBN 978-3-662-48542-2. [Google Scholar]
- Khaydukov, E.V.; Semchishen, V.A.; Seminogov, V.N.; Sokolov, V.I.; Popov, A.P.; Bykov, A.V.; Nechaev, A.V.; Akhmanov, A.S.; Panchenko, V.Y.; Zvyagin, A.V. Enhanced spatial resolution in optical imaging of biotissues labelled with upconversion nanoparticles using a fibre-optic probe scanning technique. Laser Phys. Lett. 2014, 11, 095602. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, L.; Shen, B.; Chen, X.; Wang, L.; Wang, L.; Li, F. Amphiphilic PEGylated lanthanide-doped upconversion nanoparticles for significantly passive accumulation in the peritoneal metastatic carcinomatosis models following intraperitoneal administration. Biomat. Sci. Eng. 2017, 3, 2176–2184. [Google Scholar] [CrossRef]
- Rocheva, V.V.; Sholina, N.V.; Derevyashkin, S.P.; Generalova, A.N.; Nechaev, A.V.; Khochenkov, D.A.; Semchishen, V.A.; Khaydukov, E.V.; Stepanova, E.V.; Panchenko, V.Y. Luminescence diagnostics of tumors with upconversion nanoparticles. Alm. Clin. Med. 2016, 44, 227–233. [Google Scholar] [CrossRef][Green Version]
- Park, H.S.; Kim, J.; Cho, M.Y.; Cho, Y.-J.; Suh, Y.D.; Nam, S.H.; Hong, K.S. Effectual labeling of natural killer cells with upconverting nanoparticles by electroporation for in vivo tracking and biodistribution assessment. Appl. Mater. Interfaces 2020, 12, 49362–49370. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolaeva, M.E.; Nechaev, A.V.; Shmendel, E.V.; Akasov, R.A.; Maslov, M.A.; Mironov, A.F. New Cysteine-Containing PEG-Glycerolipid Increases the Bloodstream Circulation Time of Upconverting Nanoparticles. Molecules 2022, 27, 2763. https://doi.org/10.3390/molecules27092763
Nikolaeva ME, Nechaev AV, Shmendel EV, Akasov RA, Maslov MA, Mironov AF. New Cysteine-Containing PEG-Glycerolipid Increases the Bloodstream Circulation Time of Upconverting Nanoparticles. Molecules. 2022; 27(9):2763. https://doi.org/10.3390/molecules27092763
Chicago/Turabian StyleNikolaeva, Maria E., Andrey V. Nechaev, Elena V. Shmendel, Roman A. Akasov, Mikhail A. Maslov, and Andrey F. Mironov. 2022. "New Cysteine-Containing PEG-Glycerolipid Increases the Bloodstream Circulation Time of Upconverting Nanoparticles" Molecules 27, no. 9: 2763. https://doi.org/10.3390/molecules27092763
APA StyleNikolaeva, M. E., Nechaev, A. V., Shmendel, E. V., Akasov, R. A., Maslov, M. A., & Mironov, A. F. (2022). New Cysteine-Containing PEG-Glycerolipid Increases the Bloodstream Circulation Time of Upconverting Nanoparticles. Molecules, 27(9), 2763. https://doi.org/10.3390/molecules27092763






