Delving into the Therapeutic Potential of Carica papaya Leaf against Thrombocytopenia
Abstract
1. Introduction
2. Mechanism of Action of C. papaya against Thrombocytopenia
3. Selection and Collection of Data
4. Thrombocytopenia Ameliorating Potential of C. papaya Leaf
4.1. Drug-Induced Thrombocytopenia
4.1.1. Cyclophosphamide-Induced Thrombocytopenia
4.1.2. Busulfan-Induced Thrombocytopenia
4.1.3. Carboplatin-Induced Thrombocytopenia
4.1.4. Cotrimoxazole-Induced Thrombocytopenia
4.1.5. Hydroxyurea-Induced Thrombocytopenia
4.1.6. Aspirin-Induced Thrombocytopenia
4.1.7. Gentamicin-Induced Thrombocytopenia
4.2. Chemotherapy-Induced Thrombocytopenia
4.3. Disease-Induced Thrombocytopenia
4.4. Dengue-Induced Thrombocytopenia
5. Phytochemical Screening
6. Other Therapeutic Potentials of C. papaya Leaf
6.1. Immunomodulatory Activity
6.2. Antimalarial Activity
6.3. Antiviral Efficacy
6.4. Anticancer Properties
6.5. Antidiabetic Activity
6.6. Antiangiogenic Activity
6.7. Antioxidative Activity
6.8. Antibacterial Activity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMC | Bone marrow cells |
| BW | Body weight |
| CP | Carica papaya |
| CPLE | Carica papaya leaf extract |
| CPLJ | Carica papaya leaf juice |
| CIT | Chemotherapy-induced thrombocytopenia |
| Cyp | Cyclophosphamide |
| DENV | Dengue virus |
| DHF | Dengue hemorrhagic fever |
| EPO | Erythropoietin |
| FCPLJ | Freeze-dried Carica papaya leaf juice |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| GRO-alpha | Growth regulated oncogene |
| HDL | High-density lipoprotein |
| HGB | Hemoglobin |
| HIV | Human immunodeficiency virus |
| HPTLC | High-performance thin-layer chromatography |
| IC50 | Inhibiting concentration |
| IL | Interleukin |
| LDL | Low-density lipoprotein |
| MCP-1 | Monocyte chemoattractant protein |
| MIP-1 | Macrophage inflammatory protein |
| MLCC | Mature leaf concentrate of Carica papaya |
| mm | Millimolar |
| NS | Non-structural protein |
| PCV | Packed cell volume |
| qRT-PCR | Real-time polymerase chain reaction |
| RBCl | Red blood cells |
| RNA | Ribonucleic acid |
| SC | Stem cells |
| SCPLE | Standardized Carica papaya leaf extract |
| TPO | Thrombopoietin |
| UPLC–qtof/MS | Ultra-performance liquid chromatography–quadrupole time of flight/mass spectrometer |
| VEGFR | View/edit human vascular endothelial growth factor receptor |
| WBC | White blood cells |
References
- Sailor, G.; Hirani, K.; Parmar, G.; Maheshwari, R.; Singh, R.; Kumar Seth, A. Platelet Augmentation Potential of Polyherbal Formulation in Cyclophosphamide-Induced Thrombocytopenia in Wistar Rats. Folia Med. 2021, 63, 67–73. [Google Scholar] [CrossRef]
- Nandini, C.; Madhunapantula, S.R.V.; Bovilla, V.R.; Ali, M.; Mruthunjaya, K.; Santhepete, M.N.; Jayashree, K. Platelet enhancement by Carica papaya L. leaf fractions in cyclophosphamide induced thrombocytopenic rats is due to elevated expression of CD110 receptor on megakaryocytes: Carica papaya leaf juice for the treatment of thrombocytopenia. J. Ethnopharmacol. 2021, 275, 114074. [Google Scholar] [CrossRef]
- Ahmed, M.; Memon, F.; Irum, N.; Dahri, G.M.; Mughal, A.; Kumar, G. Papaya seeds: Their effects on quantitative platetlets count in female rabbits. JPUMHS 2020, 10, 47–53. [Google Scholar]
- Babu, R.; Kubavat, S.; Poulose, K.P.; Srinlvasa, K.P.K.; Handae, S. A randomized, double-blind, placebocontrolled, proof of concept study to assess the safety and efficacy of Carica papaya and Tinospora cordifolia leaf extract (Thrombobliss) in subjects undergoing chemotherapy treatment and subjects with systemic microbia. Int. J. Clin. Trials 2017, 4, 116–121. [Google Scholar] [CrossRef]
- Anjum, V.; Arora, P.; Ansari, S.H.; Najmi, A.K.; Ahmad, S. Antithrombocytopenic and immunomodulatory potential of metabolically characterized aqueous extract of Carica papaya leaves. Pharm. Biol. 2017, 55, 2043–2056. [Google Scholar] [CrossRef]
- Lambert, M.P.; Gernsheimer, T.B. Clinical updates in adult immune thrombocytopenia. Blood 2017, 129, 2829–2835. [Google Scholar] [CrossRef]
- Bere, A.W.; Mulati, O.; Kimotho, J.; Ng’Ong’A, F. Carica papaya leaf extract silver synthesized nanoparticles inhibit dengue type 2 viral replication in vitro. Pharmaceuticals 2021, 14, 718. [Google Scholar] [CrossRef]
