Dried and Fermented Powders of Edible Algae (Neopyropia yezoensis) Attenuate Hepatic Steatosis in Obese Mice
Abstract
:1. Introduction
2. Results
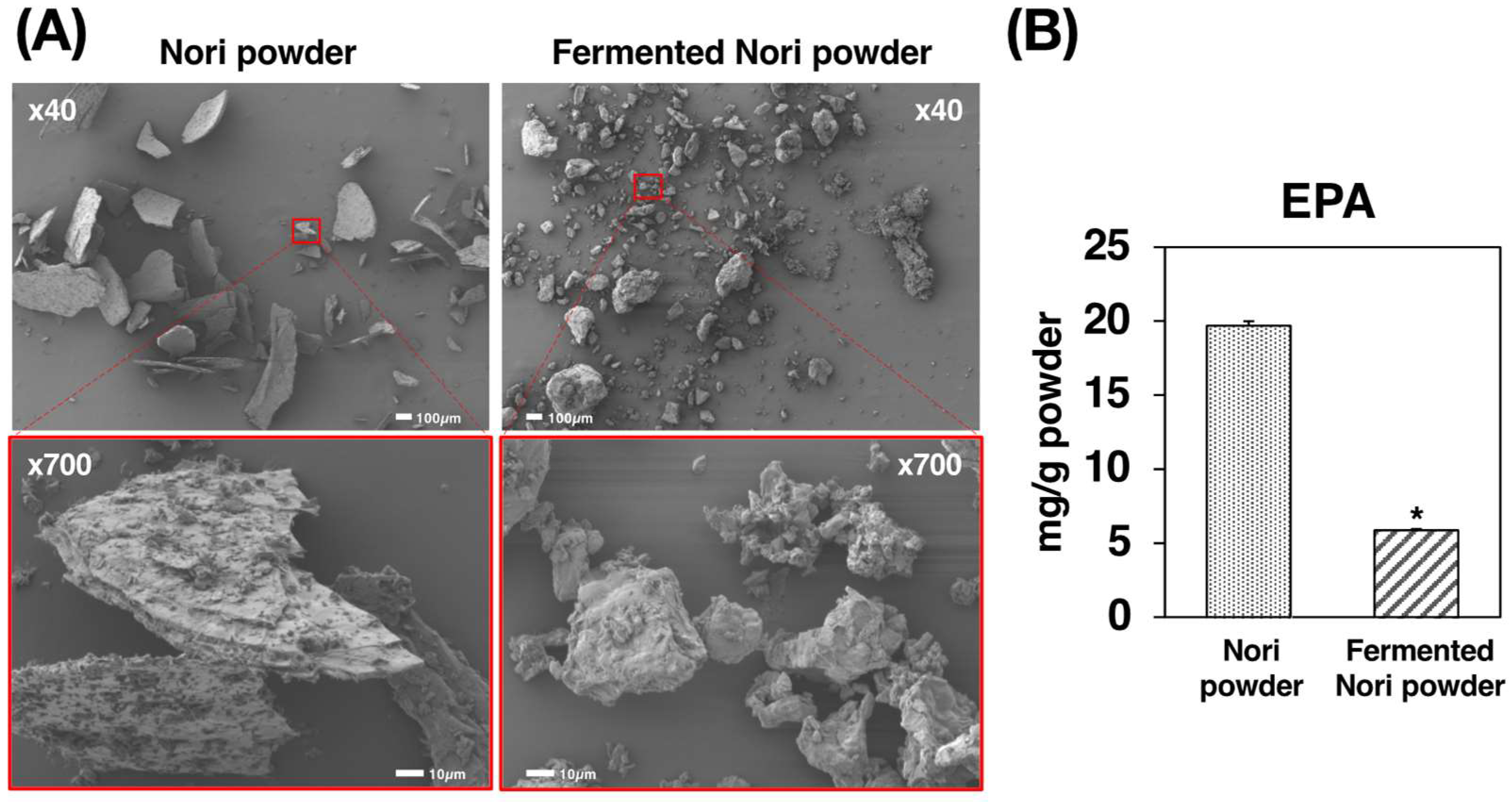
2.1. Preparation and Characterization of Nori Powder and Fermented Nori Powder
2.2. Effects of Experimental Diets on Growth Parameters
2.3. Incorporation of EPA in the Serum and Liver of db/db Mice
2.4. Effects of Experimental Diets on Liver Histology and Triglyceride Levels
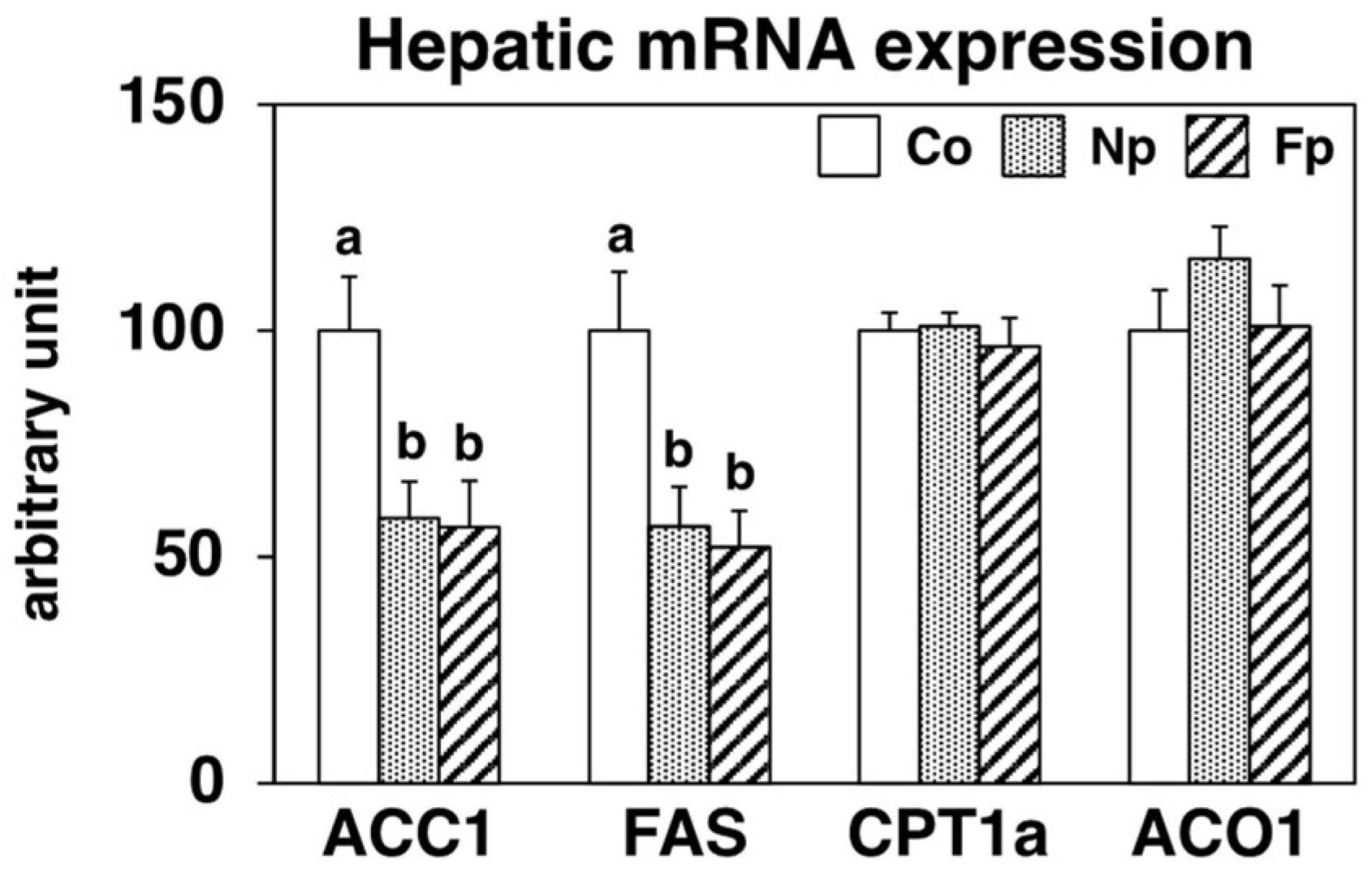
