Temozolomide Efficacy and Metabolism: The Implicit Relevance of Nanoscale Delivery Systems †
Abstract
1. Introduction
- (1)
- Inorganic nanoparticles (Fe3O4, TiO2, ZnO, SiO2, etc.);
- (2)
- Liposomes;
- (3)
- Polymer–drug, polymer–protein conjugates;
- (4)
- Micelles (block copolymer micelles);
- (5)
- Nanocrystals;
- (6)
- Viral carriers of drugs.
2. Treatment Options of Glioblastoma
3. Temozolomide (TMZ): Efficacy and Metabolism
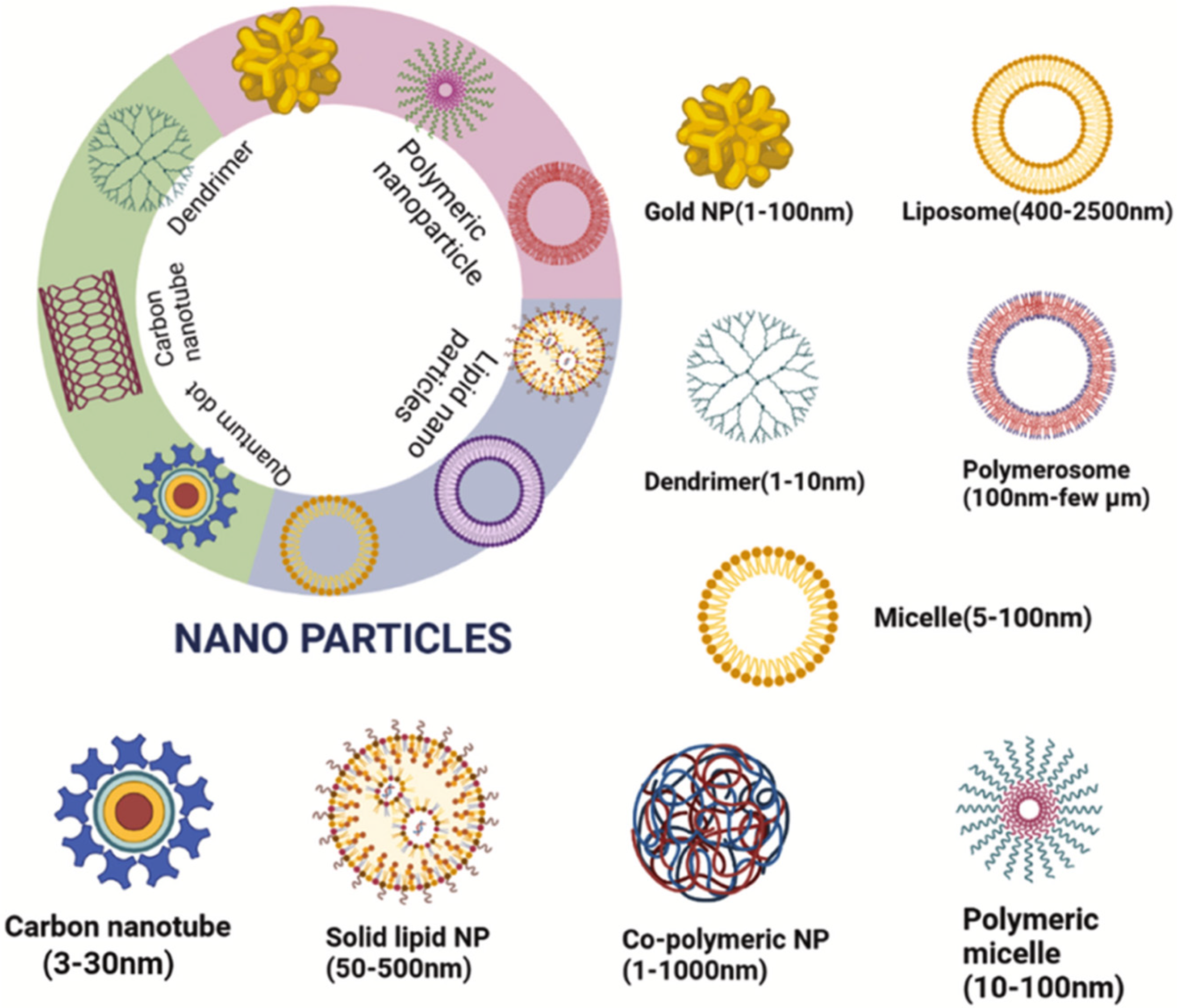
4. Nanoscale Delivery Systems
- ∗
- Metal nanoparticles (gold, silver, zinc oxide);
- ∗
- Polymer carriers (polymer micelles, polymer nanoparticles–chitosan, polymethylacrylate; nanospheres, nanocontainers, nanoglobules, dendrimers, nanoconjugates, polymerosomes);
- ∗
- Carbon nanotubes;
- ∗
- Quantum dots;
- ∗
- Lipid systems (solid lipid nanocarriers, cationic/anionic liposomes);
- ∗
- Nanoemulsions (cubosomes of 50–700 nm), etc.
