Synthesis of Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Thioctate and the Potential for Antioxidant Application
Abstract
:1. Introduction
2. Results and Discussion
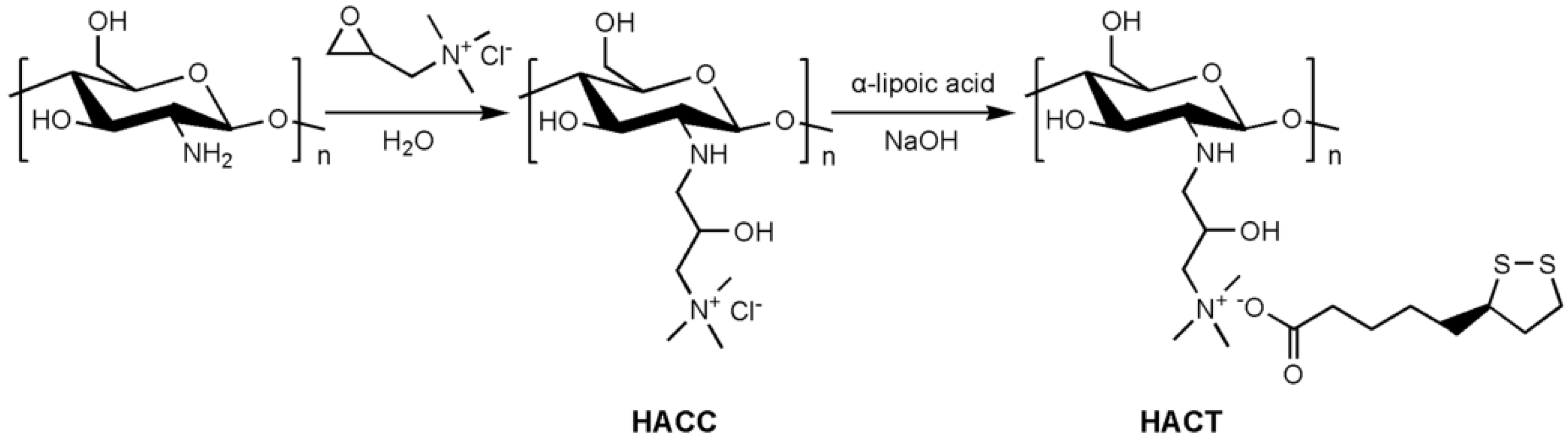
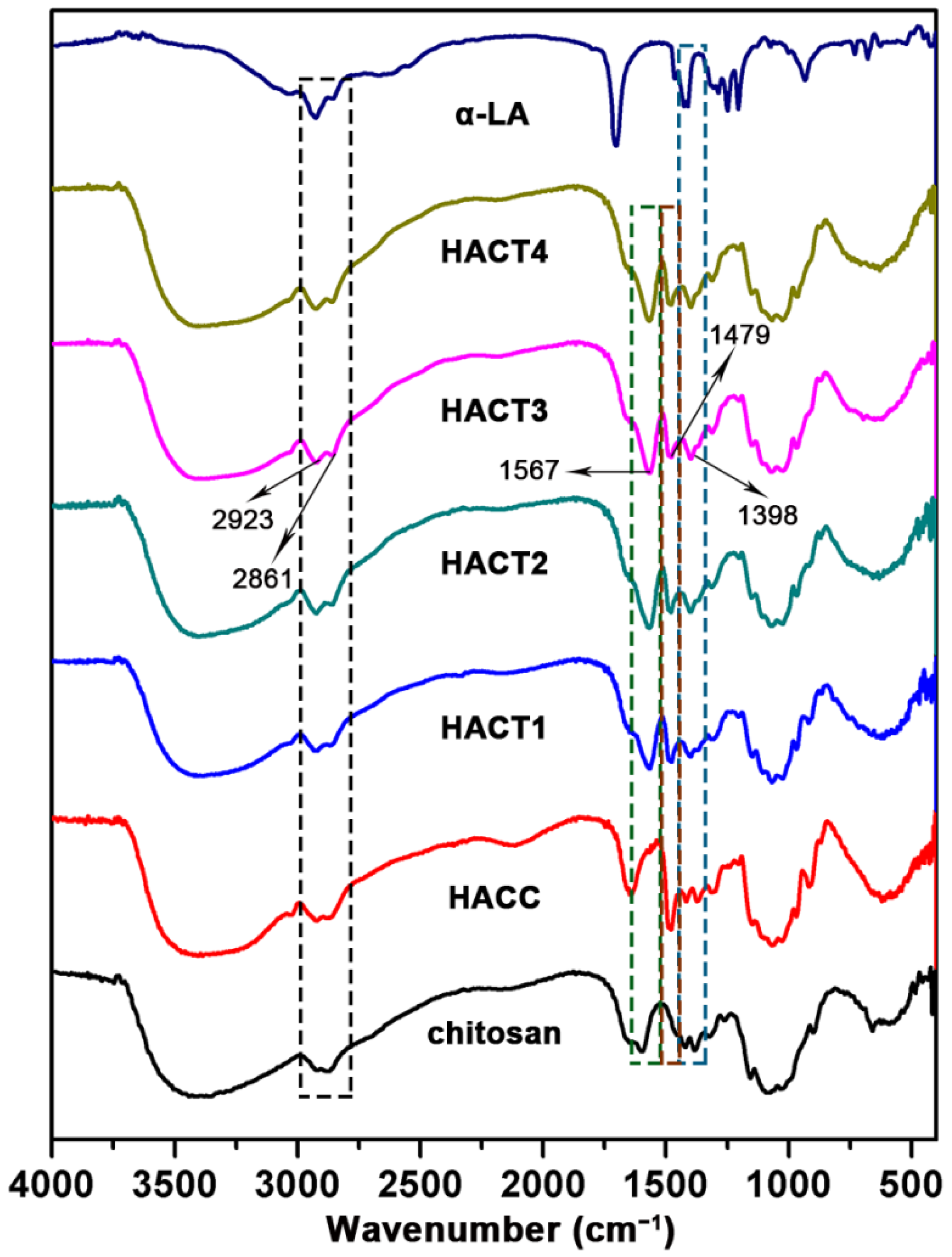
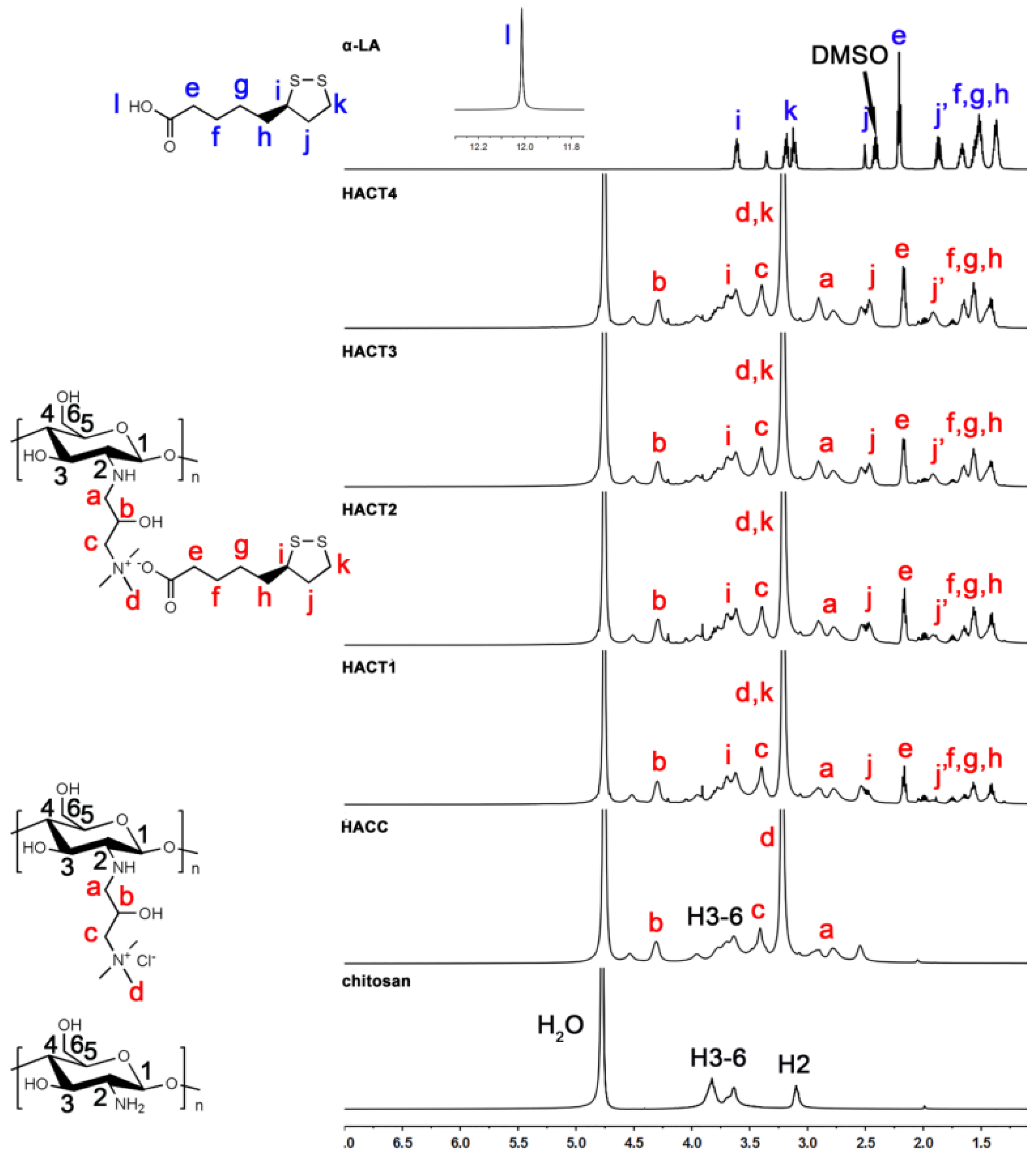
2.1. Chemical Synthesis and Characterization
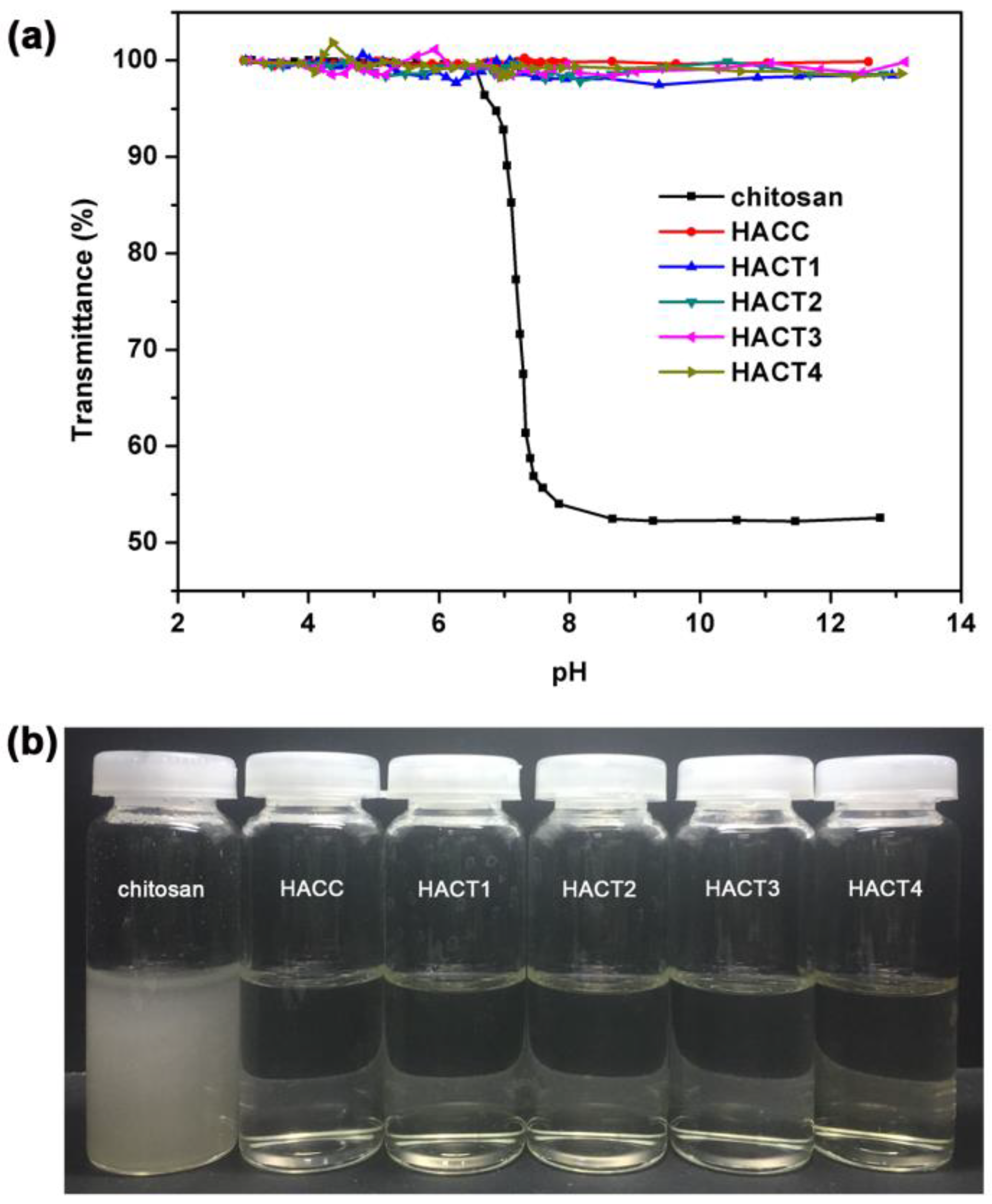
2.2. Water Solubility
2.3. Antioxidant Activity
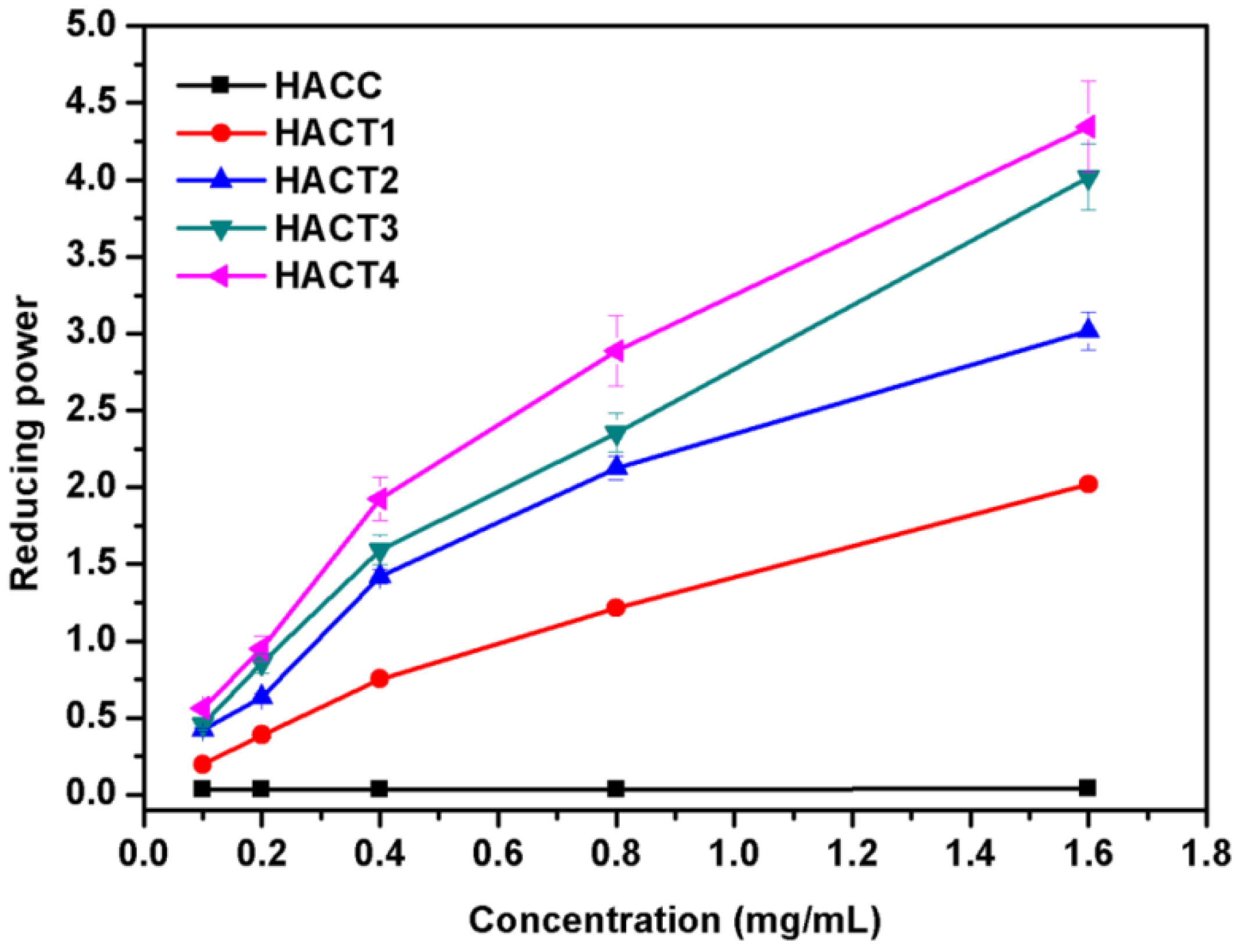
2.3.1. Reducing Power
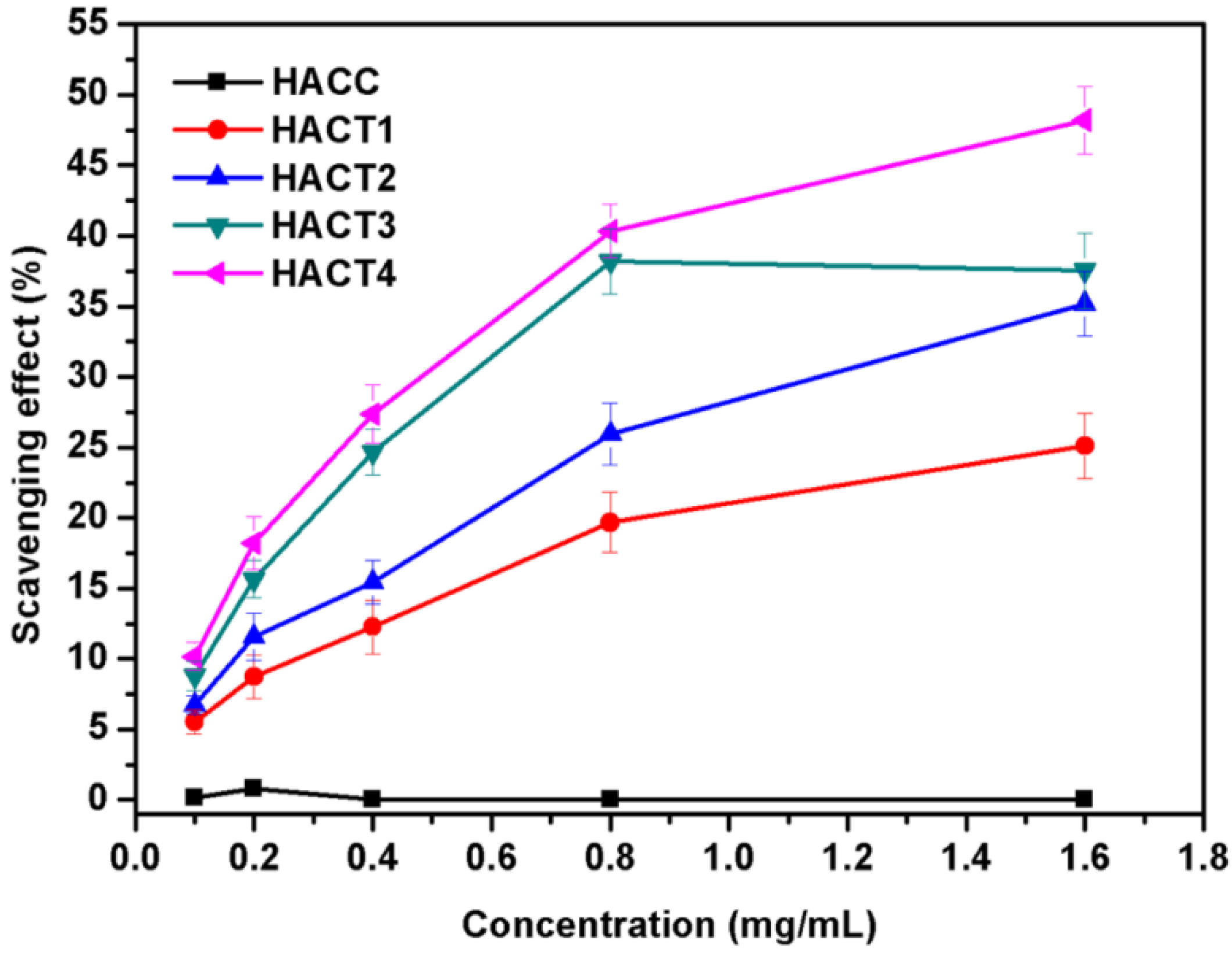
2.3.2. DPPH Radical Scavenging Activity
2.3.3. Hydroxyl Radical Scavenging Activity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chitosan Derivatives
3.2.1. Synthesis of Hydroxypropyltrimethyl Ammonium Chloride Chitosan (HACC)
3.2.2. Synthesis of Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Thioctate (HACTs)
3.3. Characterization of Chitosan Derivatives
3.3.1. Fourier Transform Infrared (FTIR) Spectroscopy
3.3.2. Nuclear Magnetic Resonance (1H NMR and 13C NMR) Spectroscopy
3.4. Water Solubility
3.5. Evaluation of Antioxidant Activity In Vitro
3.5.1. Reducing Power Assay
3.5.2. DPPH Radical Scavenging Assay
3.5.3. Hydroxyl Radical Scavenging Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Khan, A.Q.; Rashid, K.; AlAmodi, A.A.; Agha, M.V.; Akhtar, S.; Hakeem, I.; Raza, S.S.; Uddin, S. Reactive oxygen species (ROS) in cancer pathogenesis and therapy: An update on the role of ROS in anticancer action of benzophenanthridine alkaloids. Biomed. Pharmacother. 2021, 143, 112142. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Madronal, M.; Caro-Leon, J.; Espinosa-Cano, E.; Aguilar, M.R.; Vazquez-Lasa, B. Chitosan–Rosmarinic acid conjugates with antioxidant, anti-inflammatory and photoprotective properties. Carbohydr. Polym. 2021, 273, 118619. [Google Scholar] [CrossRef] [PubMed]
