Chemical Characterization, Antioxidant and Antimicrobial Properties of Different Types of Tissue of Cedrus brevifolia Henry Extracts
Abstract
1. Introduction
2. Results
2.1. Chemical Analysis of Methanol Extract of C. brevifolia
2.2. Determination of Total Phenolic Content (TPC)
2.3. Antioxidant Activity of Methanol Extracts of C. brevifolia
2.4. Antimicrobial Activity of Extracts
2.4.1. Minimum Inhibitory Concentrations and Minimum Bactericidal Concentration against S. Aureus and E. Coli Bacteria
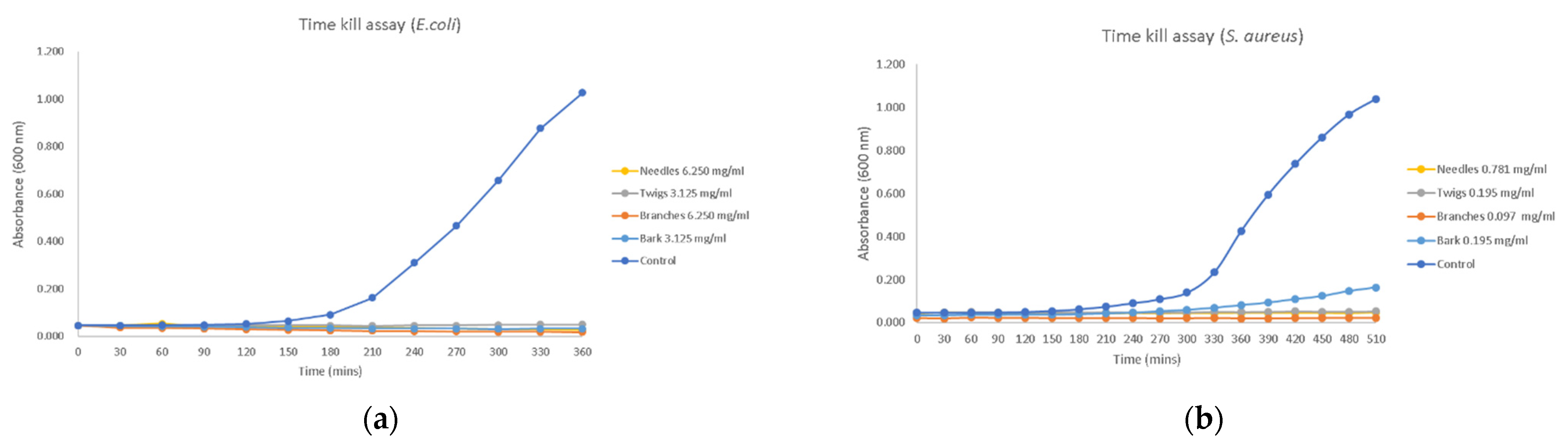
2.4.2. Time–Kill Assay
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Extracts
4.3. LC-Q-TOF/HRMS Analysis
4.4. Total Phenolic Content
4.5. Antioxidant Activity
4.6. Antibacterial Activity
4.6.1. Determination of MIC
4.6.2. Determining MBC
4.6.3. Time–Kill Assay
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pijut, P.M. Cedrus–The true cedar. Arboric. J. 2000, 26, 218–224. [Google Scholar] [CrossRef]
- Anna, K.; Jasińska, A.L.; Boratyńska, K.; Sobierajska, K.; Romo, A.; Ok, T.; Kharat, M.B.D.; Boratyński, A. Relationships among Cedrus libani, C. brevifolia and C. atlantica as revealed by the morphological and anatomical needle characters. Plant Syst. Evol. 2013, 299, 35–48. [Google Scholar] [CrossRef]
- Fabre, J.P.; Bariteau, M.; Chalon, A.; Thevenet, J. Possibilités de multiplication du puceron Cedrobium laportei Remaudière, (Homoptera, Lachnidae) sur différentes provenances du genre Cedrus et sur deux hybrides d’espèces, perspectives d’utilisation en France. In Proceedings of the International Meeting on Sylviculture of Cork Oak (Quercus suber L.), and Atlas cedar (Cedrus atlantica Manetti), Rabat, Morocco, 22–25 October 2001. [Google Scholar]
- Ladjal, M.; Huc, R.; Ducrey, M. Drought effects on hydraulic conductivity and xylem vulnerability to embolism in diverse species and provenances of Mediterranean cedars. Tree Physiol. 2005, 25, 1109–1117. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hort, A. Theophrastus: Enquiry into Plants, and Minor Works on Odours and Weather Signs; Books 1–9; Harvard University Press: Cambridge, MA, USA, 1980; Volume 2, pp. 458–465. [Google Scholar]
- Qiao, C.Y.; Ran, J.H.; Li, Y.; Wang, X.Q. Phylogeny and biogeography of Cedrus (Pinaceae) inferred from sequences of seven paternal chloroplast and maternal mitochondrial DNA regions. Ann. Bot. 2007, 100, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Croitoru, L. (Eds.) Department of Forests Cyprus. In Valuing Mediterranean Forests: Towards Total Economic Value; CABI Publishing: Wallingford, UK, 2005; pp. 213–225. [Google Scholar]
- Tsintides, T.; Christodoulou, C.S.; Delipetrou, P.; Georgiou, K. The Red Data Book of the Flora of Cyprus; Cyprus Forestry Association: Nicosia, Cyprus, 2007; pp. 149–151. [Google Scholar]