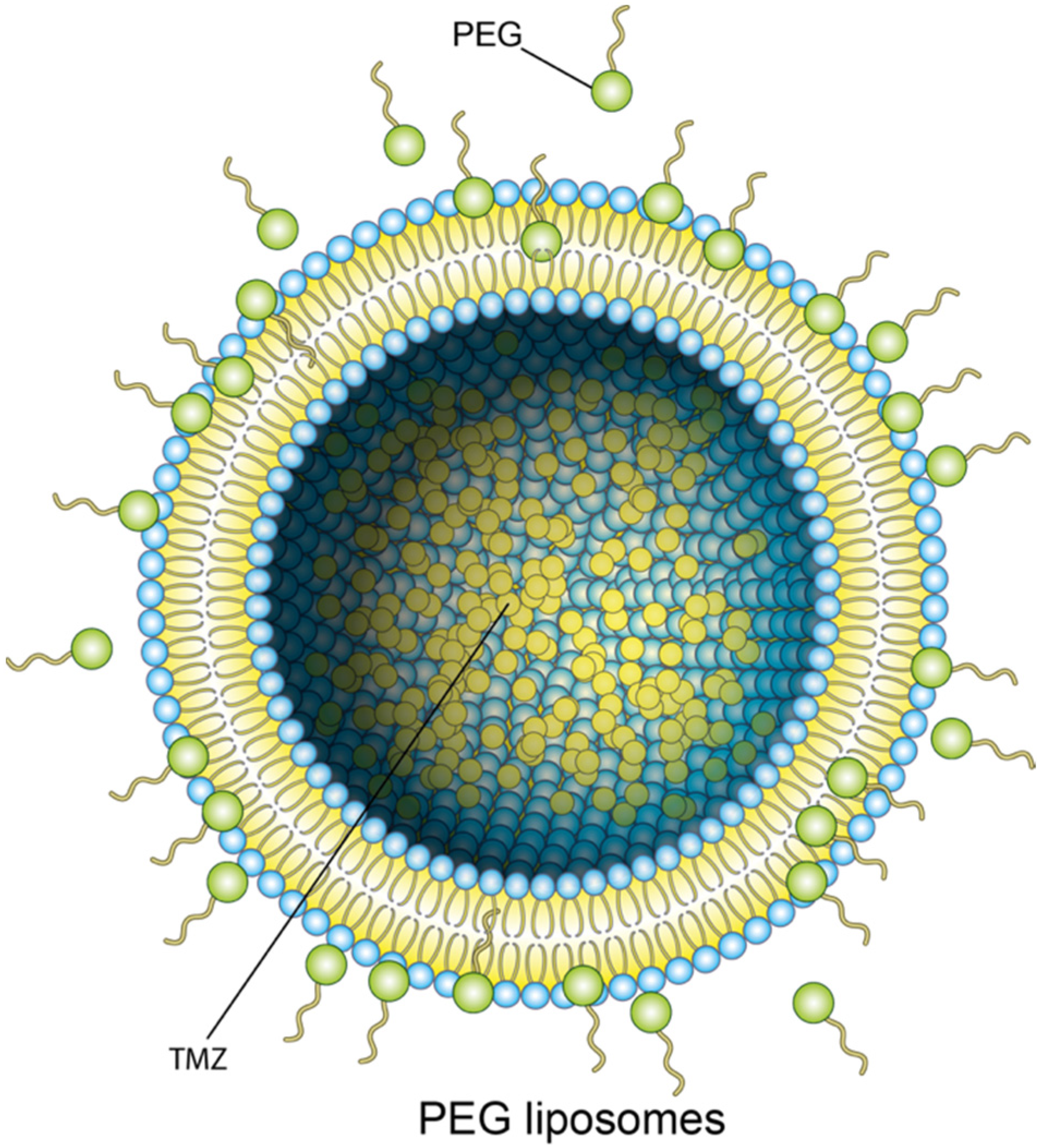
4.1. Liposomes
4.2. Solid Lipid Nanoparticles and Nanostructured Lipid Carries
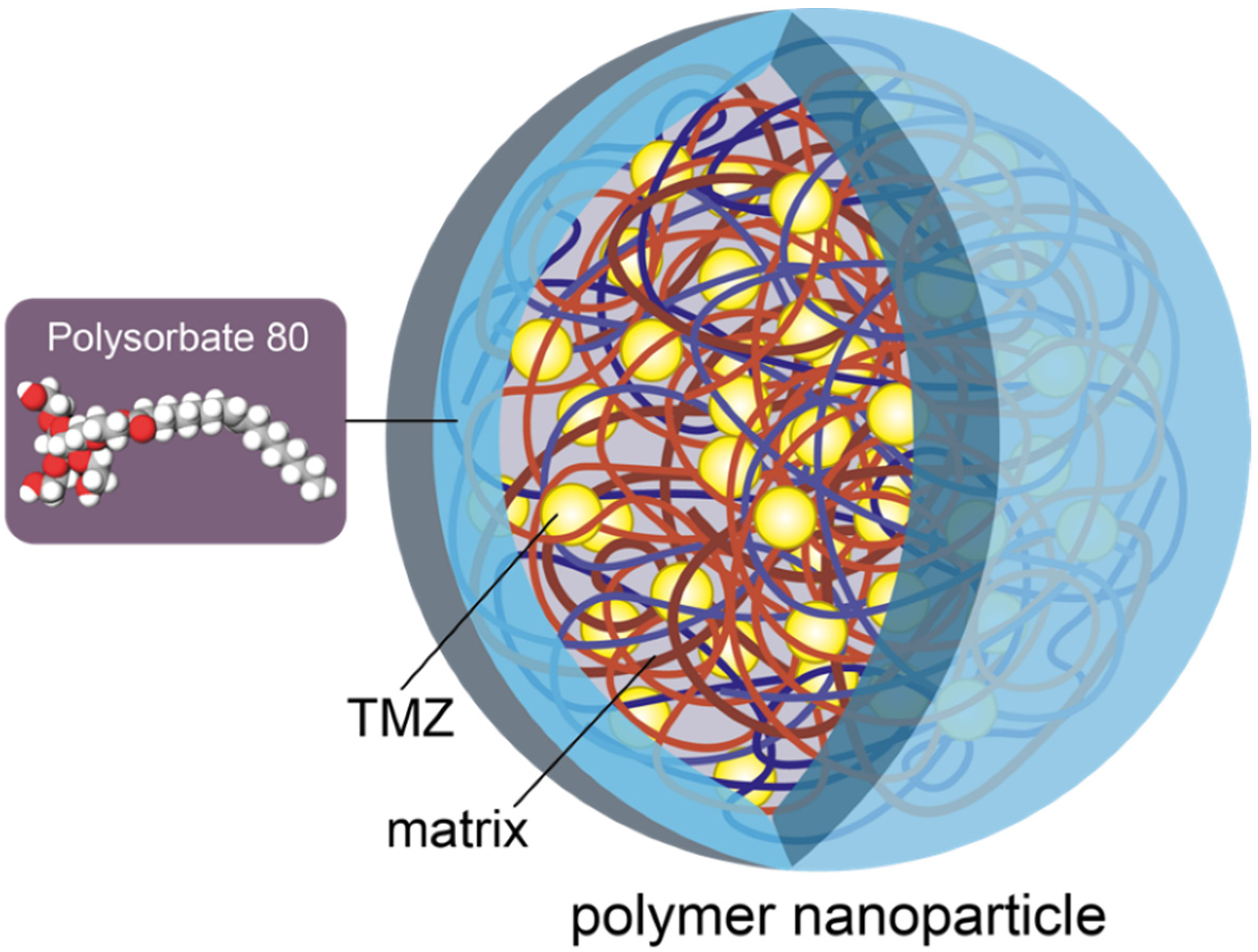
4.3. Polymeric Nanoparticles
4.4. Metal Nanoparticles
4.5. Carbon Quantum Dots
4.6. Gene Therapy
4.7. Nanocomposites and Nanotubes
4.8. Researches of TMZ Encapsulated in Nanotransporters
4.9. The Approaches to Fabricating Nanoscale Delivery Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019, 21 (Suppl. 5), v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Helligsoe, A.S.L.; Kenborg, L.; Henriksen, L.T.; Udupi, A.; Hasle, H.; Winther, J.F. Incidence and survival of childhood central nervous system tumors in Denmark, 1997–2019. Cancer Med. 2022, 11, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Meredith, D.M.; Alexandrescu, S. Embryonal and non-meningothelial mesenchymal tumors of the central nervous system–Advances in diagnosis and prognostication. Brain Pathol. 2022, e13059. [Google Scholar] [CrossRef] [PubMed]
- Pichaivel, M.; Anbumani, G.; Theivendren, P.; Gopal, M. An Overview of Brain Tumor; IntechOpen: London, UK, 2022. [Google Scholar]
- Fan, Y.; Avci, N.G.; Nguyen, D.T.; Dragomir, A.; Akay, Y.M.; Xu, F.; Akay, M. Engineering a High-Throughput 3-D In Vitro Glioblastoma Model. IEEE J. Transl. Eng. Health Med. 2015, 3, 4300108. [Google Scholar] [CrossRef] [PubMed]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to curing primary brain tumours. Nature reviews. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Stetson, L.; Virk, S.M.; Barnholtz-Sloan, J.S. Epidemiology of gliomas. Cancer Treat. Res. 2015, 163, 1–14. [Google Scholar] [CrossRef]
- Romo, C.G.; Palsgrove, D.N.; Sivakumar, A.; Elledge, C.R.; Kleinberg, L.R.; Chaichana, K.L.; Gocke, C.D.; Rodriguez, F.J.; Holdhoff, M. Widely metastatic IDH1-mutant glioblastoma with oligodendroglial features and atypical molecular findings: A case report and review of current challenges in molecular diagnostics. Diagn. Pathol. 2019, 14, 16. [Google Scholar] [CrossRef]
- Bauchet, L.; Mathieu-Daudé, H.; Fabbro-Peray, P.; Rigau, V.; Fabbro, M.; Chinot, O.; Pallusseau, L.; Carnin, C.; Lainé, K.; Schlama, A.; et al. Association des Neuro-Oncologues d’Expression Française (ANOCEF). Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro-Oncol. 2010, 12, 725–735. [Google Scholar] [CrossRef]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- Wanis, H.A.; Møller, H.; Ashkan, K.; Davies, E.A. The incidence of major subtypes of primary brain tumors in adults in England 1995–2017. Neuro-Oncology 2021, 23, 1371–1382. [Google Scholar] [CrossRef]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.I.; Sandeman, S.R. Nano carriers for drug transport across the blood-brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Rafiyath, S.M.; Rasul, M.; Lee, B.; Wei, G.; Lamba, G.; Liu, D. Comparison of safety and toxicity of liposomal doxorubicin vs. conventional anthracyclines: A meta-analysis. Exp. Hematol. Oncol. 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Storm, G.; van Bloois, L.; Steerenberg, P.A.; van Etten, E.; de Groot, G.; Crommelin, D.J.A. Liposome encapsulation of doxorubicin: Pharmaceutical and therapeutic aspects. J. Control. Release 1989, 9, 215–229. [Google Scholar] [CrossRef]
- Desai, N.; Trieu, V.; Yao, Z.; Louie, L.; Ci, S.; Yang, A.; Tao, C.; De, T.; Beals, B.; Dykes, D.; et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell traNDort of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin. Cancer Res. 2006, 12, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, K.D.; Yadav, T.P.; Prajapati, V.K.; Kumar, S.; Rai, M.; Dube, A.; Srivastava, O.N.; Sundar, S. Antileishmanial activity of nano-amphotericin B deoxycholate. J. Antimicrob. Chemother. 2008, 62, 376–380. [Google Scholar] [CrossRef]