2.5. Effects of Experimental Diets on Hepatic Expression of Genes Related to Lipid Metabolism
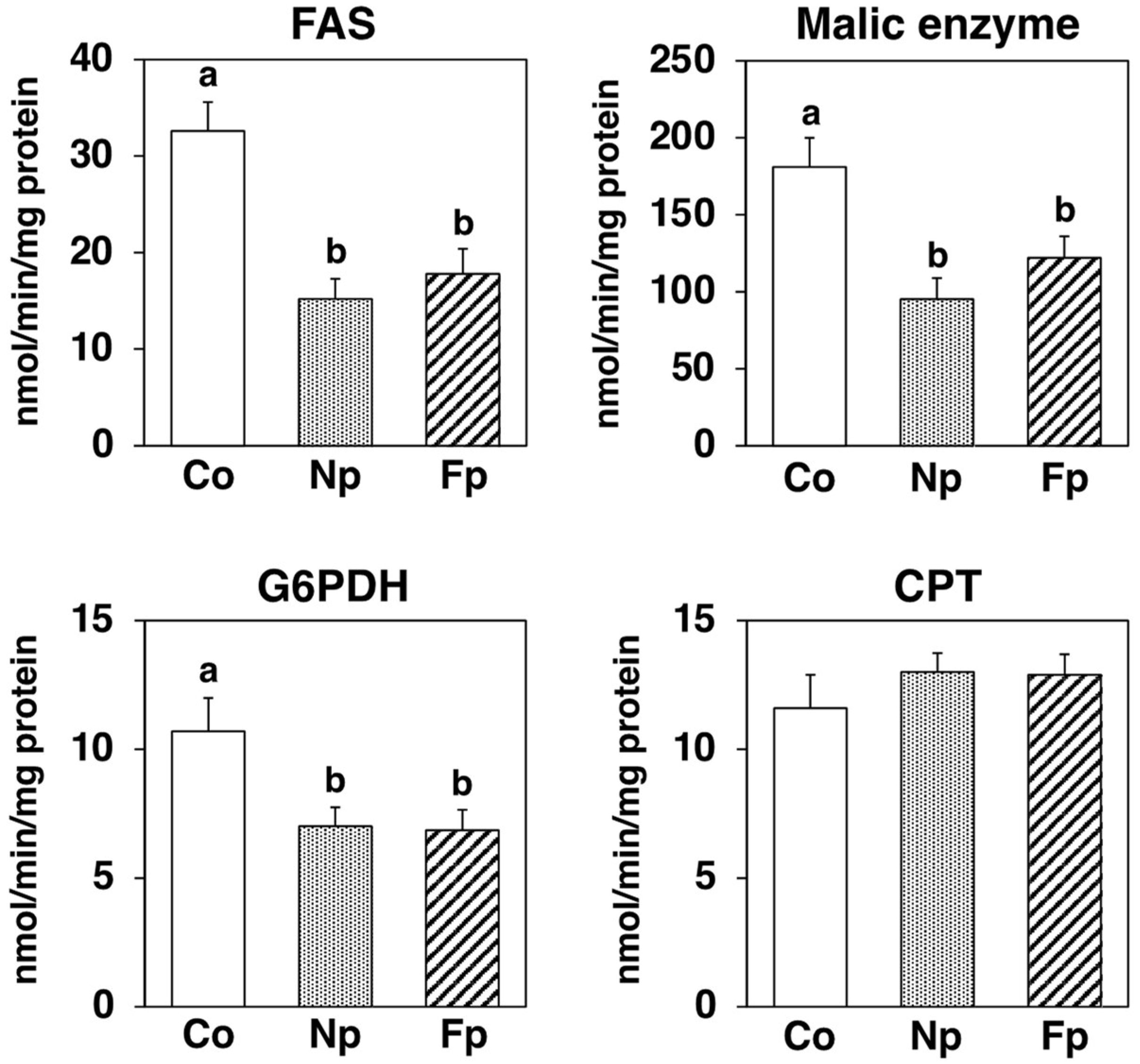
2.6. Effects of Experimental Diets on Activities of Enzymes Related to Lipid Metabolism in the Liver
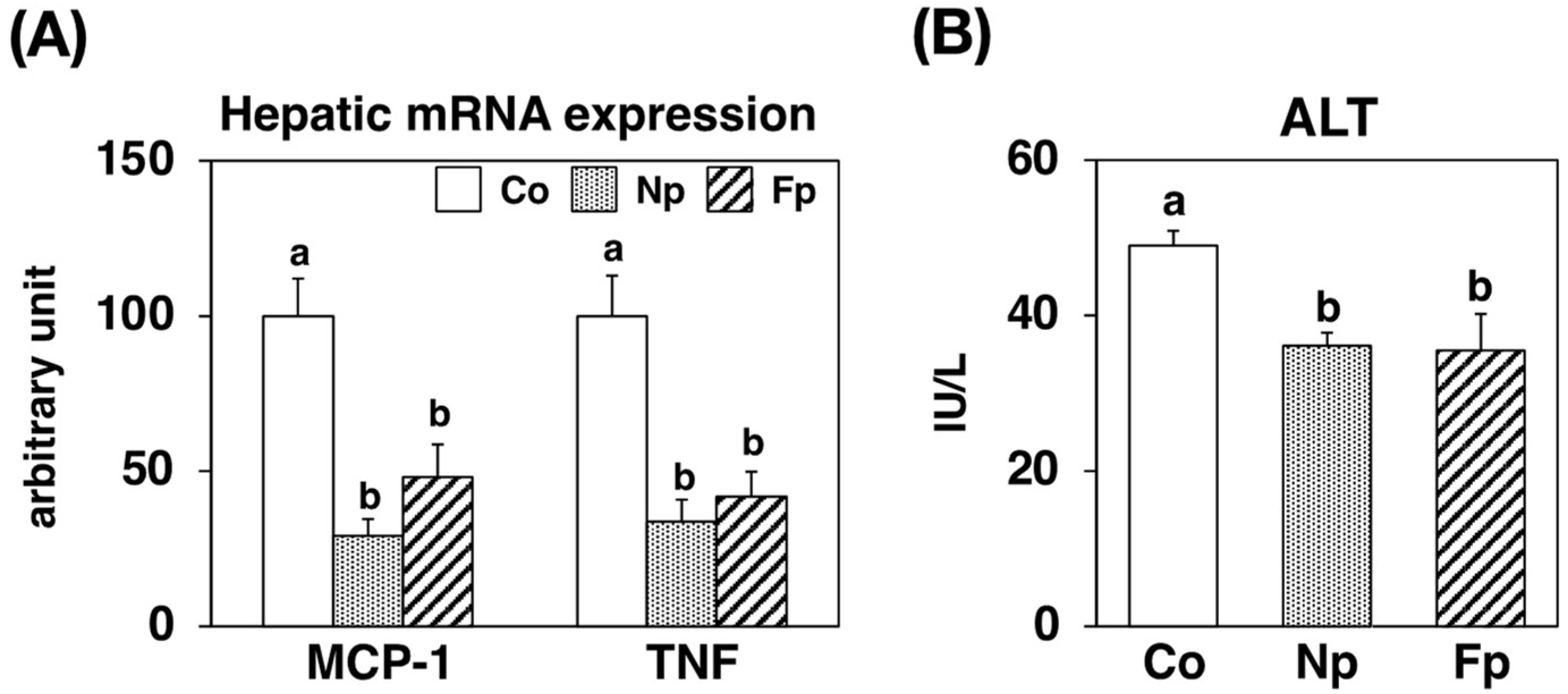
2.7. Effects of Experimental Diets on Hepatic Inflammatory and Injury Marker Levels
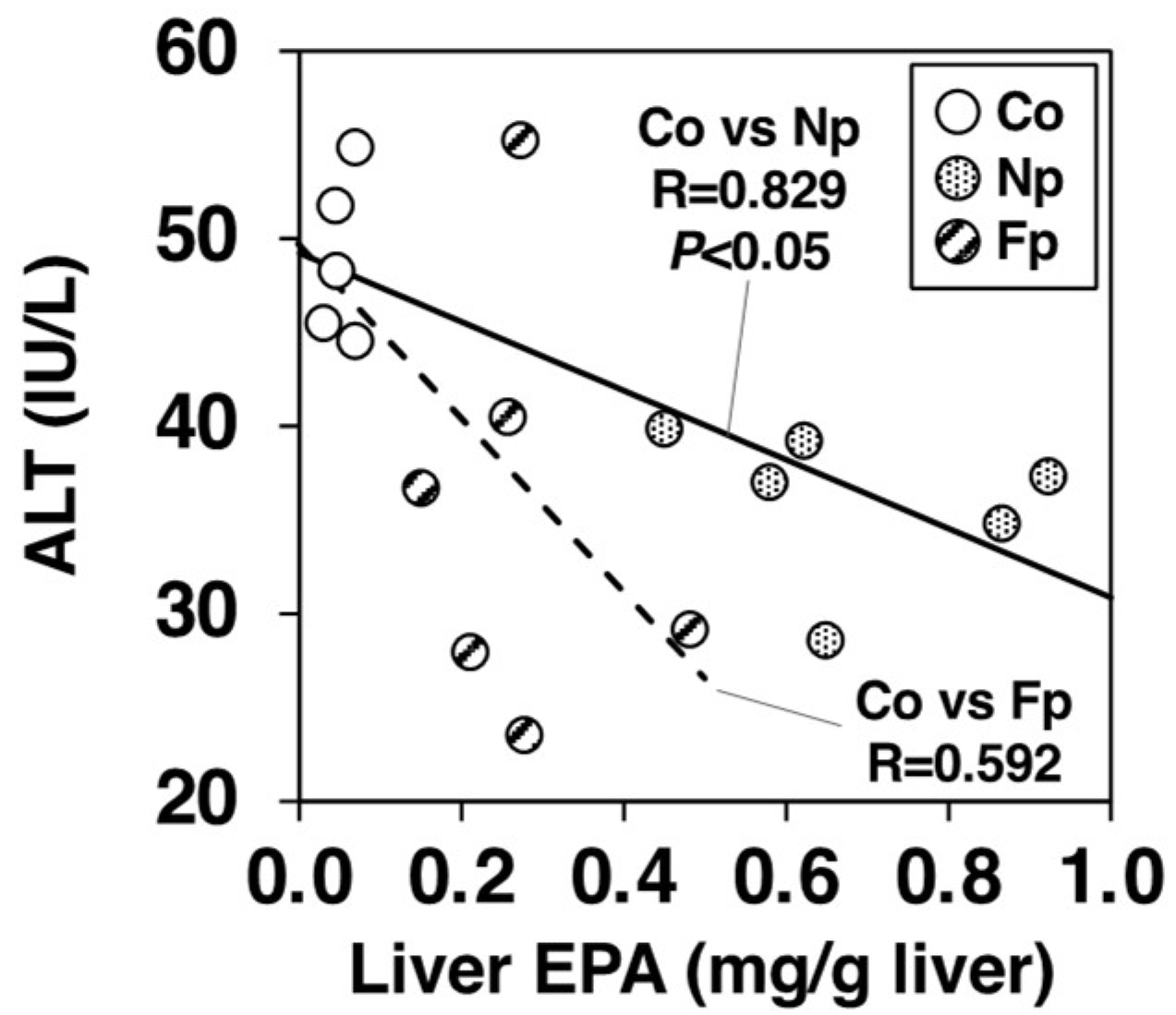
2.8. Contribution of EPA Incorporation into the Liver on the Alleviation of Hepatic Injury