- Tayal, N.; Srivastava, P.; Srivastava, N. Anti angiogenic activity of carica papaya leaf extract. J. Pure Appl. Microbiol. 2019, 13, 567–571. [Google Scholar] [CrossRef]
- Olubodun, A.; Olayemi, A.; Shade, B.; Abidat, S.; Odunayo, D. Anti-diabetic Effect of Ethanol Extract of Carica papaya leaf in Alloxan induced Diabetic Mice. Mediterr. J. Basic Appl. Sci. 2018, 2, 46–56. [Google Scholar]
- George, A.D.I.; Uedeme-Naa, B.; Okon, M.A. Comparative study of testis histology and haematology of Clarias gariepinus exposed to phytochemicals of Moringa oleifera and Carica papaya leaf powder. Int. J. Fish. Aquat. Stud. 2020, 8, 301–306. [Google Scholar]
- Cruz, A.F.; de Oliveira, B.F.; Pires, M.d.C. Optimum Level of Nitrogen and Phosphorus to Achieve Better Papaya (Carica papaya var. Solo) Seedlings Growth and Mycorrhizal Colonization. Int. J. Fruit Sci. 2017, 17, 259–268. [Google Scholar] [CrossRef]
- Mandal, S.; Jaiswal, V.; Sagar, M.K.; Kumar, S. Formulation and evaluation of carica papaya nanoemulsion for treatment of dengue and thrombocytopenia. Plant Arch. 2021, 21, 1345–1354. [Google Scholar] [CrossRef]
- Zunjar, V.; Dash, R.P.; Jivrajani, M.; Trivedi, B.; Nivsarkar, M. Antithrombocytopenic activity of carpaine and alkaloidal extract of Carica papaya Linn. leaves in busulfan induced thrombocytopenic Wistar rats. J. Ethnopharmacol. 2016, 181, 20–25. [Google Scholar] [CrossRef]
- Asghar, M.S.; Hassan, M.; Rasheed, U.; Jawed, R.; Khan, N.A.; HaiderKazmi, S.J.; Khan, S.A. A study on investigating the role of Papaya extracts in the management of acute thrombocytopenia in Dengue fever, is there any clinical significance? Merit Res. J. 2020, 8, 485–491. [Google Scholar]
- Pandita, A.; Mishra, N.; Gupta, G.; Singh, R. Use of papaya leaf extract in neonatal thrombocytopenia. Clin. Case Rep. 2019, 7, 497–499. [Google Scholar] [CrossRef]
- Adarsh, V.B.; Doddamane, K.; Kumar, V.D. Role of Carica papaya Leaf Product in Improving the Platelet Count in Patients with Dengue Fever. Int. J. Res. Med. 2017, 6, 63–68. [Google Scholar]
- Manasa, M.; Vani, R. Evaluation of CaripillTM as a component of platelet storage solution. Hematol. Transfus. Cell Ther. 2021, 43, 133–140. [Google Scholar] [CrossRef]
- Sharma, N.; Mishra, K.P.; Chanda, S.; Bhardwaj, V.; Tanwar, H.; Ganju, L.; Kumar, B.; Singh, S.B. Evaluation of anti-dengue activity of Carica papaya aqueous leaf extract and its role in platelet augmentation. Arch. Virol. 2019, 164, 1095–1110. [Google Scholar] [CrossRef]
- Abdelrahim, L.M.; Zain, Z.N.; Jalil, S.N.A.; Seman, Z.A.; Mansur, F.A.; Abdullah, N.S. The Potential Role of Thrombopoietin and Interleukin-6 in the Thrombocytosis Effect of Carica papaya Leaves. Int. J. Res. Pharm. Sci. 2020, 11, 1928–1934. [Google Scholar] [CrossRef]
- Saraf, M.; Kavimandan, B. Animal trials of carica papaya leaf extracts for increasing platelet count. Indian J. Public Health Res. Dev. 2017, 8, 782–787. [Google Scholar] [CrossRef]
- Utama, S.Y.A. The effect of papaya leaf extract (Carica papaya L.) to the bleeding time on mice with trombositopenia. J. Gizi Diet. Indones. (Indones. J. Nutr. Diet.) 2018, 6, 133–138. [Google Scholar] [CrossRef]
- Santhosh, K.M.; Geetha, M.; Mansi, J.S.; Malvika, G.; Srinivas, L.D. Evaluation of efficacy of Carica papaya leaf extracts to increase platelet count in hydroxyurea induced thrombocytopenia in Albino rats. Int. J. Basic Clin. Pharmacol. 2018, 7, 173–178. [Google Scholar]
- Aberion, M.; Elizabelle, A.; Chan, R.M.A.; Noguera, R.G.G.; Padilla, H.J.D.; Yang, J.D. A Comparative Study on the Anti-thrombocytopenic Effect of Carica papaya Fruit and Leaf Extract on Aspirin-Induced Thrombocytopenic Male Rattus Novegicus Albino Rats. Available online: https://greenprints.dlshsi.edu.ph/grade_12/57/ (accessed on 27 March 2022).
- Gheith, I.; El-Mahmoudy, A. Hemogram and iron indices in renal anemia and the amelioration with Carica papaya leaf extract applied on albino rat model. Biosci. Rep. 2019, 39, BSR20181699. [Google Scholar] [CrossRef] [PubMed]
- Sundarmurthy, D.; Jayanthi, C.R.; Lakshmaiah, K.C. Effect of Carica papaya leaf extract on platelet count in chemotherapy-induced thrombocytopenic patients: A preliminary study. Natl. J. Physiol. Pharm. Pharmacol. 2017, 7, 685–692. [Google Scholar] [CrossRef]
- Hussain, S.M.A.; Sohrab, M.H.; Al-Mahmood, A.K.; Shuayb, M.; Al-Mansur, M.A.; Hasan, C.M. Clinical use of Carica papaya leaf extract in chemotherapy induced thrombocytopaenia. Int. J. Clin. Exp. Med. 2017, 10, 3752–3756. [Google Scholar]