- Havel, H.A. Where are the nanodrugs? An industry perspective on development of drug products containing nanomaterials. AAPS J. 2016, 18, 1351–1353. [Google Scholar] [CrossRef]
- Wang, Y.X. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J. Gastroenterol. 2015, 21, 13400–13402. [Google Scholar] [CrossRef]
- Duncan, R.; Gaspar, R. Nanomedicine(s) under the microscope. Mol. Pharm. 2011, 8, 2101–2141. [Google Scholar] [CrossRef]
- Gupta, R.; Xie, H. Nanoparticles in daily life: Applications, toxicity and regulations. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 209–230. [Google Scholar] [CrossRef]
- Steinmetz, N.F. Viral nanoparticles as platforms for next-generation therapeutics and imaging devices. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Tran, S.; De Giovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hableel, G.; Ruike Zhao, E.; Jokerst, J. Multifunctional nanomedicine with silica: Role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J. Colloid Interface Sci. 2018, 521, 261–279. [Google Scholar] [CrossRef]
- Li, T.; Duan, E.-Y.; Liu, C.-J.; Ma, J.-G.; Peng, C. Application of Gd (III) complexes for magnetic resonance imaging and the improvement of relaxivities via nanocrystallization. Inorg. Chem. Commun. 2018, 98, 111–114. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Ur Rahman, A.; Tajuddin Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Gemini-Piperni, S.; Travassos, R.; Lemgruber, L.; Silva, R.; Granjeiro, J.M. Trojan-like internalization of anatase titanium dioxide nanoparticles by human osteoblast cells. Sci. Rep. 2016, 6, 23615. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.K.; Brothers, S.P.; Wahlestedt, C. Emerging treatment strategies for glioblastoma multiforme. EMBO Mol. Med. 2014, 6, 1359–1370. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant gliomas in adults. N. Engl. J. Med. 2008, 395, 492–507. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2015, 352, 987–996. [Google Scholar] [CrossRef]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yun, B.; Han, Y.; Jiang, Z.; Zhu, H.; Ren, F.; Li, Z. Dye-Sensitized Rare Earth Nanoparticles with Up/Down Conversion Luminescence for On-Demand Gas Therapy of Glioblastoma Guided by NIR-II Fluorescence Imaging. Adv. Healthc. Mater. 2022, 11, 2102042. [Google Scholar] [CrossRef] [PubMed]
- Vogelius, I.R.; Bentzen, S.M. Proton vs photon radiation therapy for glioblastoma: Maximizing information from trial. Neuro-Oncol. 2022, 24, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S. Tumour treating fields (TTFields) therapy for glioblastoma: A quantitative and qualitative summary of research and advances in our understanding. Brain Tumor Res. Treat. 2022, 10, S251. [Google Scholar]
- Lalatsa, A.; Schätzlein, A.G.; Uchegbu, I.F. Nanostructures Overcoming the Blood-Brain Barrier: Physiological Considerations and Mechanistic Issues; Royal Society of Chemistry: London, UK, 2012; Chapter 7.1. [Google Scholar]
- Lalatsa, A.; Butt, A.M. Physiology of the Blood-Brain Barrier and Mechanisms of Transport Across the BBB. In Nanotechnology-Based Targeted Drug Delivery Systems for Brain Tumors; Academic Press: Cambridge, MA, USA, 2018; pp. 49–74. [Google Scholar]
- Löscher, W.; Potschka, H. Role of drug efflux transporters in the brain for drug disposition and treatment of brain diseases. Prog. Neurobiol. 2005, 76, 22–76. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nature reviews. Neuroscience 2005, 6, 591–602. [Google Scholar] [CrossRef]
- Miller, D.S. Regulation of ABC transporters blood-brain barrier: The good, the bad, and the ugly. Adv. Cancer Res. 2015, 125, 43–70. [Google Scholar] [CrossRef]
- Noack, A.; Gericke, B.; von Köckritz-Blickwede, M.; Menze, A.; Noack, S.; Gerhauser, I.; Osten, F.; Naim, H.Y.; Löscher, W. Mechanism of drug extrusion by brain endothelial cells via lysosomal drug trapping and disposal by neutrophils. Proc. Natl. Acad. Sci. USA 2018, 115, E9590–E9599. [Google Scholar] [CrossRef]