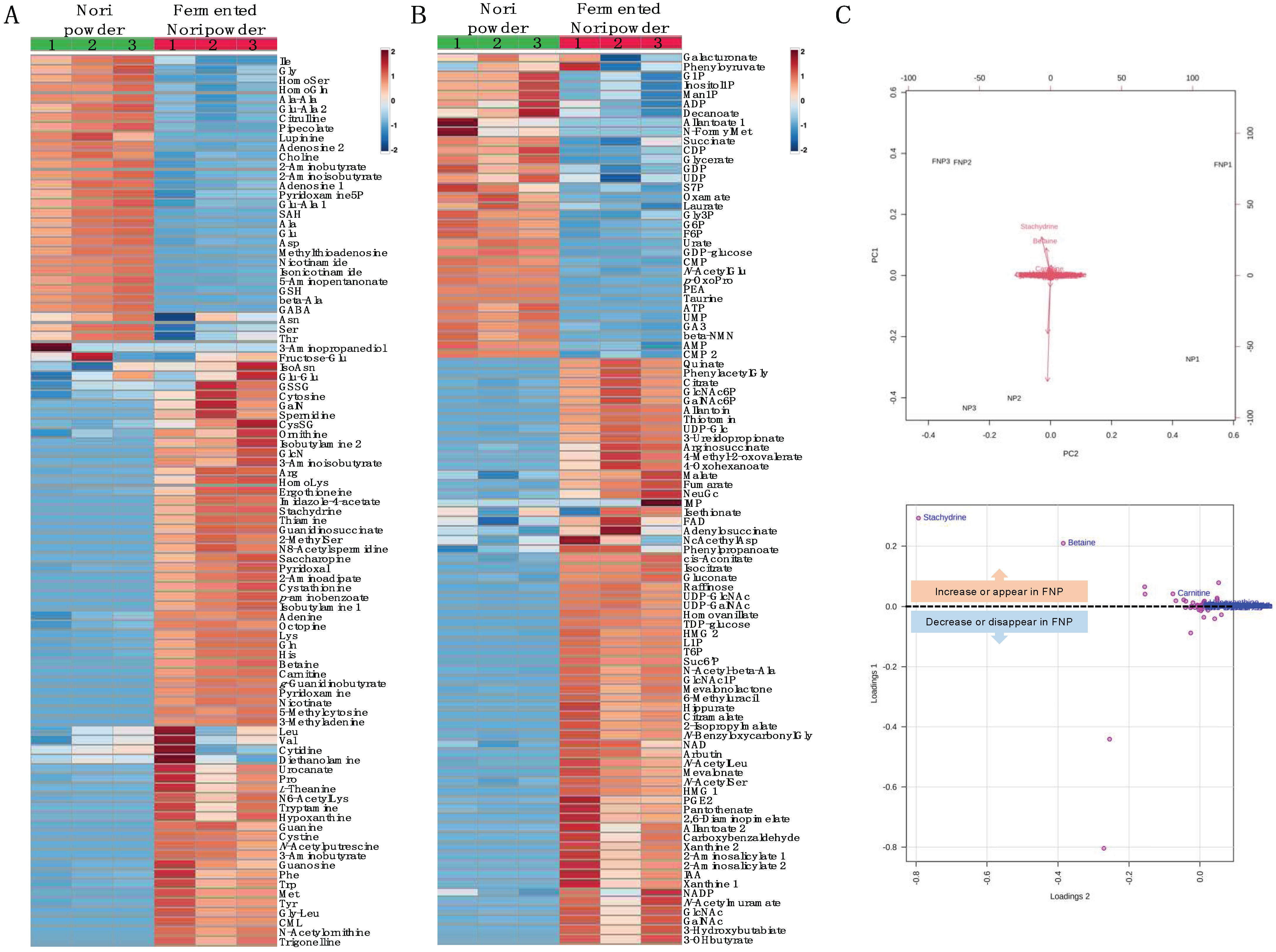
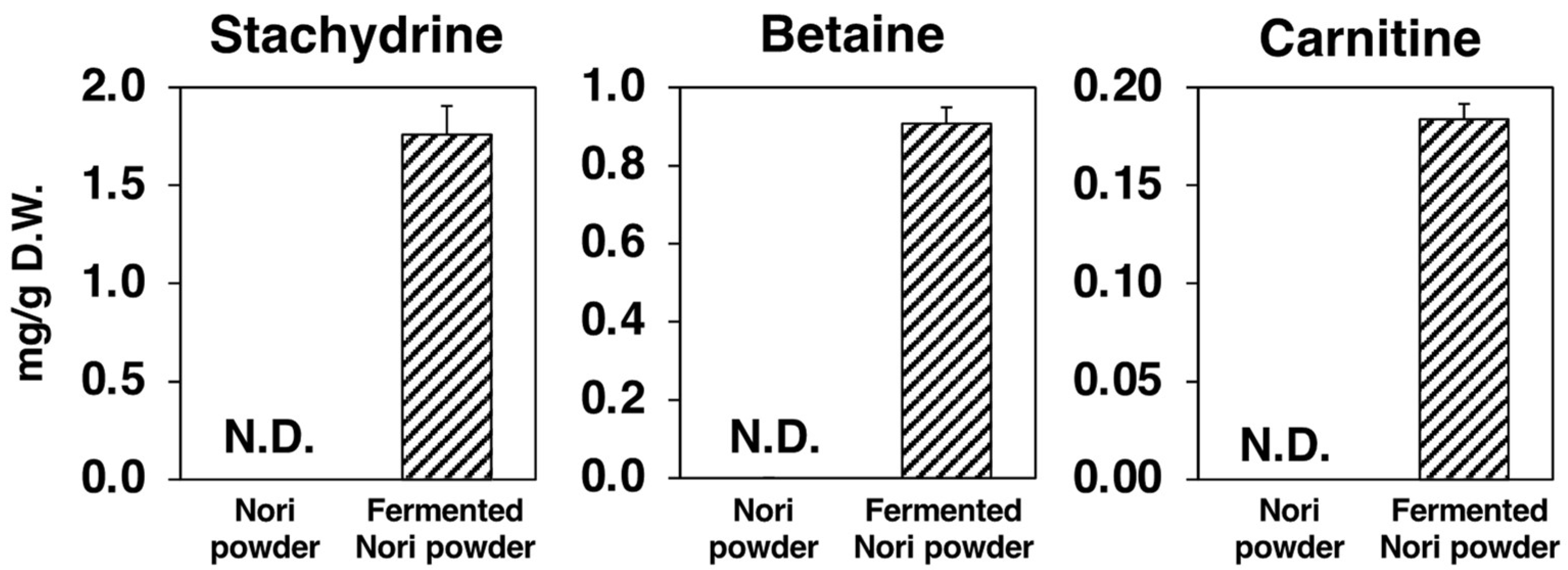
2.9. Metabolomic Changes in Nori Powder before and after Koji Fermentation
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of Nori Powder and Fermented Nori Powder
4.2. Animals
4.3. Physiological and Histochemical Evaluation of Serum and Livers
4.4. Assays of Hepatic Enzyme Activity
4.5. Analysis of mRNA Expression in the Liver
4.6. Capillary Electrophoresis Mass Spectrometry