- Sreelatha, P.; Jose, W.M. Efficacy of Carica papaya Leaf Extract in Reducing Treatment-Delay Secondary to Chemotherapy induced Thrombocytopenia. J. Clin. Diagn. Res. 2020, 14, 10–14. [Google Scholar] [CrossRef]
- Tiwari, R.; Mandal, D.K.; Patel, J. A post marketing randomized placebo controlled study to evaluate the efficacy of study product UPLAT® (Carica papaya leaf extract + Tinospora cordifolia extract) in the cancer patients with thrombocytopenia induced by chemotherapy. Int. J. Clin. Trials 2018, 5, 170. [Google Scholar] [CrossRef][Green Version]
- Panda, A.K.; Bhuyan, G.; Dattatray, D.; Rao, M.M. Phyto extracts of Carica papaya and Tinospora cordifolia can correct thrombocytopenia in alcoholic decompensate liver cirrhosis: Case Series. J. Ayurveda Integr. Med. Sci. 2018, 3, 99. [Google Scholar] [CrossRef]
- Singhai, A.; Juneja, V.; Abbas, S.; Kumar Jha, R. The effect of Carica papaya leaves extract capsules on platelets count and hematocrit levels in acute febrile illness with thrombocytopenia patient. Int. J. Med. Res. Health Sci. 2018, 41, 254–257. [Google Scholar]
- Semwal, D.K.; Dahiya, R.S.; Joshi, N.; Semwal, R.B.; Aswal, S.; Kumar, A.; Chauhan, A.; Kumar, A. Preclinical and Clinical Studies to Evaluate the Effect of Carica papaya Leaf Extract on Platelets. Curr. Tradit. Med. 2018, 4, 297–304. [Google Scholar] [CrossRef]
- Srikanth, B.; Reddy, L.; Biradar, S.; Shamanna, M.; Mariguddi, D.D.; Krishnakumar, M. An open-label, randomized prospective study to evaluate the efficacy and safety of Carica papaya leaf extract for thrombocytopenia associated with dengue fever in pediatric subjects. Pediatr. Health Med. Ther. 2019, 10, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, K.; Suresh, R.M.; Halesha, B.R. Role of Carica papaya leaf extract tablets/capsules on platelet counts in cases of dengue thrombocytopenia. Int. J. Adv. Med. 2018, 5, 845–848. [Google Scholar]
- Solanki, G.; Trivedi, P. Evaluation of the Efficacy of Carica Papaya Leaf Extract on Platelet Counts in Dengue Patients. Res. J. Adv. Sci. 2020, 30, 62–65. [Google Scholar]
- Yuson-Sunga, J.; Austria, M.P.A.B.; Caballes, N.V.; Favorito, C.E.L.; Lamera, C.C.L.; Manuba, A.J.L.; Ramos, D.L.D.; Soriano, M.J.; de los Santos Torrena, L.; Villostas, C.C. The The Effect of Carica papaya Leaf Extract on Increasing Platelet Count among Dengue Fever Patients: A Meta-Analysis. Acta Med. 2021, 52, 11–17. [Google Scholar] [CrossRef]
- Shetty, D.; Manoj, A.; Jain, D.; Narayane, M.; Rudrakar, A. The effectiveness of carica papaya leaf extract in children with european of biomedical and Pharmaceutical sciences the effectiveness of carica papaya leaf extract in children with. Eur. J. Biomed. Pharm. 2019, 6, 380–383. [Google Scholar] [CrossRef]
- Vijeth, S.B.; Kauser, M.M.; Mangsuli, V.; Kumar, V.S.R.; Sree, S.; Varghese, S.A. Effect of Carica papaya leaf extract (CPLE) on thrombocytopenia among dengue patients of tertiary care hospital, Chitradurga, India. Int. J. Adv. Med. 2018, 5, 974–977. [Google Scholar]
- Krishna, A.R.; Myreddy, N.; Uppara, S. Assessment of Platelet Levels in Serum Samples of Dengue Patients before and after Providing Papaya with Other Fruits. Ann. Int. Med. Dent. Res. 2017, 3, 1. [Google Scholar] [CrossRef]
- Sathyapalan, D.T.; Padmanabhan, A.; Moni, M.; P-prabhu, B.; Prasanna, P.; Balachandran, S.; Trikkur, S.P.; Jose, S.; Edathadathil, F.; Anilkumar, J.O.; et al. Efficacy & safety of Carica papaya leaf extract (CPLE) in severe thrombocytopenia (≤ 30,000/μL) in adult dengue—Results of a pilot study. PLoS ONE 2020, 15, e0228699. [Google Scholar]
- Abdel-Halim, S.A.; Ibrahim, M.T.; Mohsen, M.M.A.; Abou-Setta, L.M.; Sleem, A.A.; Morsy, F.A.; El-Missiry, M.M. Phytochemical and biological investigation of Carica papaya Linn. Leaves cultivated in Egypt (Family Caricaceae). J. Pharmacogn. Phytochem. 2020, 9, 47–54. [Google Scholar] [CrossRef]
- Iskandar, Y.; Mustarichie, R. Chemical Compounds’ Content Determination and a Pharmacognostic Parameter of Pepaya (Carica papaya, Linn.) Leaves Ethanol Extract. Int. J. Pharm. Res. Allied Sci. 2018, 7, 1–9. [Google Scholar]