- Demurtas, M.; Baldisserotto, A.; Lampronti, I.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Indole derivatives as multifunctional drugs: Synthesis and evaluation of antioxidant, photoprotective and antiproliferative activity of indole hydrazones. Bioorg. Chem. 2019, 85, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Baldisserotto, A.; Demurtas, M.; Lampronti, I.; Tacchini, M.; Moi, D.; Balboni, G.; Pacifico, S.; Vertuani, S.; Manfredini, S.; Onnis, V. Synthesis and evaluation of antioxidant and antiproliferative activity of 2-arylbenzimidazoles. Bioorg. Chem. 2020, 94, 103396. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Liu, Y.; Jia, M.; Wang, Q.; Sun, Z.; Ji, L.; Mayo, K.H.; Zhou, Y.; Sun, L. Pectic polysaccharides from Radix Sophorae Tonkinensis exhibit significant antioxidant effects. Carbohydr. Polym. 2021, 262, 117925. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Dong, F.; Zhang, J.; Zhao, X.; Li, Q.; Guo, Z. Physical and Antioxidant Properties of Edible Chitosan Ascorbate Films. J. Agric. Food Chem. 2019, 67, 2530–2539. [Google Scholar] [CrossRef]
- Bhoopathy, S.; Inbakandan, D.; Rajendran, T.; Chandrasekaran, K.; Kasilingam, R.; Gopal, D. Curcumin loaded chitosan nanoparticles fortify shrimp feed pellets with enhanced antioxidant activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111737. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Guan, S.; Xu, J.; Gong, W.; Liu, T.; Ma, X.; Sun, C. Preparation, characterization and antioxidant activity of protocatechuic acid grafted carboxymethyl chitosan and its hydrogel. Carbohydr. Polym. 2021, 252, 117210. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, M.; Ma, G.; Fang, Y.; Yang, W.; Ma, N.; Fang, D.; Hu, Q.; Pei, F. The antioxidant and antimicrobial activities of different phenolic acids grafted onto chitosan. Carbohydr. Polym. 2019, 225, 115238. [Google Scholar] [CrossRef]
- Arata Badano, J.; Vanden Braber, N.; Rossi, Y.; Diaz Vergara, L.; Bohl, L.; Porporatto, C.; Falcone, R.D.; Montenegro, M. Physicochemical, in vitro antioxidant and cytotoxic properties of water-soluble chitosan-lactose derivatives. Carbohydr. Polym. 2019, 224, 115158. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Dabholkar, N.; Sharma, S.; Rapalli, V.K.; Alexander, A.; Singhvi, G. Chitosan-based microneedles as a potential platform for drug delivery through the skin: Trends and regulatory aspects. Int. J. Biol. Macromol. 2021, 184, 438–453. [Google Scholar] [CrossRef]
- Balan, V.; Dodi, G.; Mihai, C.T.; Serban, A.M.; Ursachi, V.C. Biotinylated chitosan macromolecule based nanosystems: A review from chemical design to biological targets. Int. J. Biol. Macromol. 2021, 188, 82–93. [Google Scholar] [CrossRef]
- Kandile, N.G.; Mohamed, H.M. New chitosan derivatives inspired on heterocyclic anhydride of potential bioactive for medical applications. Int. J. Biol. Macromol. 2021, 182, 1543–1553. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Tan, W.; Zhang, J.; Guo, Z. Phenolic-containing chitosan quaternary ammonium derivatives and their significantly enhanced antioxidant and antitumor properties. Carbohydr. Res. 2020, 498, 108169. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Tan, W.; Wang, G.; Li, Q.; Dong, F.; Guo, Z. The antioxidant and antifungal activity of chitosan derivatives bearing Schiff bases and quaternary ammonium salts. Carbohydr. Polym. 2019, 226, 115256. [Google Scholar] [CrossRef] [PubMed]
- Nornberg, A.B.; de Aquino, T.F.B.; Martins, C.C.; Luchese, C.; Wilhelm, E.A.; Jacob, R.G.; Hartwig, D.; Fajardo, A.R. Organoselenium-chitosan derivative: Synthesis via “click” reaction, characterization and antioxidant activity. Int. J. Biol. Macromol. 2021, 191, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Anghel, N.; Dinu, M.V.; Zaltariov, M.; Pamfil, D.; Spiridon, I. New cellulose-collagen-alginate materials incorporated with quercetin, anthocyanins and lipoic acid. Int. J. Biol. Macromol. 2021, 181, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Eser Faki, H.; Tras, B.; Uney, K. Alpha lipoic acid and vitamin E improve atorvastatin-induced mitochondrial dysfunctions in rats. Mitochondrion 2020, 52, 83–88. [Google Scholar] [CrossRef]
- Mahmoudi-Nezhad, M.; Vajdi, M.; Farhangi, M.A. An updated systematic review and dose-response meta-analysis of the effects of alpha-lipoic acid supplementation on glycemic markers in adults. Nutrition 2021, 82, 111041. [Google Scholar] [CrossRef]