- European Commission. Interpretation Manual of European Union Habitats; Version EUR 28. Nature ENV B.3; DG Environment, European Commission: Brussels, Belgium, 2013; Available online: https://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int_Manual_EU28pdf (accessed on 24 May 2020).
- Hadjikyriakou, G.N. Aromatic and Spicy Plants in Cyprus-from Antiquity to the Present Day; Bank of Cyprus Cultural Foundation: Nicosia, Cyprus, 2007; pp. 44–46. [Google Scholar]
- Saab, A.M.; Gambari, R.; Sacchetti, G.; Guerrini, A.; Lampronti, I.; Tacchini, M.; El Samrani, A.; Medawar, S.; Makhlouf, H.; Tannoury, M.; et al. Phytochemical and pharmacological properties of essential oils from Cedrus species. Nat. Prod. Res. 2018, 32, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Saab, A.M.; Lampronti, I.; Grandini, A.; Borgatti, M.; Finotti, A.; Saccheti, G.; Gambari, R.; Guerrini, A. Antiproliferative and erythroid differentiation activities of Cedrus libani seed extracts against K562 human chronic myelogenus leukemia cells. Int. J. Pharm. Biol. Arch. 2011, 2, 1744–1748. [Google Scholar]
- Cretu, E.; Trifan, A.; Aprotosoaie, A.C.; Miron, A. 15-Lipoxygenase inhibition, superoxide and xydroxyl radicals scavenging activities of Cedrus brevifolia bark extracts. Rev. Med. Chir. Soc. Med. Nat. Iasi 2013, 117, 250–256. [Google Scholar]
- Douros, A.; Hadjipavlou-Litina, D.; Nikolaou, K.; Skaltsa, H. The occurrence of flavonoids and related compounds in Cedrus brevifolia A. Henry ex Elwes & A. Henry needles. Inhibitory potencies on lipoxygenase, linoleic acid lipid peroxidation and antioxidant activity. Plants 2018, 7, 1. [Google Scholar] [CrossRef]
- Douros, A.; Christopoulou, A.; Kikionis, S.; Nikolaou, K.; Skaltsa, H. Volatile components of heartwood, sapwood, and resin from dated Cedrus brevifolia. Nat. Prod. Commun. 2019, 14, 1934578X19859125. [Google Scholar] [CrossRef]
- Boutos, S.; Tomou, E.M.; Rancic, A.; Socović, M.; Hadjipavlou-Litina, D.; Nikolaou, K.; Skaltsa, H. Composition of the essential oil of Cedrus brevifolia needles. Evaluation of its antimicrobial and antioxidant activities. Am. J. Essent. Oil. Nat. Prod. 2020, 8, 1–5. [Google Scholar]
- Kanthal, L.K.; Dey, A.; Satyavathi, K.; Bhojaraju, P. GC-MS analysis of bio-active compounds in methanolic extract of Lactuca runcinata DC. Pharmacogn. Res. 2014, 6, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Motsumi, P.T.; Qwebani-Ogunleye, T.; Ejidike, I.P.; Mtunzi, F.M.; Nate, Z. Teedia lucida root extracts by ultrasonication and maceration techniques: Phytochemical screening, antimicrobial and antioxidant potentials. Rasāyan J. Chem. 2020, 13, 423–433. [Google Scholar] [CrossRef]
