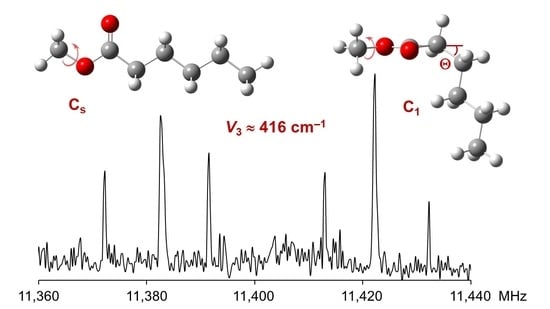
Methyl Internal Rotation in Fruit Esters: Chain-Length Effect Observed in the Microwave Spectrum of Methyl Hexanoate
Abstract
:1. Introduction
2. Results
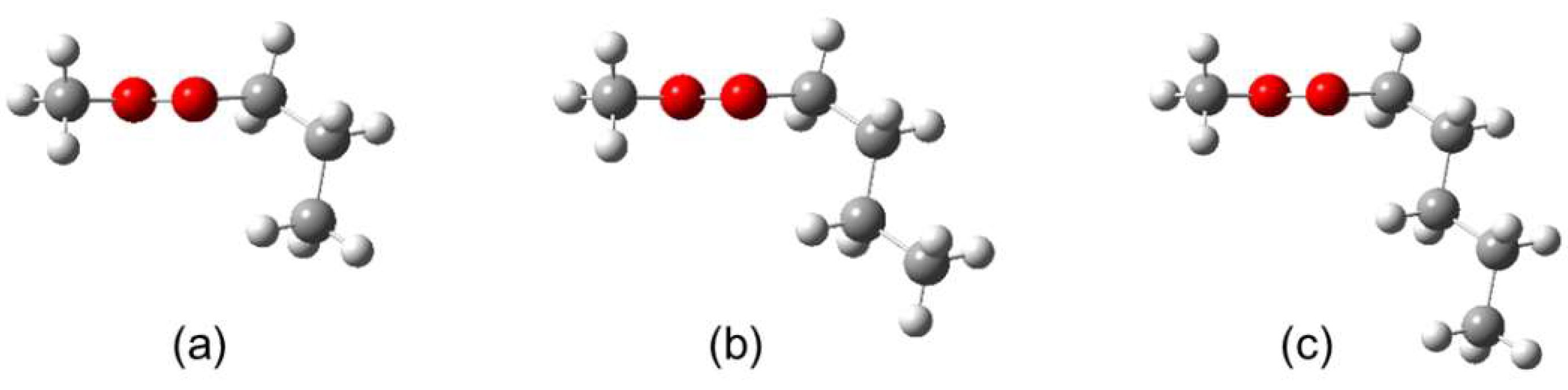
2.1. Quantum Chemical Calculations
2.2. Microwave Spectroscopy
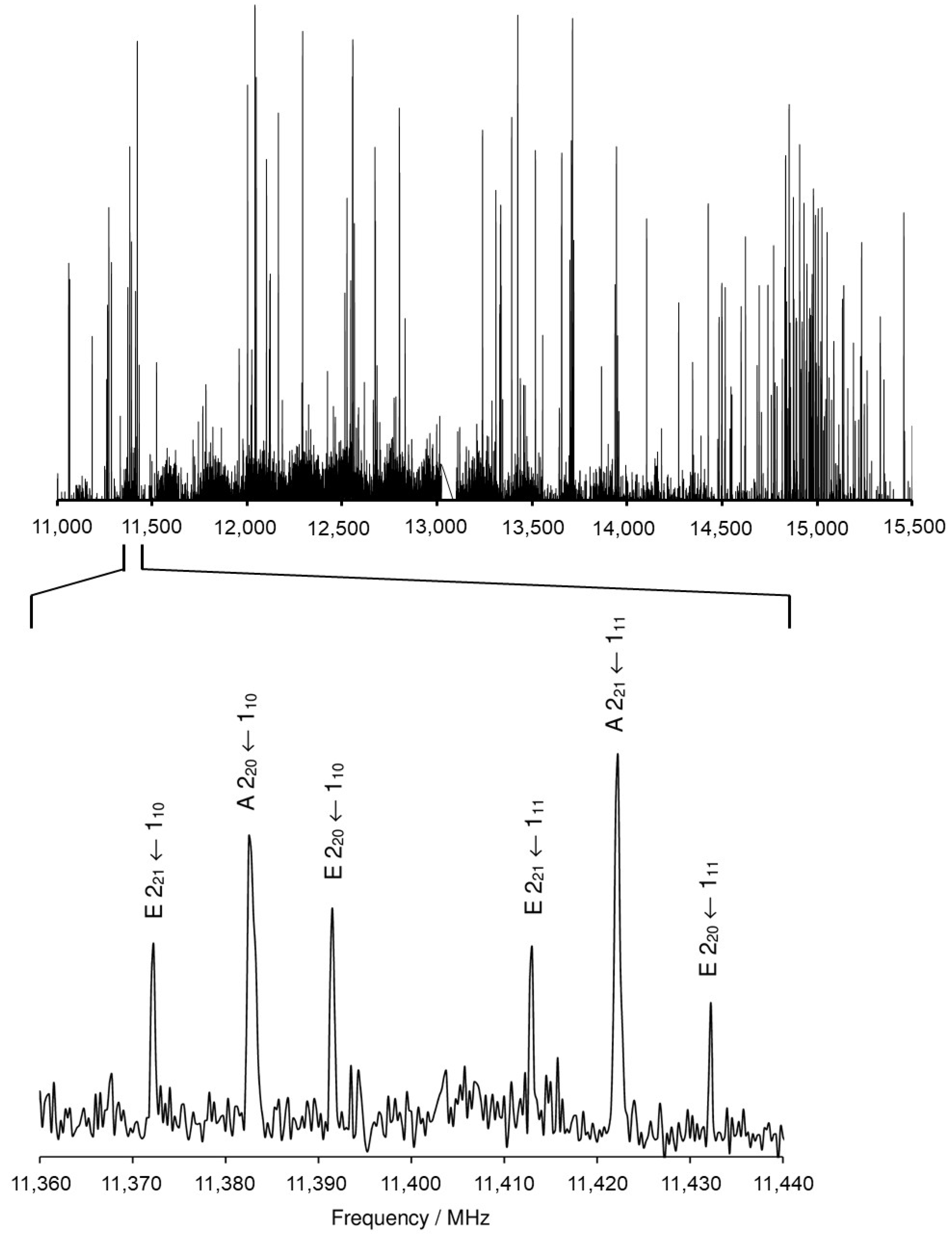
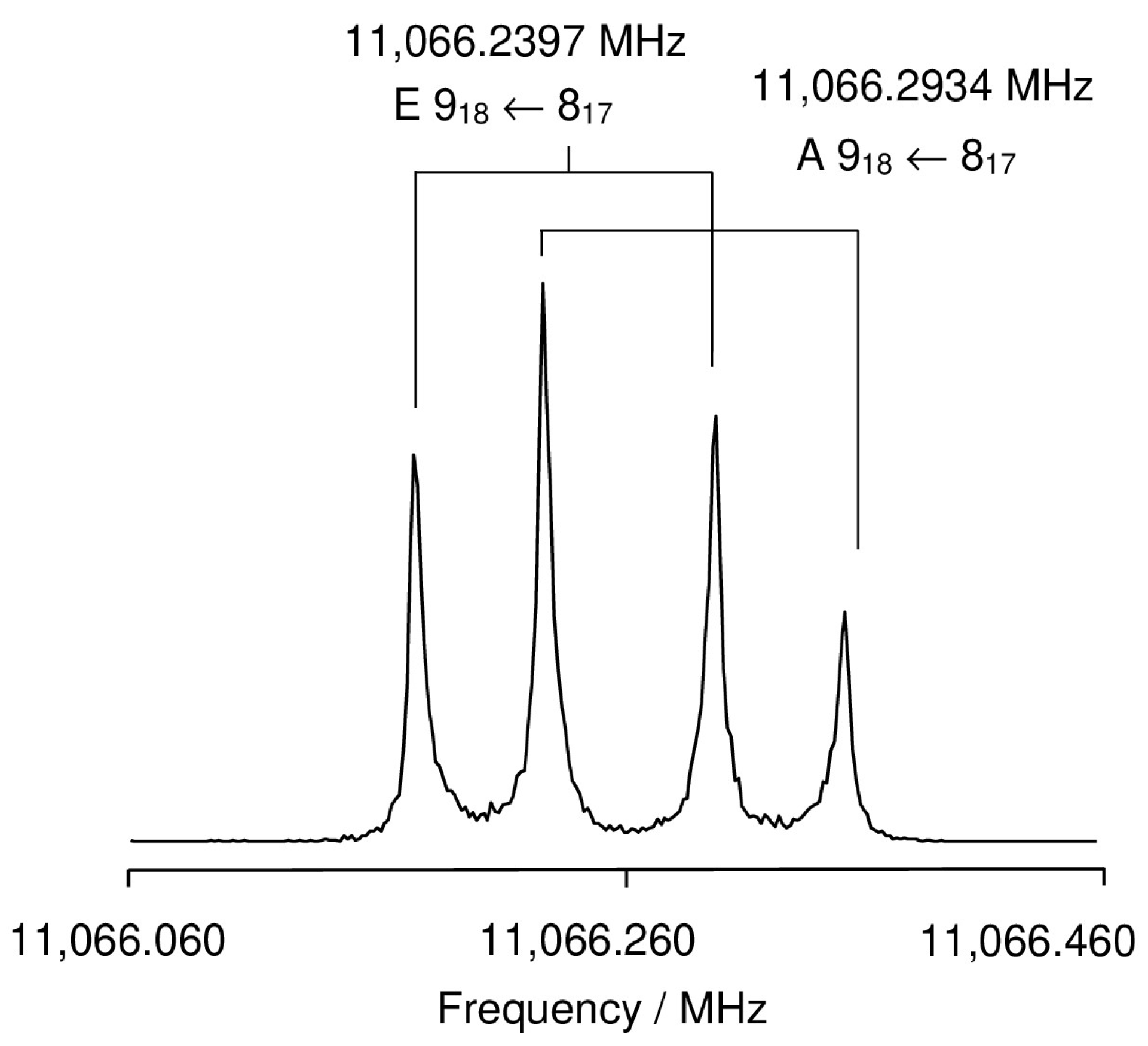
2.2.1. Measurements
2.2.2. The C1 Conformer
2.2.3. The Cs Conformer
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Elss, S.; Preston, C.; Hertzig, C.; Heckel, F.; Richling, E.; Schreier, P. Aroma Profiles of Pineapple Fruit (Ananas Comosus Merr.) and Pineapple Products. LWT-Food Sci. Technol. 2005, 38, 263–274. [Google Scholar] [CrossRef]
- Macoris, M.S.; Janzantti, N.S.; Garruti, D.d.S.; Monteiro, M. Volatile Compounds from Organic and Conventional Passion Fruit (Passiflora edulis F. flavicarpa) Pulp. Food Sci. Technol. 2011, 31, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Vendramini, A.L.; Trugo, L.C. Chemical Composition of Acerola Fruit (Malpighia Punicifolia L.) at Three Stages of Maturity. Food Chem. 2000, 71, 195–198. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Wang, S.Y.; Wang, C.Y.; González-Aguilar, G.A. Methyl Jasmonate in Conjunction With Ethanol Treatment Increases Antioxidant Capacity, Volatile Compounds and Postharvest Life of Strawberry Fruit. Eur. Food Res. Technol. 2005, 221, 731–738. [Google Scholar] [CrossRef]
- Schipilliti, L.; Dugo, P.; Bonaccorsi, I.; Mondello, L. Headspace-Solid Phase Microextraction Coupled to Gas Chromatography-Combustion-Isotope Ratio Mass Spectrometer and to Enantioselective Gas Chromatography for Strawberry Flavoured Food Quality Control. J. Chromatogr. A 2011, 1218, 7481–7486. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Reddy, B.R.; Chang, S.S. Chemical Reactions Involved in the Deep Fat Frying of Foods: IV. Identification of Acidic Volatile Decomposition Products of Hydrogenated Cottonseed Oil. J. Am. Oil. Chem. Soc. 1968, 45, 625–628. [Google Scholar] [CrossRef]