- Sheikholeslami, S.; Khodaverdian, S.; Dorri-Giv, M.; Mohammad Hosseini, S.; Souri, S.; Abedi-Firouzjah, R.; Zamani, H.; Dastranj, L.; Farhood, B. The radioprotective effects of alpha-lipoic acid on radiotherapy-induced toxicities: A systematic review. Int. immunopharmacol. 2021, 96, 107741. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.; Molavi, N.; Tavalaee, M.; Abbasi, H.; Nasr-Esfahani, M.H. Alpha-lipoic acid improves sperm motility in infertile men after varicocelectomy: A triple-blind randomized controlled trial. Reprod. Biomed. Online 2020, 41, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Zhong, H.; Ge, W.; Ren, J.; Wang, X. Quercetin/chitosan-graft-alpha lipoic acid micelles: A versatile antioxidant water dispersion with high stability. Carbohydr. Polym. 2020, 234, 115927. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Han, Q.; Chen, L.; Fan, X.; Wang, Y.; Fei, Z.; Zhang, H.; Wang, Y. Redox response, antibacterial and drug package capacities of chitosan-alpha-lipoic acid conjugates. Int. J. Biol. Macromol. 2020, 154, 1166–1174. [Google Scholar] [CrossRef]
- Mi, Y.; Tan, W.; Zhang, J.; Wei, L.; Chen, Y.; Li, Q.; Dong, F.; Guo, Z. Synthesis, Characterization, and Antifungal Property of Hydroxypropyltrimethyl Ammonium Chitosan Halogenated Acetates. Mar. Drugs 2018, 16, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, Y.; Chen, Y.; Gu, G.; Miao, Q.; Tan, W.; Li, Q.; Guo, Z. New synthetic adriamycin-incorporated chitosan nanoparticles with enhanced antioxidant, antitumor activities and pH-sensitive drug release. Carbohydr. Polym. 2021, 273, 118623. [Google Scholar] [CrossRef]