- Reid, J.M.; Stevens, D.C.; Rubin, J.; Ames, M.M. Pharmacokinetics of 3-methyl-(triazen-1-yl)imidazole-4-carboximide following administration of temozolomide to patients with advanced cancer. Clin. Cancer Res. 1997, 3, 2393–2398. [Google Scholar]
- Estlin, E.J.; Lashford, L.; Ablett, S.; Price, L.; Gowing, R.; Gholkar, A.; Kohler, J.; Lewis, I.J.; Morland, B.; Pinkerton, C.R.; et al. Phase I study of temozolomide in paediatric patients with advanced cancer. Br. J. Cancer 1998, 78, 652–661. [Google Scholar] [CrossRef]
- Brada, M.; Judson, I.; Beale, P.; Moore, S.; Reidenberg, P.; Statkevich, P.; Dugan, M.; Batra, V.; Cutler, D. Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. Br. J. Cancer 1999, 81, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- ABrandes, A.; Ermani, M.; Basso, U.; Amistà, P.; Berti, F.; Scienza, R.; Rotilio, A.; Pinna, G.; Gardiman, M.; Monfardini, S. Temozolomide as a second-line systemic regimen in recurrent high-grade glioma: A phase II study. Ann. Oncol. 2001, 12, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Messaoudi, K.; Clavreul, A.; Lagarce, F. Toward an effective strategy in glioblastoma treatment. Part I: Resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discov. Today 2015, 20, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Denny, B.J.; Wheelhouse, R.T.; Stevens, M.F.; Tsang, L.L.; Slack, J.A. NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry 1994, 33, 9045–9051. [Google Scholar] [CrossRef] [PubMed]
- Portnow, J.; Badie, B.; Chen, M.; Liu, A.; Blanchard, S.; Synold, T.W. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: Potential implications for the current approach to chemoradiation. Clin. Cancer Res. 2009, 15, 7092–7098. [Google Scholar] [CrossRef] [PubMed]
- Soni, V.; Jain, A.; Khare, P.; Gulbake, A.; Jain, S.K. Potential approaches for drug delivery to the brain: Past, present, and future. Crit. Rev. Ther. Drug Carrier Syst. 2010, 27, 187–236. [Google Scholar] [CrossRef] [PubMed]
- Cha, G.D.; Kang, T.; Baik, S.; Kim, D.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Advances in drug delivery technology for the treatment of glioblastoma multiforme. J. Control. Release 2020, 328, 350–367. [Google Scholar] [CrossRef]
- Strobel, H.; Baisch, T.; Fitzel, R.; Schilberg, K.; Siegelin, M.D.; Karpel-Massler, G.; Debatin, K.M.; Westhoff, M.A. Temozolomide and Other Alkylating Agents in Glioblastoma Therapy. Biomedicines 2019, 7, 69. [Google Scholar] [CrossRef]
- Yasaswi, P.S.; Shetty, K.; Yadav, K.S. Temozolomide nano enabled medicine: Promises made by the nanocarriers in glioblastoma therapy. J. Control. Release 2021, 336, 549–571. [Google Scholar] [CrossRef]
- Vanza, J.; Jani, P.; Pandya, N.; Tandel, H. Formulation and statistical optimization of intravenous temozolomide-loaded PEGylated liposomes to treat glioblastoma multiforme by three-level factorial design. Drug Dev. Ind. Pharm. 2018, 44, 923–933. [Google Scholar] [CrossRef]
- Kurano, T.; Kanazawa, T.; Ooba, A.; Masuyama, Y.; Maruhana, N.; Yamada, M.; Suzuki, T. Nose-to-brain/spinal cord delivery kinetics of liposomes with different surface properties. J. Control. Release 2022, 344, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.A.; Baby Chakrapani, P.S. Containers Based Drug Delivery for Neuroscience. In Micro- and Nano-Containers for Smart Applications; Springer: Singapore, 2022; pp. 309–325. [Google Scholar]
- De Lange, E.C.; Hammarlund Udenaes, M. Understanding the Blood-Brain Barrier and Beyond: Challenges and Opportunities for Novel CNS Therapeutics. Clin. Pharmacol. Ther. 2022, 111, 758–773. [Google Scholar] [CrossRef] [PubMed]