- Ntshanka, M.N.; Ejidike, I.P.; Mthunzi, F.M.; Moloto, M.J.; Mubiayi, K.P. Investigation into the phytochemical profile, antioxidant and antibacterial potentials of Combretum molle and Acacia mearnsii leaf parts. Biomed. Pharmacol. J. 2020, 13, 1683–1694. [Google Scholar] [CrossRef]
- Falowo, T.T.; Ejidike, I.P.; Lajide, L.; Clayton, H.S. Polyphenolic content of Musa acuminata and Musa paradisiaca bracts: Chemical composition, antioxidant and antimicrobial potentials. Biomed. Pharmacol. J. 2021, 14, 1767–1780. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Ahmad, S.I.; Mazumder, A. Cedrus deodara (Roxb.) Loud.: A review on its ethnobotany, phytochemical and pharmacological profile. Polym. J. 2011, 3, 12–17. [Google Scholar] [CrossRef]
- Agrawal, P.K.; Agarwal, S.K.; Rastogi, R.P. Dihydroflavonols from Cedrus deodara. Phytochemistry 1980, 19, 893–896. [Google Scholar] [CrossRef]
- Wu, Y.P.; Liang, X.; Liu, X.Y.; Zhong, K.; Gao, B.; Huang, Y.N.; Gao, H. Cedrus deodara pine needle as a potential source of natural antioxidants: Bioactive constituents and antioxidant activities. J. Funct. Foods 2015, 14, 605–612. [Google Scholar] [CrossRef]
- Shirazi, O.U.; Khattak, M.M.A.K.; Shukri, N.A.M.; Nasyriq, M.N. Determination of total phenolic, flavonoid content and free radical scavenging activities of common herbs and spices. J. Pharmacogn. Phytochem. 2014, 3, 104–108. [Google Scholar]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of two Origanum Species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef]
- Fabry, W.; Okemo, P.O.; Ansorg, R. Antibacterial activity of East African medicinal plants. J. Ethnopharmacol. 1998, 60, 79–84. [Google Scholar] [CrossRef]
- Mogana, R.; Adhikari, A.; Tzar, M.M.; Ramliza, R.; Wiart, C. Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq. against bacterial clinical isolates. BMC Complement Altern. Med. 2020, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, H.; Tan, G.; Zhu, Z.; Chai, Y. Liquid chromatography coupled with time-of-flight and ion trap mass spectrometry for qualitative analysis of herbal medicines. J. Pharm. Anal. 2011, 1, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Jain, S.; Misra, A.; Dwivedi, J.; Paliwal, S.; Sharma, S. Cedrus deodara (Roxb. ex D.Don) G.Don: A review of traditional use, phytochemical composition and pharmacology. J. Ethnopharmacol. 2021, 279, 114361. [Google Scholar] [CrossRef] [PubMed]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Pharmacognosy Res. 2014, 1, 35–40. [Google Scholar]
- Zhang, L.; Zhu, C.; Liu, X.; Su, E.; Cao, F.; Zhao, L. Study on synergistic antioxidant effect of typical functional components of hydroethanolic leaf extract from Ginkgo Biloba in vitro. Molecules 2022, 27, 439. [Google Scholar] [CrossRef] [PubMed]