- Xu, C.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. The immunostimulatory effects of hydroxypropyltrimethyl ammonium chloride chitosan-carboxymethyl chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 181, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Lyu, R.; Li, Z.; Liang, C.; Zhang, C.; Xia, T.; Wu, M.; Wang, Y.; Wang, L.; Luo, X.; Xu, C. Acylated carboxymethyl chitosan grafted with MPEG-1900 as a high-efficiency demulsifier for O/W crude oil emulsions. Carbohyd. Polym. Technol. Appl. 2021, 2, 100144. [Google Scholar] [CrossRef]
- Gao, K.; Zhan, J.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, P. Synthesis and effects of the selective oxidation of chitosan in induced disease resistance against Botrytis cinerea. Carbohydr. Polym. 2021, 265, 118073. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Chitosan, hydroxypropyltrimethyl ammonium chloride chitosan and sulfated chitosan nanoparticles as adjuvants for inactivated Newcastle disease vaccine. Carbohydr. Polym. 2020, 229, 115423. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, K.; Yu, H.; Liu, W.; Qin, Y.; Xing, R.; Liu, S.; Li, P. C-coordinated O-carboxymethyl chitosan Cu(II) complex exerts antifungal activity by disrupting the cell membrane integrity of Phytophthora capsici Leonian. Carbohydr. Polym. 2021, 261, 117821. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ye, W.; Wang, X.; Lin, W.; Lin, Q.; Zhang, Q.; Wu, F. Preparation of copper-chelate quaternized carboxymethyl chitosan/organic rectorite nanocomposites for algae inhibition. Carbohydr. Polym. 2016, 151, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Sauraj; Kumar, A.; Kumar, B.; Kulshreshtha, A.; Negi, Y.S. Redox-sensitive nanoparticles based on xylan-lipoic acid conjugate for tumor targeted drug delivery of niclosamide in cancer therapy. Carbohydrate Res. 2021, 499, 108222. [Google Scholar] [CrossRef]