- Katona, G.; Sabir, F.; Sipos, B.; Naveed, M.; Schelz, Z.; Zupkó, I.; Csóka, I. Development of Lomustine and n-Propyl Gallate Co-Encapsulated Liposomes for Targeting Glioblastoma Multiforme via Intranasal Administration. Pharmaceutics 2022, 14, 631. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, Z.; Liu, H.; Wang, L.; Huang, G. Liposome encapsulated of temozolomide for the treatment of glioma tumor: Preparation, characterization and evaluation. Drug Discov. Ther. 2015, 9, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Gabay, M.; Weizman, A.; Zeineh, N.; Kahana, M.; Obeid, F.; Allon, N.; Gavish, M. Liposomal Carrier Conjugated to APP-Derived Peptide for Brain Cancer Treatment. Cell. Mol. Neurobiol. 2021, 41, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Amarandi, R.M.; Ibanescu, A.; Carasevici, E.; Marin, L.; Dragoi, B. Liposomal-Based Formulations: A Path from Basic Research to Temozolomide Delivery Inside Glioblastoma Tissue. Pharmaceutics 2022, 14, 308. [Google Scholar] [CrossRef]
- Lin, C.Y.; Li, R.J.; Huang, C.Y.; Wei, K.C.; Chen, P.Y. Controlled release of liposome-encapsulated temozolomide for brain tumour treatment by convection-enhanced delivery. J. Drug Target. 2018, 26, 325–332. [Google Scholar] [CrossRef]
- Nordling-David, M.M.; Yaffe, R.; Guez, D.; Meirow, H.; Last, D.; Grad, E.; Salomon, S.; Sharabi, S.; Levi-Kalisman, Y.; Golomb, G.; et al. Liposomal temozolomide drug delivery using convection enhanced delivery. J. Control. Release 2017, 261, 138–146. [Google Scholar] [CrossRef]
- Bi, H.; Xue, J.; Jiang, H.; Gao, S.; Yang, D.; Fang, Y.; Shi, K. Current developments in drug delivery with thermosensitive liposomes. Asian J. Pharm. Sci. 2019, 14, 365–379. [Google Scholar] [CrossRef]
- Arcella, A.; Palchetti, S.; Digiacomo, L.; Pozzi, D.; Capriotti, A.L.; Frati, L.; Oliva, M.A.; Tsaouli, G.; Rota, R.; Screpanti, I.; et al. Brain targeting by liposome-biomolecular corona boosts anticancer efficacy of temozolomide in glioblastoma cells. ACS Chem. Neurosci. 2018, 9, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Rapalli, V.K.; Singhvi, G.; Gorantla, S.; Khosa, A.; Dubey, S.K.; Saha, R.N. Design of temozolomide-loaded proliposomes and lipid crystal nanoparticles with industrial feasible approaches: Comparative assessment of drug loading, entrapment efficiency, and stability at plasma pH. J. Liposome Res. 2021, 31, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Javed, M.; Deeb, H.H.; Nicola, M.K.; Mirza, M.; Alam, M.; Waziri, A. Lipid Nanocarriers for Neurotherapeutics: Introduction, Challenges, Blood-brain Barrier, and Promises of Delivery Approaches. CNS Neurol. Disord. Drug Targets 2021. [Google Scholar] [CrossRef] [PubMed]
- Muntoni, E.; Marini, E.; Ferraris, C.; Garelli, S.; Capucchio, M.T.; Colombino, E.; Battaglia, L. Intranasal lipid nanocarriers: Uptake studies with fluorescently labeled formulations. Colloids Surf. B Biointerfaces 2022, 214, 112470. [Google Scholar] [CrossRef]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnurch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Famta, P.; Raghuvanshi, R.S.; Singh, S.B.; Srivastava, S. Lipid polymer hybrid nanocarriers: Insights into synthesis aspects, characterization, release mechanisms, surface functionalization and potential implications. Colloid Interface Sci. Commun. 2022, 46, 100570. [Google Scholar] [CrossRef]
- Khosa, A.; Krishna, K.V.; Dubey, S.K.; Saha, R.N. Lipid Nanocarriers for Enhanced Delivery of Temozolomide to the Brain. In Methods in Molecular Biology 2059; Springer: Berlin/Heidelberg, Germany, 2020; pp. 285–298. [Google Scholar]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, L.; Chen, Z.; Mao, G.; Gao, Z.; Lai, X.; Zhu, X.; Zhu, J. Nanostructured lipid carriers, solid lipid nanoparticles, and polymeric nanoparticles: Which kind of drug delivery system is better for glioblastoma chemotherapy? Drug Deliv. 2016, 12, 3408–3416. [Google Scholar] [CrossRef]