- Cretu, E.; Salminen, J.P.; Karonen, M.; Miron, A.; Charalambous, C.; Constantinou, A.I.; Aprotosoaie, A.C. In vitro antioxidant activity and phenolic content of Cedrus brevifolia bark. Nat. Prod. Commun. 2014, 9, 481–482. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.; Qu, H.; Wang, K.; Jing, S.; Guan, S.; Su, L.; Li, Q.; Wang, D. Taxifolin, an inhibitor of Sortase A, interferes with the adhesion of Methicillin-resistant Staphylococcal aureus. Front. Microbiol. 2021, 12, 1876. [Google Scholar] [CrossRef]
- Wu, T.; He, M.; Zang, X.; Zhou, Y.; Qiu, T.; Pan, S.; Xu, X. A structure–activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, X.; Huo, L.; Zhang, S.; Chen, Y.; Zou, Z.; Tan, H. Sesquiterpenes and steroids from an endophytic Eutypella scoparia. J. Nat. Prod. 2021, 84, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, G.F.; Loesgen, S.J. Antibacterial drimane sesquiterpenes from Aspergillus ustus. Nat. Prod. 2021, 84, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhao, L.; Chen, K.; Shang, Y.; Wu, J.; Guo, X.; Chen, Y.; Liu, H.; Tan, H.; Qiu, S.X. Antibacterial sesquiterpenes from the stems and roots of Thuja sutchuenensis. Bioorg. Chem. 2020, 96, 103645. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Pisteli, L.; Giorgi, I. Antimicrobial action of flavonoids. In Dietary Phytochemicals and Microbes; Patra, A.K., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 33–61. [Google Scholar]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic effects of plant derivatives and conventional chemotherapeutic agents: An update on the cancer perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef]
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.H.; Zhao, J.; Zhang, W.D.; Ma, B.L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: Focus on natural occurring nanoparticles. Pharmaceutics 2020, 12, 128. [Google Scholar] [CrossRef]
- Naimi, F.; Bousta, D.; Balouiri, M.; Meskaoui, A.E. Antioxidant and free radical-scavenging properties of seeds flavonoids extract of Cedrus atlantica Manetti, Linum usitatissimum L. and Ocimum basilicum L. species. J. Appl. Pharm. Sci. 2015, 5, 95–99. [Google Scholar] [CrossRef][Green Version]
| Compound Number | tR | Molecular Formula | Observed ion (m/z) [M-H]− | ms/ms Productions | Compound Name | Twigs | Needles | Branches | Bark | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.72 | C14H18O9 | 329.0874 | 167.0346 152.0110 123.0085 | vanilloyl hexoside | + | + | - | + | NA |
| 2 | 1.89 | C13H16O8 | 299.0769 | 137.0239 121.0282 119.0363 | hydroxybenzoic acid hexoside | + | - | - | + | [14] |
| 3 | 2.83 | C16H20O9 | 355.1023 | 297.0569 193.0506 119.0475 | ferrulic acid hexoside | + | + | - | + | [21] |
| 4 | 3.26 | C15H14O6 | 289.0713 | SS | catechin | + | + | - | + | [14] |
| 5 | 3.59 | C21H22O13 | 481.0972 | 463.085 289.0711 163.0348 | epigallocatechin glucuronate | + | - | - | - | NA |