- Relkin, P.; Fabre, M.; Guichard, E. Effect of Fat Nature and Aroma Compound Hydrophobicity on Flavor Release from Complex Food Emulsions. J. Agric. Food Chem. 2004, 52, 6257–6262. [Google Scholar] [CrossRef]
- Gordy, W.; Cook, R.L. Microwave Molecular Spectra, 3rd ed.; Wiley: New York, NY, USA, 1984; Volume 18. [Google Scholar]
- Nguyen, H.V.L.; Kleiner, I. Theoretical and Computational Chemistry; Gulaczyk, I., Tylkowski, B., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021; pp. 41–78. [Google Scholar]
- Mouhib, H.; Stahl, W. Conformational Analysis of Green Apple Flavour: The Gas-Phase Structure of Ethyl Valerate Validated by Microwave Spectroscopy. Chem. Phys. Chem. 2012, 13, 1297–1301. [Google Scholar] [CrossRef]
- Mouhib, H.; Stahl, W.; Lüthy, M.; Büchel, M.; Kraft, P. Cassis Odor through Microwave Eyes: Olfactory Properties and Gas-Phase Structures of all the Cassyrane Stereoisomers and its Dihydro Derivatives. Angew. Chem. Int. Ed. 2011, 50, 5576–5580. [Google Scholar] [CrossRef]
- Sell, C.S. On the Unpredictability of Odor. Angew. Chem. Int. Ed. 2006, 45, 6254–6261. [Google Scholar] [CrossRef]
- Lin, C.C.; Swalen, J.D. Internal Rotation and Microwave Spectroscopy. Rev. Mod. Phys. 1959, 31, 841. [Google Scholar] [CrossRef]
- Tudorie, M.; Kleiner, I.; Hougen, J.T.; Melandri, S.; Sutikdja, L.W.; Stahl, W. A Fitting Program for Molecules With Two Inequivalent Methyl Tops and a Plane of Symmetry at Equilibrium: Application to New Microwave and Millimeter-Wave Measurements of Methyl Acetate. J. Mol. Spectrosc. 2011, 269, 211–225. [Google Scholar] [CrossRef]