- Khadam, S.; Afzal, U.; Gul, H.; Hira, S.; Satti, M.; Yaqub, A.; Ajab, H.; Gulfraz, M. Phytochemical screening and bioactivity assessment of leaves and fruits extract of Carica papaya. Pak. J. Pharm. Sci. 2019, 32, 1941–1948. [Google Scholar] [PubMed]
- Sarjono, P.R.; Putri, L.D.; Budiarti, C.E.; Mulyani, N.S.; Ngadiwiyana; Ismiyarto; Kusrini, D.; Adiwibawa Prasetya, N.B. Antioxidant and antibacterial activities of secondary metabolite endophytic bacteria from papaya leaf (Carica papaya L.). IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012112. [Google Scholar] [CrossRef]
- Gastélum-Martínez, E.; Ayora-Talavera, T.R.; González-Flores, T.; Sánchez-Contreras, A.; Ocampo-Thomason, P.; Pacheco-López, N.A. Use and exploitation of Carica papaya ‘Maradol’ and by-products for food industry application. Acta Hortic. 2019, 1250, 209–217. [Google Scholar] [CrossRef]
- Airaodion, A.I.; Okoroukwu, V.N.; Ogbuagu, E.O.; Ekenjoku, J.A.; Ogbuagu, U.; Airaodion, E.O. Antifertility Effect of Ethanolic Leaf Extract of Carica papaya in Male Wistar Rats. Merit Res. J. Med. Med. Sci. 2019, 7, 374–381. [Google Scholar] [CrossRef]
- Bin Hyun, S.; Ko, M.N.; Hyun, C.G. Carica papaya leaf water extract promotes innate immune response via mapk signaling pathways. J. Appl. Biol. Chem. 2021, 64, 277–284. [Google Scholar] [CrossRef]
- Shirole, A.; Agarwal, H.; Singh Parihar, P.; Brahma, S.; Vahikar, E. Benefits of Papaya Fruit and its leaves to Treat Malaria or Dengue and Various Other Uses for Human Health Meesha Deshpande (Guide). Int. Res. J. Eng. Technol. 2021, 8, 56–72. [Google Scholar]
- Singh, S.P.; Mishra, A.; Shyanti, R.K.; Singh, R.P.; Acharya, A. Silver Nanoparticles Synthesized Using Carica papaya Leaf Extract (AgNPs-PLE) Causes Cell Cycle Arrest and Apoptosis in Human Prostate (DU145) Cancer Cells. Biol. Trace Elem. Res. 2021, 199, 1316–1331. [Google Scholar] [CrossRef]
- Airaodion, A.I. Antidiabetic Effect of Ethanolic Extract of Carica papaya Leaves in Alloxan-Induced Diabetic Rats. Am. J. Biomed. Sci. Res. 2019, 5, 227–234. [Google Scholar] [CrossRef]
- Luiz, T.C.; da Cunha, A.P.S.; Aguiar, D.H.; Sugui, M.M.; de Campos Bicudo, R.; Sinhorin, A.P.; Sinhorin, V.D.G. Antioxidant potential of Carica papaya Linn (Caricaceae) leaf extract in mice with cyclophosphamide induced oxidative stress. Sci. Med. 2020, 30, e34702. [Google Scholar] [CrossRef]
- Razak, M.R.M.A.; Norahmad, N.A.; Jelas, N.H.M.; Afzan, A.; Misnan, N.M.; Ripen, A.M.; Thayan, R.; Zainol, M.; Mohamed, A.F.S. Immunomodulatory activities of Carica papaya L. Leaf juice in a non-lethal, symptomatic dengue mouse model. Pathogens 2021, 10, 501. [Google Scholar] [CrossRef]
- Jayasinghe, C.D.; Gunasekera, D.S.; De Silva, N.; Jayawardena, K.K.M.; Udagama, P.V. Mature leaf concentrate of Sri Lankan wild type Carica papaya Linn. modulates nonfunctional and functional immune responses of rats. BMC Complement. Altern. Med. 2017, 17, 230. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.C.; Chan, W.; Suwanarusk, R.; Ong, A.; Ho, H.K.; Russell, B.; Rénia, L.; Koh, H.L. In vitro antimalarial evaluations and cytotoxicity investigations of carica papaya leaves and carpaine. Nat. Prod. Commun. 2018, 14, 33–36. [Google Scholar] [CrossRef]
- Momoh, J.O.; Damazio, O.A.; Oyegbami, O.M. GC–MS Analysis and Antimalarial Activity of Methanolic Leaf Extract of Carica papaya against Plasmodium berghei NK65 Infection in Swiss Mice. Annu. Res. Rev. Biol. 2020, 35, 183–197. [Google Scholar] [CrossRef]
- Razak, M.R.M.A.; Mohmad Misnan, N.; Md Jelas, N.H.; Norahmad, N.A.; Muhammad, A.; Ho, T.C.D.; Jusoh, B.; Sastu, U.R.; Zainol, M.; Wasiman, M.I.; et al. The effect of freeze-dried Carica papaya leaf juice treatment on NS1 and viremia levels in dengue fever mice model 11 Medical and Health Sciences 1108 Medical Microbiology 11 Medical and Health Sciences 1103 Clinical Sciences. BMC Complement. Altern. Med. 2018, 18, 320. [Google Scholar] [CrossRef]
- Galappaththi, M.O.; Indeewari, W.M.A.; Abeykoon, A.M.S.B.; Muthuwaththa, M.G.C.M.; Kothwela, V.K.; Kumari, A.H.M.L.N.; Harasgama, H.D.R.P.D.; Noordeen, F. Antiviral activity of Carica papaya extract against experimental dengue virus 1 infection. Sri Lankan J. Infect. Dis. 2021, 11, 8374. [Google Scholar] [CrossRef]
- Pandey, S.; Walpole, C.; Cabot, P.J.; Shaw, P.N.; Batra, J.; Hewavitharana, A.K. Selective anti-proliferative activities of Carica papaya leaf juice extracts against prostate cancer. Biomed. Pharmacother. 2017, 89, 515–523. [Google Scholar] [CrossRef]