| 6 | 5.45 | C21H22O12 | 465.1026 | 447.0897 303.0504 151.0035 | quercitrin hydrate | + | - | - | + | NA |
| 7 | 5.91 | C22H21O13 | 495.1126 | 315.0507 163.0349 121.0284 | methyl epigallocathechin glucuronate | - | + | - | - | NA |
| 8 | 6.195 | C15H12O7 | 303.0501 | 285.0381 193.0450 151.0360 | taxifolin | - | - | + | + | [14,21] |
| 9 | 6.27 | C15H12O8 | 319.0453 | 301.0353 245.0408 151.0026 | dihydromyricetin | + | + | - | + | [21,22] |
| 10 | 6.92 | C27H30O15 | 593.1492 | 285.0391 229.1479 151.0001 | kaempferol rutinoside | - | + | - | - | [14] |
| 11 | 7.02 | C28H32O16 | 623.1587 | 315.0511 287.0555 151.0030 | isorhamnetin rutinoside | - | + | - | - | NA |
| 12 | 7.12 | C16H14O8 | 333.0606 | 315.0471 209.0407 166.0245 | cedrin | + | + | - | - | [21,22,23] |
| 13 | 7.15 | C16H12O8 | 331.0451 | 313.0334 271.0621 151.0053 | methylmyricetin | + | + | - | - | NA |
| 14 | 7.26 | C21H20O11 | 447.0921 | 285.0376 241.0491 151.0034 | astragalin | - | + | - | - | [14] |
| 15 | 7.36 | C22H24O12 | 479.1183 | 461.1055 271.0589 137.024 | methyl epicathechin glucuronate | + | + | - | + | NA |
| 16 | 7.46 | C22H22O12 | 477.1018 | 315.0368 299.0541 151.0392 | isorhamnetin hexoside | + | + | - | - | [14] |
| 17 | 7.60 | C26H32O11 | 519.1854 | 357.1317 219.0702 206.0531 | matairesinoside | + | + | - | - | NA |
| 18 | 7.78 | C20H18O10 | 417.0817 | 285.0336 213.0571 151.0029 | kaempherol pentoside | - | + | - | - | NA |
| 19 | 7.86 | C23H24O13 | 507.1129 | 344.0505 271.0181 151.0064 | syringetin glucoside | - | + | - | - | [14] |
| 20 | 8.01 | C16H14O7 | 317.0656 | 300.0569 165.0195 151.0398 | deodarin or cedeodarin 1 | + | - | - | + | [21,22] |
| 21 | 8.66 | C23H22O12 | 489.103 | 300.0346 151.0069 107.0485 | quercetin acetyl pentoside | - | + | - | - | NA |
| 22 | 8.75 | C16H14O7 | 317.0659 | 300.0557 165.0189 151.0408 | deodarin or cedeodarin 1 | + | - | - | + | [21,22] |
| 23 | 8.88 | C24H24O13 | 519.1128 | 315.1827 285.0388 151.0007 | isorhamnetin acetyl glucoside | - | + | - | - | NA |
| 24 | 9.13 | C20H22O7 | 373.1281 | 312.1009 237.0729 93.0366 | wikstromol | + | + | + | + | [21,23] |
| 25 | 9.19 | C25H26O14 | 549.1233 | 345.0614 271.0263 151.0037 | cedrusone A | - | + | - | - | [23] |
| 26 | 9.84 | C22H22O12 | 477.1035 | 315.6988 165.0195 121.0285 | rhamnetin hexoside | + | - | - | - | NA |
| 271 | 9.9 | C15H10O7 | 301.0349 | SS | quercetin | - | + | - | - | [23] |
| 28 | 10.19 | C30H22O13 | 593.1279 | 447.0896 307.093 285.1532 | tiliroside | + | + | - | - | [14] |
| 29 | 10.38 | C31H28O14 | 623.1387 | 447.0969 285.0400 151.0044 | kaempferol 3-(6″-ferulylglucoside) | - | + | - | - | NA |
| 30 | 11.10 | C20H22O6 | 357.1343 | 342.1077 123.0436 122.0371 | matairesinol | + | + | + | + | [21,23] |
| 31 | 11.22 | C15H12O5 | 271.0606 | SS | naringenin | + | - | + | + | NA |
| 32 | 11.84 | C15H10O6 | 285.0412 | SS | kaempferol | + | - | - | - | NA |