- Nguyen, H.V.L.; Kleiner, I.; Shipman, S.T.; Mae, Y.; Hirose, K.; Hatanaka, S.; Kobayashi, K. Extension of the Measurement, Assignment, and Fit of the Rotational Spectrum of the Two-Top Molecule Methyl Acetate. J. Mol. Spectrosc. 2014, 299, 17–21. [Google Scholar] [CrossRef]
- Nguyen, H.V.L.; Stahl, W.; Kleiner, I. Structure and Rotational Dynamics of Methyl Propionate Studied by Microwave Spectroscopy. Mol. Phys. 2012, 110, 2035–2042. [Google Scholar] [CrossRef]
- Hernandez-Castillo, A.O.; Abeysekera, C.; Hays, B.M.; Kleiner, I.; Nguyen, H.V.L.; Zwier, T.S. Conformational Preferences and Internal Rotation of Methyl Butyrate by Microwave Spectroscopy. J. Mol. Spectrosc. 2017, 337, 51–58. [Google Scholar] [CrossRef]
- Nguyen, H.V.L.; Andresen, M.; Stahl, W. Conformational Sampling and Large Amplitude Motion of Methyl Valerate. Phys. Chem. Chem. Phys. 2021, 23, 2930–2937. [Google Scholar] [CrossRef]
- Andresen, M.; Schöngen, D.; Kleiner, I.; Schwell, M.; Stahl, W.; Nguyen, H.V.L. Internal Rotation of the Acetyl Methyl Group in Methyl Alkyl Ketones: The Microwave Spectrum of Octan-2-one. Chem. Phys. Chem. 2020, 21, 2206–2216. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular Orbital Methods 25. Supplementary Functions for Gaussian Basis Sets. J. Chem. Phys. 1984, 80, 3265. [Google Scholar] [CrossRef]
- Grabow, J.-U.; Mata, S.; Alonso, J.L.; Peña, I.; Blanco, S.; López, J.C.; Cabezas, C. Rapid Probe of the Nicotine Spectra by High-Resolution Rotational Spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 21063–21069. [Google Scholar] [CrossRef] [Green Version]