- Arif, M.; Yustisia, I.; Padlianah. The combination from ethanol extract of moringa leaves (Moringa oleifera L.) and ethanol extract of papaya leaves (Carica papaya L.) slows the tumor growth in sprague dawley rats induced 7,12-dimethylbenz(a)anthracene. Med. Clin. Pract. 2020, 3, 100100. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Aly Ahmed El Sayed, R.; Eid Madboly Hanafy, Z.; Fouad Abd El Fattah, H.; Kutb Mohamed Amer, A. Possible antioxidant and anticancer effects of plant extracts from Anastatica hierochuntica, Lepidium sativum and Carica papaya against Ehrlich ascites carcinoma cells. Cancer Biol. 2020, 10, 1–16. [Google Scholar] [CrossRef]
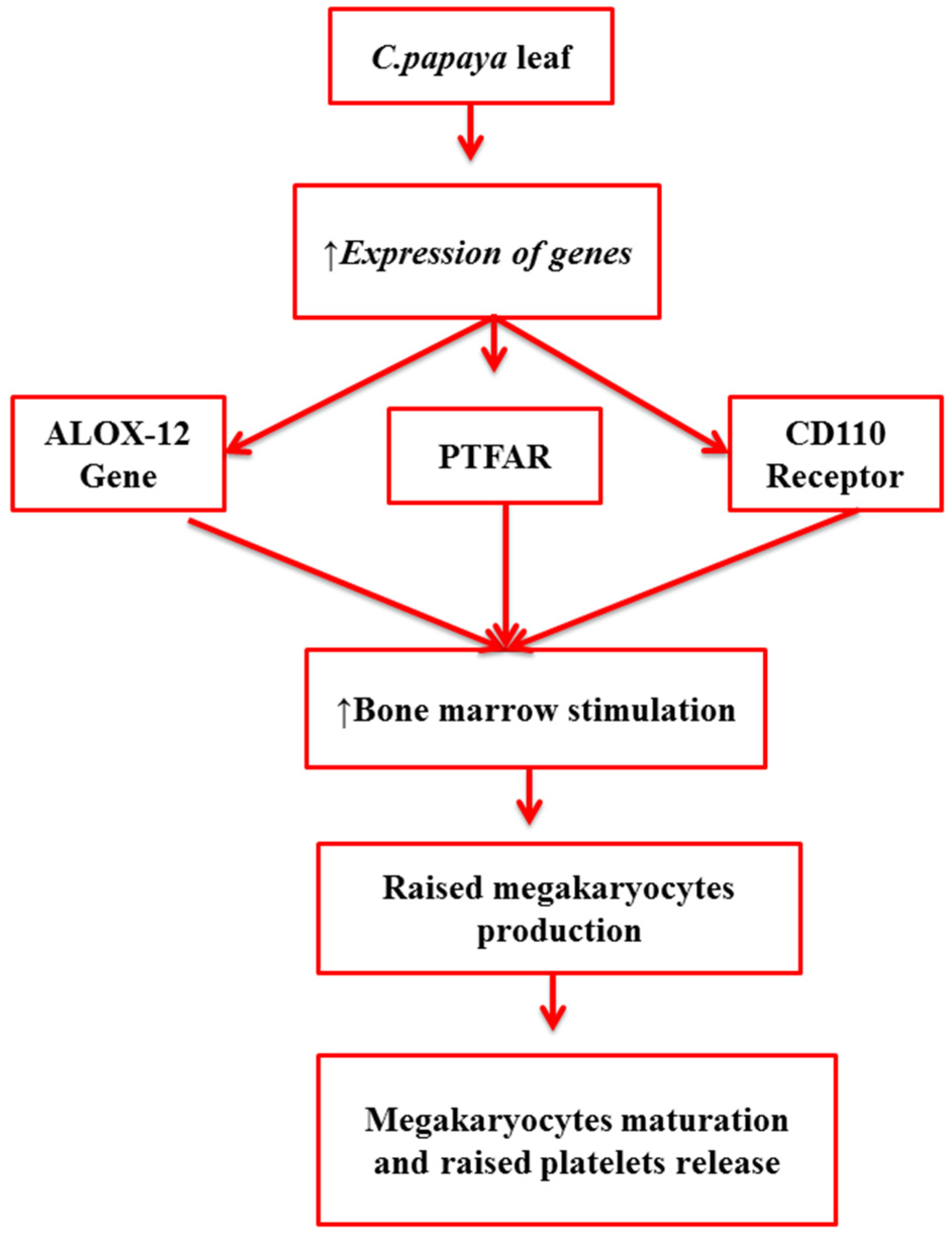
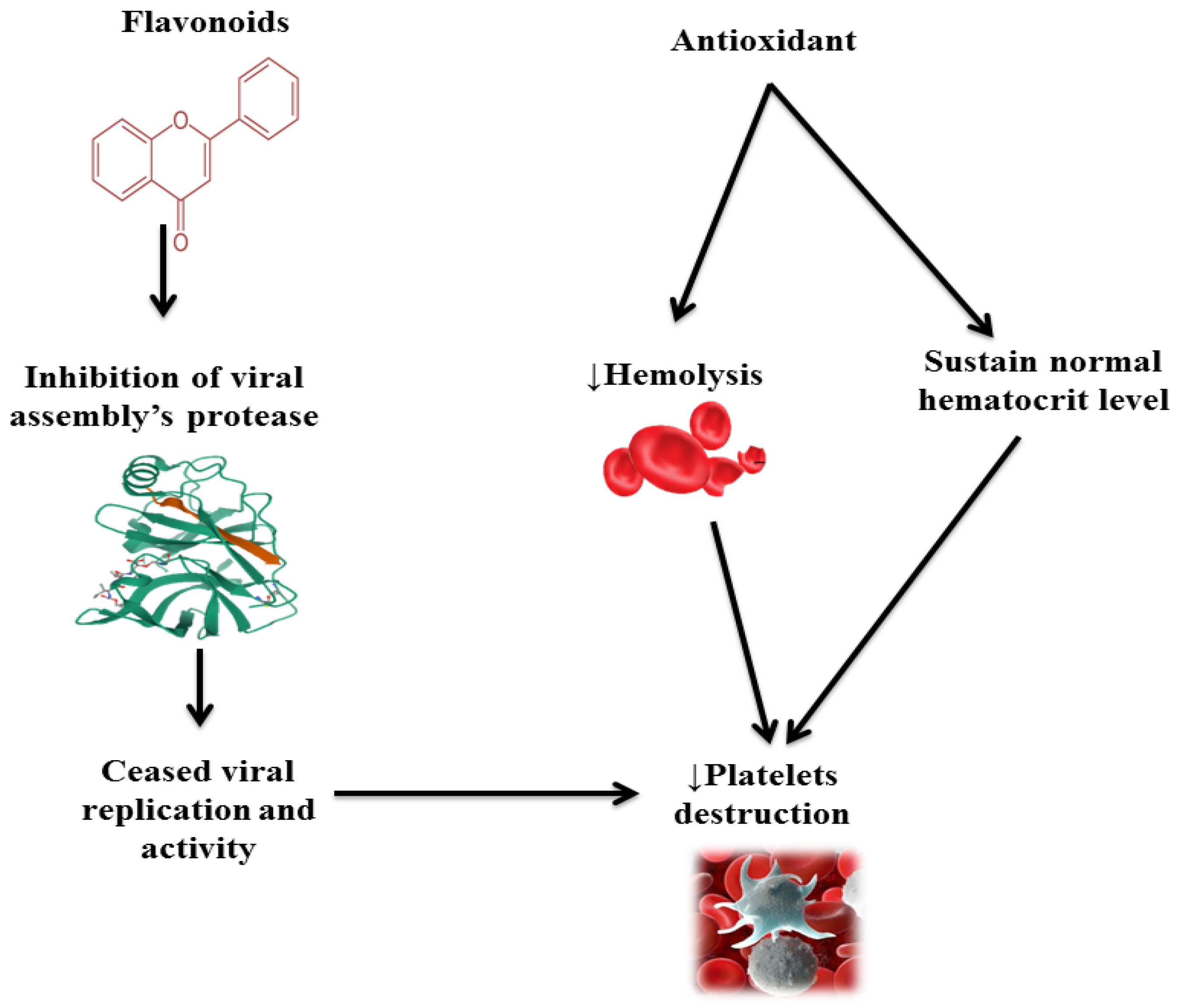
| Bioactive Component | Mechanism of Action | Reference |
|---|---|---|
| Carpaine | An alkaloid Stimulates ALOX-12 and PTFAR genes to produce megakaryocytes that mature and release more platelets Stimulates CD110, i.e., a receptor on megakaryocytes results in high platelet production | [13,14] |
| Quercetin | A flavonoid Decrease platelet destruction by binding viral proteases Inhibit viral replication that ceases platelet destruction by the viruses | [5] |
| Antioxidants | Ascorbic acid and beta-carotene Stabilize plate membrane Inhibit hemolysis Decrease platelet destruction | [15] |
| Thrombocytopenic Settings | Route of Exposure | Methodology | Findings | Reference |
|---|---|---|---|---|
| Human pediatric subjects aging 1–12 years | Thrombocytopenia associated with DHF grades I and II | n = 294, 5 days of treatment Control group (n = 147): standard therapy Investigation group (n = 147): CPLE syrup + standard therapy | Significant increase in platelets (p < 0.05), with good tolerance of extract in peds | [32] |
| Adult human subjects | Severe thrombocytopenia induced by dengue | n = 51, 5 days of treatment Control group (n = 24): placebo Investigation group (n = 26): CPLE 1100 mg capsule thrice daily | Enhanced platelet aggregation (p = 0.023); immunomodulatory and antiviral activity | [39] |
| Rat model | Cyclophosphamide-induced (6 days of subcutaneous injections of dose 70 mg/kg BD) thrombocytopenia | n = 88 (11 groups, 8 animals each), 14 days study Normal; thrombocytopenia and control; Hydrocortisone; CPJ1; CPJ2; CPJ-BT1; CPJ-BT2; CPJ-EA1; CPJ-EA2; CPJ-PE1; CPJ-PE2 (respective doses of 200 and 400 mg/kg bw in study groups) | Enhanced platelet count, reduced bleeding and clotting time, decreased oxidative markers, and increased serum thrombopoietin | [2] |
| Male Sprague Dawley rats | Cyclophosphamide-induced (s/c injections 50 mg/kg BW for 2 consecutive days) thrombocytopenia | 14 days of study, CPLE administration Hematology and histopathology evaluation | Significant platelets reduced NS1 and enveloped protein in DENV-infected THP-1 cells, and increased TPO levels | [18] |
| Human subjects aged 18 years | Chemotherapy-induced thrombocytopenia | n = 40, 7 days of treatment, 28-day follow-up Control group (n = 20): placebo Investigation group (n = 20): 1100 mg CPLE | Enhanced platelet count (p < 0.001), with improved hematological parameters | [25] |
| Male mice | Cotrimoxazole-induced (249.6 mg/kg BW for 8 days) thrombocytopenia | n = 30, 5 groups Groups 1 and 2: negative and positive control group Group 3: 0.5g CPLE/kg BW Group 4: 1 g/kg CPLE Group 5: 2 g/kg CPLE | 2 g CPLE/kg BW showed the highest significance, fast bleeding time (p < 0.0001) | [21] |
| Children aged 1 to 16 years | Dengue-associated thrombocytopenia | n = 30, 5 days dosage Control group: placebo Investigation group: Above 12 (1100 mg tablet thrice), 6–12 years (10 mL syrup thrice), below 6 (5 mL thrice) | Increasing trends in platelet count, with fast recovery from dengue (p = 0.15) | [36] |
| Humans | Dengue-associated thrombocytopenia | n = 988, 6 randomized trials, 5 days of CPLE administration | Platelets count increased; fast recovery from dengue | [35] |
| Humans | Dengue-associated thrombocytopenia | n = 100, 3 days dosage Control group (n = 50): Placebo Test group (n = 50): adult (CPLE 10 mL thrice daily + 1 kiwi fruit) Children (CPLE 5 mL thrice + 1 kiwi fruit) | Increased platelets and WBC count (p = 0.000), reduced muscle pain, and skin rashes | [34] |