- Khan, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y.; Ali, A.; Khan, K. Temozolomide loaded nano lipid based chitosan hydrogel for nose to brain delivery: Characterization, nasal absorption, histopathology and cell line study. Int. J. Biol. Macromol. 2018, 116, 1260–1267. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Castro, K.C.D.; Costa, J.M.; Campos, M.G.N. Drug-loaded polymeric nanoparticles: A review. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 1–13. [Google Scholar] [CrossRef]
- Cheng, H.; Jiang, Z.; Sun, C.; Wang, Z.; Han, G.; Chen, X.; Ding, Y. Protein stabilized polymeric nanoparticles inspired relay drug delivery for tackling post-chemotherapeutic metastasis. Chem. Eng. J. 2022, 427, 131672. [Google Scholar] [CrossRef]
- Eshaghi, B.; Fofana, J.; Nodder, S.B.; Gummuluru, S.; Reinhard, B.M. Virus-Mimicking Polymer Nanoparticles Targeting CD169+ Macrophages as Long-Acting Nanocarriers for Combination Antiretrovirals. ACS Appl. Mater. Interfaces 2022, 14, 2488–2500. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Mao, G.; Du, J.; Zhu, X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016, 23, 1404–1408. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.H.; Lin, X.N.; Wei, F.; Feng, W.; Huang, Z.C.; Wang, P.; Ren, L.; Diao, Y. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int. J. Nanomed. 2011, 6, 445–452. [Google Scholar] [CrossRef]
- Kudarha, R.R.; Sawant, K.K. Hyaluronic acid conjugated albumin nanoparticles for efficient receptor mediated brain targeted delivery of temozolomide. J. Drug Deliv. Sci. Technol. 2021, 61, 102129. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Zang, G.; Zhang, T.; Wu, Q.; Zhang, H.; Wang, G. trans-2-Enoyl-CoA Reductase Tecr-Driven Lipid Metabolism in Endothelial Cells Protects against Transcytosis to Maintain Blood-Brain Barrier Homeostasis. Research 2022, 2022, 9839368. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Transcellular routes of blood–brain barrier disruption. Exp. Biol. Med. 2022, 247, 788–796. [Google Scholar] [CrossRef]
- Ling, Y.; Wei, K.; Zou, F.; Zhong, S. Temozolomide loaded PLGA-based superparamagnetic nanoparticles for magnetic resonance imaging and treatment of malignant glioma. Int. J. Pharm. 2012, 430, 266–275. [Google Scholar] [CrossRef]
- Shanavas, A.; Sasidharan, S.; Bahadur, D.; Srivastava, R. Magnetic core-shell hybrid nanoparticles for receptor targeted anti-cancer therapy and magnetic resonance imaging. J. Colloid Interface Sci. 2017, 486, 112–120. [Google Scholar] [CrossRef]
- Lap, R. Nanocarrier Drug-Delivery Systems for Temozolomide in the Treatment of Glioblastoma Multiforme. Ph.D. Thesis, Rijksuniversiteit Groningen, Groningen, The Netherlands, 2021. [Google Scholar]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.J.; Chen, H.; Wu, X.; Mao, H. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef] [PubMed]
- Van Meir, E.G.; Hadjipanayis, C.G.; Norden, A.D.; Shu, H.K.; Wen, P.Y.; Olson, J.J. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J. Clin. 2010, 60, 166–193. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Miner, A.; Hennis, L.; Mittal, S. Mechanisms of temozolomide resistance in glioblastoma-a comprehensive review. Cancer Drug Resist. 2021, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.H.; Hsueh, W.T.; Chuang, J.Y.; Chang, K.Y. Dissecting the mechanism of temozolomide resistance and its association with the regulatory roles of intracellular reactive oxygen species in glioblastoma. J. Biomed. Sci. 2021, 28, 18. [Google Scholar] [CrossRef]
- Di Filippo, L.D.; Azambuja, J.H.; Dutra JA, P.; Luiz, M.T.; Duarte, J.L.; Nicoleti, L.R.; Chorilli, M. Improving temozolomide biopharmaceutical properties in glioblastoma multiforme (GBM) treatment using GBM-targeting nanocarriers. Eur. J. Pharm. Biopharm. 2021, 168, 76–89. [Google Scholar] [CrossRef]