- Andreica, B.-I.; Ailincai, D.; Sandu, A.-I.; Marin, L. Amphiphilic chitosan-g-poly(trimethylene carbonate)—A new approach for biomaterials design. Int. J. Biol. Macromol. 2021, 193, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. Synthesis, characterization, and antifungal evaluation of diethoxyphosphoryl polyaminoethyl chitosan derivatives. Carbohydr. Polym. 2018, 190, 1–11. [Google Scholar] [CrossRef]
- Yang, Y.; Xing, R.; Liu, S.; Qin, Y.; Li, K.; Yu, H.; Li, P. Hydroxypropyltrimethyl ammonium chloride chitosan activates RAW 264.7 macrophages through the MAPK and JAK-STAT signaling pathways. Carbohydr. Polym. 2019, 205, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zhang, J.; Zhao, X.; Li, Q.; Dong, F.; Guo, Z. Preparation and physicochemical properties of antioxidant chitosan ascorbate/methylcellulose composite films. Int. J. Biol. Macromol. 2020, 146, 53–61. [Google Scholar] [CrossRef]
- Lu, C.; Kim, B.M.; Chai, K.Y. Design, synthesis and evaluation of PEGylated lipoic acid derivatives with functionality as potent anti-melanogenic agents. Eur. J. Med. Chem. 2011, 46, 5184–5188. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, L.; Gao, J.; Huang, J.; Yang, Y.; Xu, Y.; Liu, S.; Yu, W. Characterization, antioxidant and immunomodulatory effects of selenized polysaccharides from dandelion roots. Carbohydr. Polym. 2021, 260, 117796. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Synthesis, characterization, and evaluation of antifungal and antioxidant properties of cationic chitosan derivative via azide-alkyne click reaction. Int. J. Biol. Macromol. 