- Pérez, C.; Muckle, M.T.; Zaleski, D.P.; Seifert, N.A.; Temelso, B.; Shields, G.C.; Kisiel, Z.; Pate, B.H. Structures of Cage, Prism, and Book Isomers of Water Hexamer from Broadband Rotational Spectroscopy. Science 2012, 336, 897–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evangelisti, L.; Caminati, W. Modeling the Internal Rotation Tunnelling in Benzyl Alcohol by Ring Fluorination: The Rotational Spectrum of 3,5-Difluorobenzyl Alcohol. Chem. Phys. Lett. 2019, 737S, 100004. [Google Scholar] [CrossRef]
- Van, V.; Stahl, W.; Schwell, M.; Nguyen, H.V.L. Gas-phase Conformations of 2-Methyl-1,3-dithiolane Investigated by Microwave Spectroscopy. J. Mol. Struct. 2018, 1156, 348–352. [Google Scholar] [CrossRef]
- Oki, M.; Nakanishi, H. Conformations of the Ester Group. Bull. Chem. Soc. Jpn. 1970, 43, 2558–2566. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Paar, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Dunning, T.H., Jr. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange-Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.L.K.; McCarthy, M. Bayesian Analysis of Theoretical Rotational Constants from Low-Cost Electronic Structure Methods. J. Phys. Chem. A 2020, 124, 898–910. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.A.; Medcraft, C.; Gougoula, E.; Walker, N.R. Conformational Isomers of Trans-Urocanic Acid Observed by Rotational Spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 9495–9503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neeman, E.M.; Huet, T.R. Conformational Changes in Hydroxyl Functional Groups Upon Hydration: The Case Study of Endo Fenchol. Phys. Chem. Chem. Phys. 2021, 23, 2179–2185. [Google Scholar] [CrossRef]
- Van, V.; Stahl, W.; Nguyen, M.T.; Nguyen, H.V.L. The Smell of Coffee: The Carbon Atom Microwave Structure of Coffee Furanone Validated by Quantum Chemistry. Can. J. Phys. 2020, 98, 538–542. [Google Scholar] [CrossRef]