| Infants | Neonatal thrombocytopenia | Neonate with sepsis, 7 of days treatment, follow-ups up to 18 months, 20 mg CPLE thrice daily | Platelet’s production increased with no side effects or discomfort | [15] |
| Humans | Chemotherapy-induced thrombocytopenia | n = 60, 5 days of study Control group (n = 30): placebo Case group (n = 30): CPLE capsules (290 mg twice daily) | CP leaf extracts significantly increased thrombocytes in post-chemotherapy cancer patients (p = 0.11) No adverse effects | [26] |
| Male patients | Alcoholic decompensate liver-cirrhosis-induced thrombocytopenia | n = 3 Cariden (phytoextracts of CP—1100 mg + Tinospora cordifolia—500 mg) | Enhances platelets in 15 days and normalizes level in 90 days of therapy | [29] |
| Humans | Chemotherapy- and microbial-infection-induced thrombocytopenia | n = 250, 5 days randomized study Control group (n = 125): placebo Intervention group (n = 125): 5 mL syrup twice daily | C. papaya and T. cordifolia leaves extracts combination effectively increases platelet count (p < 0.05) | [4] |
| Male albino rats | Nephrotoxicity due to subcutaneous administration of gentamicin | 21 days CPLE administration (150 and 300 mg/kg BW orally) Kidney biomarkers and blood profiling performed | Exhibit nephroprotective effect; increased RBCs, platelet, WBCs, HGB, and iron-binding capacity | [24] |
| Sprague Dawley rats | Intraperitoneal injection of carboplatin | n = 48, 7 groups (n = 6 each) 21 days dosage Control group: 0.3 mL distilled water Investigation group: 400 mg/kg BW | Raised platelets’ levels significantly | [20] |
| Humans | Chemotherapy-induced thrombocytopenia | n = 45, treatment period varied (7–14 days) 1100 mg tablets twice daily till recovery | Improved platelet count, mitigation of treatment delay in CIT | [27] |
| Female albino Wistar rats | Cyclophosphamide-induced thrombocytopenia | n = 24, 4 groups (n = 6 each group), 14 days treatment Groups I and II: normal and toxic control—0.8 mL saline water Group III: 50 mg/kg BW SCPLE Group IV: 150 mg/kg BW SCPLE | ↑ Platelet, leukocyte count in treatment groups (p < 0.01) ↓ Bleeding and clotting time in Groups III and IV Immunomodulation in SCPLE treated groups Highest significance in Group IV | [5] |
| Humans | Dengue-associated thrombocytopenia | n = 100, 5 days treatment Study group (n = 50): CPLE capsules at 500 mg thrice daily Control group (n = 50): placebo capsules thrice daily | ↑ Platelet count significantly in the study group (p < 0.01) ↓ Thrombocytopenia complications | [16] |
| Humans | Chemotherapy-induced thrombocytopenia | n = 60, 10 days of treatment Control group (n = 20): placebo Case group (n = 40): UPLAT® twice daily | Cases group showed an increased thrombocyte count in post-chemotherapy, no adverse effects | [28] |
| Preclinical: Wistar albino rats Clinical: Humans | Dengue-associated thrombocytopenia | Preclinical: 400 mg/kg BW of ME CPLE and AC CPLE and heparin 100 mg/kg BW Clinical: 1g leaf powder thrice daily | Pre-clinical: faster bleeding time by 3, 4, and 7 s respectively Clinical: platelet count increased by platelet attenuation | [31] |
| Sprague Dawley rats | Busulfan-induced thrombocytopenia (20 mg/kg BW intraperitoneal injections for 3 days) | n = 32, 7 days treatment, 4 groups (n = 8) Group A and B: positive and negative control Group C: 2 mL/day AQ CPLE Group D: 2 mL/day ME CPLE | Increased platelets (p = 0.00) and TPO (p = 0.149) levels in treatment groups, AQ CPLE showed the best results | [19] |
| Humans | Dengue-associated thrombocytopenia | n = 80, Control group (n = 40): placebo Investigation group (n = 40): 2 CPLE capsules thrice daily | Significant rise in platelet count (p < 0.05) Normal hematocrit level stability | [30] |
| Humans | Dengue-induced thrombocytopenia | n = 500, 5 days of dosage Study group: 1100 mg thrice daily | The study group showed a clear increase in Thrombocytes, and reduced dengue complications | [33] |
| Humans | Dengue-induced thrombocytopenia | n = 200 Control group (n = 100): standard treatment Investigation group (n = 100): CPLE | Increased platelet count (p = 0.045), reduced hospitalization, used as a supplement | [37] |
| Albino rats | Hydroxyurea (15 mg/kg BW orally) | n = 48, 5 days dosage, eight groups (n = 6) Group 1 and 2: control and toxic control The remaining 6 groups were fed with different doses of commercial and fresh CPLE | Significant increase in mean platelet, RBCs, and WBCs (p < 0.05) Decreased bleeding and clotting time | [22] |