- Saul, J.M.; Annapragada, A.; Natarajan, J.V.; Bellamkonda, R.V. Controlled targeting of liposomal doxorubicin via the folate receptor in vitro. J. Control. Release Off. J. Control. Release Soc. 2003, 92, 49–67. [Google Scholar] [CrossRef]
- Lainé, A.L.; Passirani, C. Novel metal-based anticancer drugs: A new challenge in drug delivery. Curr. Opin. Pharmacol. 2012, 12, 420–426. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic Effects Between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 1–23. [Google Scholar] [CrossRef]
- Aallaei, M.; Molaakbari, E.; Mostafavi, P.; Salarizadeh, N.; Maleksah, R.E.; Afzali, D. Investigation of Cu metal nanoparticles with different morphologies to inhibit SARS-CoV-2 main protease and spike glycoprotein using Molecular Docking and Dynamics Simulation. J. Mol. Struct. 2022, 1253, 132301. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Shi, H.; Zhu, W.; Gui, Q.; Xu, Y.; Meng, J.; Guo, X.; Gong, Z.; Chen, H. Silver nanoparticles enhance the sensitivity of temozolomide on human glioma cells. Oncotarget 2017, 8, 7533–7539. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Wang, S.; Liu, Z.; Xie, L.; Deng, Y. Development of temozolomide coated nano zinc oxide for reversing the resistance of malignant glioma stem cells. Mater. Sci. Eng. C 2018, 83, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A critical review of synthesis parameters affecting the properties of zinc oxide nanoparticle and its application in wastewater treatment. Appl. Water Sci. 2021, 11, 48. [Google Scholar] [CrossRef]
- Shamsipour, M.; Mansouri, A.M.; Moradipour, P. Temozolomide conjugated carbon quantum dots embedded in core/shell nanofibers prepared by coaxial electrospinning as an implantable delivery system for cell imaging and sustained drug release. AAPS PharmSciTech 2019, 20, 259. [Google Scholar] [CrossRef]
- Hettiarachchi, S.D.; Graham, R.M.; Mintz, K.J.; Zhou, Y.; Vanni, S.; Penga, Z.; Leblan, R.M. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 2019, 11, 6192–6205. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Ouyang, J.; Zhang, L.; Xue, J.; Zhang, H.; Tao, W. Electroactive electrospun nanofibers for tissue engineering. Nano Today 2021, 39, 101196. [Google Scholar] [CrossRef]
- Sonali Viswanadh, M.K.; Singh, R.P.; Agrawal, P.; Mehata, A.K.; Pawde, D.M.; Narendra Sonkar, R.; Muthu, M.S. Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics 2018, 2, 70–86. [Google Scholar] [CrossRef]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Zhang, F.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, S.; Ding, F.; Guo, S.; Ying, G.; Yan, Z. Ursolic acid attenuates temozolomide resistance in glioblastoma cells by downregulating O6- methylguanine-DNA methyltransferase (MGMT) expression. Am. J. Transl. Res. 2016, 8, 3299–3308. [Google Scholar] [PubMed]
- Kim, S.S.; Harford, J.B.; Moghe, M.; Rait, A.; Pirollo, K.F.; Chang, E.H. Targeted nanocomplex carrying siRNA against MALAT1 sensitizes glioblastoma to temozolomide. Nucleic Acids Res. 2018, 46, 1424–1440. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhou, Y.; Chen, J.; Huang, N.; Wang, Z.; Cheng, Y. Gene Therapy for Drug-Resistant Glioblastoma via Lipid-Polymer Hybrid Nanoparticles Combined with Focused Ultrasound. Int. J. Nanomed. 2021, 16, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; You, C.; Ma, L.; Li, H.; Ju, Y.; Guo, X.; Shi, S.; Zhang, T.; Zhou, R.; Lin, Y. Enhanced Efficacy of Temozolomide Loaded by a Tetrahedral Framework DNA Nanoparticle in the Therapy for Glioblastoma. ACS Appl. Mater. Interfaces 2019, 11, 39525–39533. [Google Scholar] [CrossRef]