2018, 120, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, X.; Chen, Y.; Mi, Y.; Tan, W.; Miao, Q.; Li, Q.; Dong, F.; Guo, Z. Preparation of 2,6-diurea-chitosan oligosaccharide derivatives for efficient antifungal and antioxidant activities. Carbohydr. Polym. 2020, 234, 115903. [Google Scholar] [CrossRef] [PubMed]


| Compound | Chitosan | HACC | HACT1 | HACT2 | HACT3 | HACT4 |
|---|---|---|---|---|---|---|
| Degree of Substitution | 0.95 a | 1.55 b | 0.51 c | 0.68 c | 0.80 c | 0.86 c |
| Water Solubility (mg/mL) | / * | ≥50 | ≥50 | ≥50 | ≥50 | ≥50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, W.; Lin, C.; Zhang, J.; Li, Q.; Guo, Z. Synthesis of Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Thioctate and the Potential for Antioxidant Application. Molecules 2022, 27, 2682. https://doi.org/10.3390/molecules27092682
Tan W, Lin C, Zhang J, Li Q, Guo Z. Synthesis of Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Thioctate and the Potential for Antioxidant Application. Molecules. 2022; 27(9):2682. https://doi.org/10.3390/molecules27092682
Chicago/Turabian StyleTan, Wenqiang, Conghao Lin, Jingjing Zhang, Qing Li, and Zhanyong Guo. 2022. "Synthesis of Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Thioctate and the Potential for Antioxidant Application" Molecules 27, no. 9: 2682. https://doi.org/10.3390/molecules27092682
APA StyleTan, W., Lin, C., Zhang, J., Li, Q., & Guo, Z. (2022). Synthesis of Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Thioctate and the Potential for Antioxidant Application. Molecules, 27(9), 2682. https://doi.org/10.3390/molecules27092682