| Male Rattus novergicus albino rats | Aspirin-induced thrombocytopenia | n = 15, 3 groups (n = 5), Group 1: control Group 2: CPFE Group 3: CPLE | Leaf extract showed a more significant increase in platelet, reduced bleeding and clotting time | [23] |
| Human | Dengue thrombocytopenia | n = 1 C. papaya + guava + apple + 1 spoonful ground leaf every 8 h | Increased platelets, reduced incidence of dengue | [38] |
| Activity | Bioactive Component | Mechanism of Action | Reference |
|---|---|---|---|
| Immunomodulation | Rutin and Narirutin | Stimulate JNK and ERK pathways in macrophages Increase prostaglandins 2 and pro-inflammatory cytokines Act as immunity-enhancing supplement | [46] |
| Antimalarial | Papain and Chymopapain | Cause immunity against insect attack Show larvicidal activity against Anopheles stephensi Combat malaria | [47] |
| Antiviral | Quercetin | Binds viral proteases Ceases viral replication Inhibits and destroy viral activity | [7] |
| Anticancer | Flavonoids | Cause cell-cycle arrest in cancer cells Promote apoptosis of cancer cells Inhibit reactive oxygen species Prevent cancer | [48] |
| Anticancer | Flavonoids | Cause cell-cycle arrest in cancer cells Promote apoptosis of cancer cells Inhibit reactive oxygen species Prevent cancer | [49] |
| Antidiabetic | Polyphenols | Hypoglycemic effect Inhibition of carbohydrate-hydrolyzing enzyme, α-amylase, and α-glucosidase Lower and retard glucose absorption | [45] |
| Antiangiogenic | Lycopene and Quercetin | Reduce length, size, and junction of blood vessels Inhibit the formation of abnormal new blood vessels Combat angiogenesis | [8] |
| Antioxidation | Ascorbic acid and tocopherol | Destroy ROS activity Inhibit oxidation of cell integrity Restraining oxidative chain reaction | [50] |
| Antibacterial | Alkaloids | Inhibit bacterial growth Prevent bacterial colonies formation Destroy bacteria | [40] |
| Immunomodulation | Rutin and Narirutin | Stimulate JNK and ERK pathways in macrophages Increase prostaglandins 2 and pro-inflammatory cytokines Act as immunity enhancing supplement | [46] |
| Antimalarial | Papain and Chymopapain | Cause immunity against insect attack Show larvicidal activity against Anopheles stephensi Combat malaria | [47] |
| Antiviral | Quercetin | Binds viral proteases Ceases viral replication Inhibits and destroy viral activity | [7] |
| Antidiabetic | Polyphenols | Hypoglycemic effect Inhibition of carbohydrate-hydrolyzing enzyme, α-amylase, and α-glucosidase Lower and retard glucose absorption | [49] |
| Antiangiogenic | Lycopene and Quercetin | Reduce length, size, and function of blood vessels Inhibit the formation of abnormal new blood vessels Combat angiogenesis | [8] |
| Antioxidation | Ascorbic acid and tocopherol | Destroy ROS activity Inhibit oxidation of cell integrity Restrain oxidative chain reaction | [50] |
| Antibacterial | Alkaloids | Inhibit bacterial growth Prevent bacterial colonies formation Destroy bacteria | [40] |
| Activity | Specimen | Route of Exposure | Methods | Findings | Reference |
|---|---|---|---|---|---|
| Immunomodulation | AG 129 Mice | Clinical DENV-2 (DMOF015) Isolate infection | 2-phase study, n = 25, 5 groups, 3 days post-infection dosing of 500 and 1000 mg/kg BW of freeze-dried CPLJ | WBC and neutrophils augmentation, anti-inflammatory activity, adjunctive immunotherapy | [51] |
| Wistar rats | No disease induction | n = 24. 4 groups, 3 days dosing of distilled water, 0.18, 0.36, and 0.72 mL mature leaf concentrate/100g of BW | Increased blood cells, improved functional and non-functional immune parameters | [52] | |
| Antimalarial | In vitro, leukocyte-depleted RBC | P. falciparum 3D7 and Dd2 strains | Bioassays/fractionation, carpaine isolation from leaf extraction, cytotoxicity evaluation against NL20 cells, and hemolysis assay performance on carpaine | Carpaine showed good activity against both strains, selective against the parasite, non-toxic to healthy RBCs, cured malaria | [53] |
| Swiss Mice | Intraperitoneal inoculation of P. berghei NK65 strain | n = 36, 6 groups, alternative dosages of standard drugs (10 mg/kg BW) and methanol CPLE (400 and 600 mg/kg BW) | Methanol CPLE act as an antimalarial entity, reduced WBC, increased HGB and HCT | [54] | |