- Andresen, M.; Kleiner, I.; Schwell, M.; Stahl, W.; Nguyen, H.V.L. Acetyl Methyl Torsion in the Microwave Spectrum of Pentan-2-one. J. Phys. Chem. A 2018, 122, 7071–7078. [Google Scholar] [CrossRef]
- Andresen, M.; Kleiner, I.; Schwell, M.; Stahl, W.; Nguyen, H.V.L. Sensing the Molecular Structures of Hexan-2-one by Internal Rotation and Microwave Spectroscopy. ChemPhysChem 2019, 20, 2063–2073. [Google Scholar]
- Andresen, M.; Kleiner, I.; Schwell, M.; Stahl, W.; Nguyen, H.V.L. Microwave Spectrum and Internal Rotations of Heptan-2-one: A Pheromone in the Gas Phase. J. Phys. Chem. A 2020, 124, 1353–1361. [Google Scholar] [CrossRef]
- Tulimat, L.; Mouhib, H.; Nguyen, H.V.L.; Stahl, W. Laboratory Rotational Spectroscopy of Methyl n-Propyl Sulfide: Conformational Analysis and Methyl Internal Rotations. J. Mol. Spectrosc. 2020, 373, 111356. [Google Scholar] [CrossRef]
- Grabow, J.-U.; Stahl, W.; Dreizler, H. A Multioctave Coaxially Oriented Beam-Resonator Arrangement Fourier-Transform Microwave Spectrometer. Rev. Sci. Instrum. 1996, 67, 4072–4084. [Google Scholar] [CrossRef]
- Grabow, J.-U.; Stahl, W. Notizen: A Pulsed Molecular Beam Microwave Fourier Transform Spectrometer with Parallel Molecular Beam and Resonator Axes. Z. Naturforsch. 1990, 45a, 1043–1044. [Google Scholar] [CrossRef]
- Hartwig, H.; Dreizler, H. The Microwave Spectrum of trans-2,3-Dimethyloxirane in Torsional Excited States. Z. Naturforsch. 1996, 51a, 923–932. [Google Scholar] [CrossRef]
- Kleiner, I.; Hougen, J.T. Rho-Axis-Method Hamiltonian for Molecules Having One Methyl Rotor and 𝐶1 Point-Group Symmetry at Equilibrium. J. Chem. Phys. 2003, 119, 5505. [Google Scholar] [CrossRef]
- Attig, T.; Sutikdja, L.W.; Kannengießer, R.; Kleiner, I.; Stahl, W. The Microwave Spectrum of n-Butyl Acetate. J. Mol. Spectrosc. 2013, 284–285, 8–15. [Google Scholar] [CrossRef]