- Patil, R.; Portilla-Arias, J.; Ding, H.; Inoue, S.; Konda, B.; Hu, J.; Wawrowsky, K.A.; Shin, P.K.; Black, K.L.; Holler, E.; et al. Temozolomide delivery to tumor cells by a multifunctional nano vehicle based on poly(β-L-malic acid). Pharm. Res. 2010, 27, 2317–2329. [Google Scholar] [CrossRef]
- Sahli, F.; Courcelle, M.; Palama, T.; Djaker, N.; Savarin, P.; Spadavecchia, J. Temozolomide, Gemcitabine, and Decitabine Hybrid Nanoconjugates: From Design to Proof-of-Concept (PoC) of Synergies toward the Understanding of Drug Impact on Human Glioblastoma Cells. J. Med. Chem. 2020, 63, 7410–7421. [Google Scholar] [CrossRef]
- Kriel, J.; Müller-Nedebock, K.; Maarman, G.; Mbizana, S.; Ojuka, E.; Klumperman, B.; Loos, B. Coordinated autophagy modulation overcomes glioblastoma chemoresistance through disruption of mitochondrial bioenergetics. Sci. Rep. 2018, 8, 10348. [Google Scholar] [CrossRef]
- Panigrahi, B.K.; Nayak, A.K. Carbon Nanotubes: An Emerging Drug Delivery Carrier in Cancer Therapeutics. Curr. Drug Deliv. 2020, 17, 558–576. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon Nanotubes: Properties and Application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Ilijeva, R.; Grozdanov, A.; Ulanski, P.; Kadlubowski, S.; Runceva, M.; Ivceska, A.; Mladenovska, K. Effect of irradiation on the physicochemical and biopharmaceutical properties of Temozolomide loaded carbon nanotubes. Maced. Pharm. Bull. 2020, 66 (Suppl. 1), 115–116. [Google Scholar] [CrossRef]
- Abrudan, C.; Florian, I.S.; Baritchii, A.; Soritau, O.; Dreve, S.; Tomuleasa, C.; Petrushev, B. Assessment of temozolomide action encapsulated in chitosan and polymer nanostructures on glioblastoma cell lines. Rom. Neurosurg. 2014, 21, 18–29. [Google Scholar] [CrossRef][Green Version]
- Jain, D.S.; Athawale, R.B.; Bajaj, A.N.; Shrikhande, S.S.; Goel, P.N.; Nikam, Y.; Gude, R.P. Unraveling the cytotoxic potential of Temozolomide loaded into PLGA nanoparticles. DARU J. Pharm. Sci. 2014, 22, 18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tao, J.; Hua-Nan, P.; Jin-Na, Y.; Dong-Bo, D.; Fan-Zhu, L. Preparation and brain delivery evaluation of temozolomide-loaded albumin nanoparticles. Chin. J. Pharm. 2013, 44, 41–45. [Google Scholar]
- Rónavári, A.; Igaz, N.; Adamecz, D.I.; Szerencsés, B.; Molnar, C.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Green Silver and Gold Nanoparticles: Biological Synthesis Approaches and Potentials for Biomedical Applications. Molecules 2021, 26, 844. [Google Scholar] [CrossRef]
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, Chemical-Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules 2021, 26, 5823. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrenko, D.; Chubarev, V.; Syzrantsev, N.; Ismail, N.; Merkulov, V.; Sologova, S.; Grigorevskikh, E.; Smolyarchuk, E.; Alyautdin, R. Temozolomide Efficacy and Metabolism: The Implicit Relevance of Nanoscale Delivery Systems. Molecules 2022, 27, 3507. https://doi.org/10.3390/molecules27113507
Petrenko D, Chubarev V, Syzrantsev N, Ismail N, Merkulov V, Sologova S, Grigorevskikh E, Smolyarchuk E, Alyautdin R. Temozolomide Efficacy and Metabolism: The Implicit Relevance of Nanoscale Delivery Systems. Molecules. 2022; 27(11):3507. https://doi.org/10.3390/molecules27113507
Chicago/Turabian StylePetrenko, Daria, Vladimir Chubarev, Nikita Syzrantsev, Nafeeza Ismail, Vadim Merkulov, Susanna Sologova, Ekaterina Grigorevskikh, Elena Smolyarchuk, and Renad Alyautdin. 2022. "Temozolomide Efficacy and Metabolism: The Implicit Relevance of Nanoscale Delivery Systems" Molecules 27, no. 11: 3507. https://doi.org/10.3390/molecules27113507
APA StylePetrenko, D., Chubarev, V., Syzrantsev, N., Ismail, N., Merkulov, V., Sologova, S., Grigorevskikh, E., Smolyarchuk, E., & Alyautdin, R. (2022). Temozolomide Efficacy and Metabolism: The Implicit Relevance of Nanoscale Delivery Systems. Molecules, 27(11), 3507. https://doi.org/10.3390/molecules27113507