| Antiviral | AG 129 Mice | Non-mouse adapted New Guinea C Strain Dengue virus inoculation | n = 20, 4 groups, 3-day post-infection dosing of mock doses, 500 and 1000 mg/kg BW of freeze-dried CPLJ | Decreased morbidity levels in the infected specimen by FCPLJ | [55] |
| C6/36 Cell containing culture | DENV-1 infected cells | Controls and 4 treated with four different dilutions of CPLE, qRT-PCR performed | Inhibition of DENV-1 viral infection in all four samples | [56] | |
| Kidney Vero E2 Cell lines | DENV-2 NS5 protein | Molecular docking, methanol CPLE Silver synthesized nanoparticles evaluation against dengue | Anti-dengue efficacy of silver synthesized nanoparticles of CPLE was observed in vitro | [7] | |
| Anticancer | Cell lines of prostate origin | Cell proliferation assays | In vitro, time-course analysis, CPL juice (1–0.01 mg/mL) with various extracts validated against the range of proliferative cell lines | Antiproliferative response and antimetastatic potential | [57] |
| Rats (breast gland) | 1 mL/rat DMBA induction with a dose of 25 mg | Combination of ethanolic extracts of moringa and C. papaya leaves, 150 and 200 mg/kg BW dosing | 150 mg/kg BW slowed cancer and tumor growth | [58] | |
| Antidiabetic | Albino rats | Intraperitoneal Alloxan monohydrate injection (150 mg/kg BW) | n = 30, 5 groups of distilled water, drugs, and ethanolic CPLE (250 and 500 mg/kg BW), 28 days dosing | Antihyperglycemic efficacy and improved lipid profiles | [9] |
| Albino rats | Alloxan monohydrate + sterile saline induced (150 mg/kg BW) | n = 30, 6 groups, 14 days dosing with DW, drugs, and ethanolic CPLE (200, 400, and 600 mg/kg BW) | Decreased blood glucose, hypolipidemia, lower TC, LDL, HDL, and TG | [49] | |
| Antiangiogenic | Fertilized chicken eggs | No induction | Humidified incubation, small window cut, control group, and various aqueous CPLE treated groups, 3-day incubation | Attenuate angiogenesis, reduction in length, size, and the junction of blood vessels | [8] |
| Antioxidant | Male Swiss Mice | Cyclophosphamide induced (75 mg/kg BW) | 15 days treatment, groups received water, drug, and CPLE (500 mg/kg BW) | Exhibit potential against oxidative events, reduce free radicals, and prevent DNA damage | [50] |
| Female Swiss albino mice | Intraperitoneal injections of Ehrlich ascites carcinoma cells | n = 90, 9 groups, provided with placebo, drugs, and 500 mg/kg BW of AH, LP, and CP extracts | Antioxidant, antimutagenic, and antitumor activity | [59] | |
| Antibacterial | In vitro, Petri-dish culture | Staphylococcus aureus, Bacillus subtilis, Salmonella typhi, and Escherichia coli inoculation | Positive and negative control and methanolic CPLE treated groups validation against various bacterial strains, MCPLE 7 component fractionation | Antibacterial activity, highest against E. coli by 5 components of MCPLE, treat gastroenteritis, urethritis, and wound infections | [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, S.; Liu, Z.-W.; Tariq, T.; Rabail, R.; Kowalczewski, P.Ł.; Lewandowicz, J.; Blecharczyk, A.; Abid, M.; Inam-Ur-Raheem, M.; Aadil, R.M. Delving into the Therapeutic Potential of Carica papaya Leaf against Thrombocytopenia. Molecules 2022, 27, 2760. https://doi.org/10.3390/molecules27092760
Munir S, Liu Z-W, Tariq T, Rabail R, Kowalczewski PŁ, Lewandowicz J, Blecharczyk A, Abid M, Inam-Ur-Raheem M, Aadil RM. Delving into the Therapeutic Potential of Carica papaya Leaf against Thrombocytopenia. Molecules. 2022; 27(9):2760. https://doi.org/10.3390/molecules27092760
Chicago/Turabian StyleMunir, Seemal, Zhi-Wei Liu, Tayyaba Tariq, Roshina Rabail, Przemysław Łukasz Kowalczewski, Jacek Lewandowicz, Andrzej Blecharczyk, Muhammad Abid, Muhammad Inam-Ur-Raheem, and Rana Muhammad Aadil. 2022. "Delving into the Therapeutic Potential of Carica papaya Leaf against Thrombocytopenia" Molecules 27, no. 9: 2760. https://doi.org/10.3390/molecules27092760
APA StyleMunir, S., Liu, Z.-W., Tariq, T., Rabail, R., Kowalczewski, P. Ł., Lewandowicz, J., Blecharczyk, A., Abid, M., Inam-Ur-Raheem, M., & Aadil, R. M. (2022). Delving into the Therapeutic Potential of Carica papaya Leaf against Thrombocytopenia. Molecules, 27(9), 2760. https://doi.org/10.3390/molecules27092760