- Attig, T.; Kannengießer, R.; Kleiner, I.; Stahl, W. Conformational Analysis of n-Pentyl Acetate Using Microwave Spectroscopy. J. Mol. Spectrosc. 2013, 290, 24–30. [Google Scholar] [CrossRef]
- Attig, T.; Kannengießer, R.; Kleiner, I.; Stahl, W. The Microwave Spectrum of n-Hexyl Acetate and Structural Aspects of n-Alkyl Acetates. J. Mol. Spectrosc. 2014, 298, 47–53. [Google Scholar] [CrossRef]
- Hougen, J.T.; Kleiner, I.; Godefroid, M. Selection Rules and Intensity Calculations for a Cs Asymmetric Top Molecule Containing a Methyl Group Internal Rotor. J. Mol. Spectrosc. 1994, 163, 559–586. [Google Scholar] [CrossRef]
- Silva, W.G.D.P.; Evangelisti, L.; van Wijngaarden, J. Internal Motions and Sulfur Hydrogen Bonding in Methyl 3-Mercaptopropionate. J. Phys. Chem. A 2019, 123, 9840–9849. [Google Scholar] [CrossRef]
- Nair, K.P.R.; Herbers, S.; Bailey, W.C.; Obenchain, D.A.; Lesarri, A.; Grabow, J.-U.; Nguyen, H.V.L. Internal Rotation and Chlorine Nuclear Quadrupole Coupling in 2-Chloro-4-fluorotoluene Explored by Microwave Spectroscopy and Quantum Chemistry. Spectrochim. Acta A 2021, 247, 119120. [Google Scholar] [CrossRef]
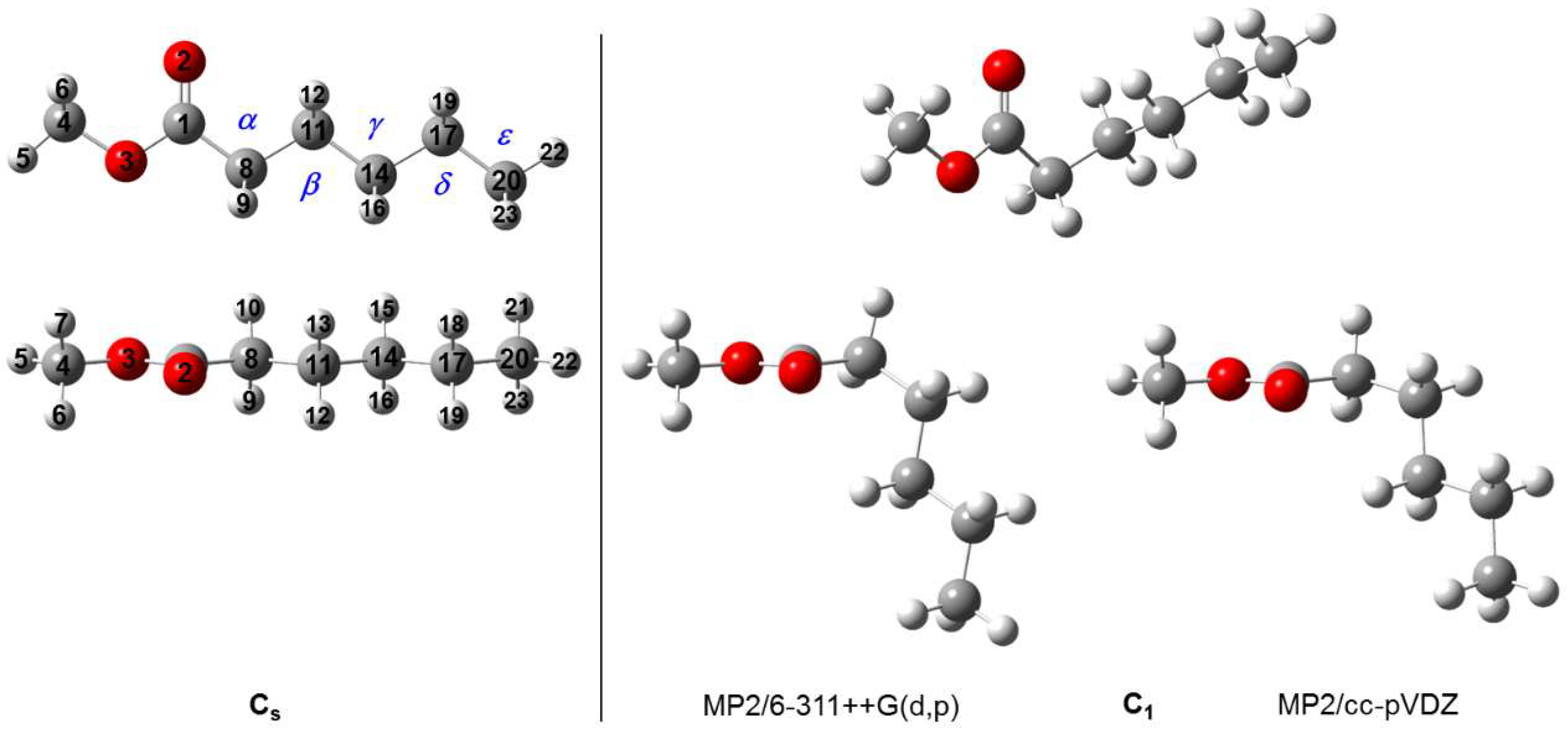
| Conf. | A | B | C | μa | μb | μc | ϑ1 | ϑ2 | ϑ3 | ϑ4 | E |
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | 3101.3 | 664.2 | 616.4 | –0.37 | 0.33 | –1.62 | –146.54 | 66.52 | 179.98 | 179.95 | 0.00 |
| I a | 3598.9 | 626.6 | 584.6 | –0.83 | 0.58 | –9.11 | –166.18 | 69.11 | 179.53 | 179.90 | |
| XIII | 6990.7 | 483.4 | 459.9 | 0.07 | –1.72 | 0.00 | 180.00 | 180.00 | 180.00 | 180.00 | 3.25 |
| Exp. b | 3599.0 | 625.4 | 585.8 |
| Par. a | Unit | I = C1 | XIII = Cs |
|---|---|---|---|
| A | MHz | 3599.03841(24) | 6987.3630(32) |
| B | MHz | 625.35951(20) | 483.652(15) |
| C | MHz | 585.83319(20) | 460.364(16) |
| ΔJ | kHz | 0.17529(66) | 0.0393(91) |
| ΔJK | kHz | −4.7630(45) | |