| 33 | 12.27 | C16H12O7 | 315.0502 | SS | isorhamnetin | + | - | - | + | NA |
| 34 | 13.48 | C16H14O5 | 285.0764 | 271.1185 151.0380 119.0501 | isosakuranetin | - | - | + | - | NA |
| 35 | 15.10 | C15H22O2 | 233.1543 | 217.1204 165.1230 107.0479 | himaphenolone | - | + | + | - | [23] |
| 36 | 15.33 | C15H22O3 | 249.1495 | 149.091 121.1011 68.9963 | deodardione | - | - | + | + | [23] |
| Tissues of C. brevifolia | TPC (mg GAE 1/g Crude Extract) ±SD |
|---|---|
| Needles | 16.656 c ± 1.058 |
| Twigs | 29.726 b ± 2.725 |
| Branches | 17.980 c ± 1.310 |
| Bark | 38.405 a ± 4.687 |
| Tissues of C. brevifolia | IC50 Concentration (mg/mL) ±SD | TEAC % ±SD |
|---|---|---|
| Needles | 0.078 c ± 0.007 | 10.287 ± 0.454 |
| Twigs | 0.031 b ± 0.006 | 27.630 ± 3.118 |
| Branches | 0.062 c ± 0.008 | 13.120 ± 1.076 |
| Bark | 0.011 a ± 0.001 | 97.667 ± 4.041 |
| Trolox (control) | 0.009 ± 0.001 | - |
| Tissues of C. brevifolia | E. coli | S. aureus | ||||
|---|---|---|---|---|---|---|
| MIC 2 (mg/mL) | MBC 3 (mg/mL) | MBC/ MIC 4 | MIC 2 (mg/mL) | MBC 3 (mg/mL) | MBC/ MIC 4 | |
| Needles | 6.250 | 12.5 | 2 | 0.781 | 0.781 | 1 |
| Twigs | 3.125 | 6.25 | 2 | 0.195 | 0.390 | 2 |
| Branches | 6.250 | 12.5 | 2 | 0.097 | 0.195 | 2 |
| Bark | 3.125 | 12.5 | 4 | 0.195 | 0.390 | 2 |
| Amp (control) 1 | 0.004 | 0.004 | 1 | - | - | - |
| Gen (control) 1 | - | - | - | 0.004 | 0.008 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charalambous, D.; Eliades, N.-G.H.; Christoforou, M.; Kakouri, E.; Kanakis, C.; Tarantilis, P.A.; Pantelidou, M. Chemical Characterization, Antioxidant and Antimicrobial Properties of Different Types of Tissue of Cedrus brevifolia Henry Extracts. Molecules 2022, 27, 2717. https://doi.org/10.3390/molecules27092717
Charalambous D, Eliades N-GH, Christoforou M, Kakouri E, Kanakis C, Tarantilis PA, Pantelidou M. Chemical Characterization, Antioxidant and Antimicrobial Properties of Different Types of Tissue of Cedrus brevifolia Henry Extracts. Molecules. 2022; 27(9):2717. https://doi.org/10.3390/molecules27092717
Chicago/Turabian StyleCharalambous, Despina, Nicolas-George Homer Eliades, Michalis Christoforou, Eleni Kakouri, Charalabos Kanakis, Petros A. Tarantilis, and Maria Pantelidou. 2022. "Chemical Characterization, Antioxidant and Antimicrobial Properties of Different Types of Tissue of Cedrus brevifolia Henry Extracts" Molecules 27, no. 9: 2717. https://doi.org/10.3390/molecules27092717
APA StyleCharalambous, D., Eliades, N.-G. H., Christoforou, M., Kakouri, E., Kanakis, C., Tarantilis, P. A., & Pantelidou, M. (2022). Chemical Characterization, Antioxidant and Antimicrobial Properties of Different Types of Tissue of Cedrus brevifolia Henry Extracts. Molecules, 27(9), 2717. https://doi.org/10.3390/molecules27092717