| ΔK | kHz | 45.278(21) | 6.13(65) |
| δJ | kHz | 0.01954(32) | 0.0027(11) |
| δK | kHz | −0.824(82) | 27.8(76) |
| V3 b | cm−1 | 415.15(13) | 416.890(96) |
| ∠(i,a) | ° | 134.280(79) | 158.82(12) |
| ∠(i,b) | ° | 133.80(40) | 68.82(12) |
| ∠(i,c) | ° | 79.46(95) | 90.0 c |
| NA/NEd | 100/80 | 21/21 | |
| σe | kHz | 4.0 | 4.4 |
| Molecule | Cs Conformer | C1 Conformer |
|---|---|---|
| Methyl acetate [14] | 424.581(56) | |
| Methyl propionate [16] | 422.801(22) a | |
| Methyl butyrate [17] | 420.155(71) | 419.447(59) |
| Methyl valerate [18] | 418.059(27) | 417.724(70) |
| Methyl hexanoate b | 416.890(96) | 415.15(13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, N.-N.; Pham, H.-N.; Kleiner, I.; Schwell, M.; Grabow, J.-U.; Nguyen, H.V.L. Methyl Internal Rotation in Fruit Esters: Chain-Length Effect Observed in the Microwave Spectrum of Methyl Hexanoate. Molecules 2022, 27, 2639. https://doi.org/10.3390/molecules27092639
Dang N-N, Pham H-N, Kleiner I, Schwell M, Grabow J-U, Nguyen HVL. Methyl Internal Rotation in Fruit Esters: Chain-Length Effect Observed in the Microwave Spectrum of Methyl Hexanoate. Molecules. 2022; 27(9):2639. https://doi.org/10.3390/molecules27092639
Chicago/Turabian StyleDang, Nhu-Ngoc, Hoang-Nam Pham, Isabelle Kleiner, Martin Schwell, Jens-Uwe Grabow, and Ha Vinh Lam Nguyen. 2022. "Methyl Internal Rotation in Fruit Esters: Chain-Length Effect Observed in the Microwave Spectrum of Methyl Hexanoate" Molecules 27, no. 9: 2639. https://doi.org/10.3390/molecules27092639
APA StyleDang, N.-N., Pham, H.-N., Kleiner, I., Schwell, M., Grabow, J.-U., & Nguyen, H. V. L. (2022). Methyl Internal Rotation in Fruit Esters: Chain-Length Effect Observed in the Microwave Spectrum of Methyl Hexanoate. Molecules, 27(9), 2639. https://doi.org/10.3390/molecules27092639