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jahangir, E.; De Schutter, A.; Lavie, C.J. The relationship between obesity and coronary artery disease. Transl. Res. 2014, 164, 336–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc. Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Diehl, A.M. Fat and the liver—A molecular overview. Semin. Gastrointest. Dis. 2002, 13, 3–16. [Google Scholar] [PubMed]
- Youssef, W.; McCullough, A.J. Diabetes mellitus, obesity, and hepatic steatosis. Semin. Gastrointest. Dis. 2002, 13, 17–30. [Google Scholar] [PubMed]
- Hummel, K.P.; Dickie, M.M.; Coleman, D.L. Diabetes, a new mutation in the mouse. Science 1966, 153, 1127–1128. [Google Scholar] [CrossRef]
- Chen, H.; Charlat, O.; Tartaglia, L.A.; Woolf, E.A.; Weng, X.; Ellis, S.J.; Lakey, N.D.; Culpepper, J.; More, K.J.; Breitbart, R.E.; et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996, 84, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadah, J.G.; Lee, G.I.; Freidman, J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996, 379, 632–635. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Goldin, R.D. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Paht. 2006, 87, 1–16. [Google Scholar] [CrossRef]
- Cho, T.J.; Rhee, M.S. Health functionality and quality control of laver (Porphyra, Phyropia): Current issues and future perspectives as an edible seaweed. Mar. Drugs 2019, 18, 14. [Google Scholar] [CrossRef] [Green Version]
- Araki, S.; Sakurai, T.; Omata, T.; Kawaguchi, A.; Murata, N. Lipid and fatty acid composition in the red alga, Porphyra yezoensis. Jpn. J. Phycol. 1986, 34, 94–100. [Google Scholar]
- Araki, S.; Sakurai, T.; Omata, T.; Kawaguchi, A.; Murata, N. Positional distribution of fatty acids in glycerolipids of the marine red alga, Porphyra yezoensis. Plant Cell Physiol. 1987, 28, 761–766. [Google Scholar] [CrossRef]
- Yanagita, T.; Tsuge, K.; Koga, M.; Inoue, N.; Nagao, K. Eicosapentaenoic acid-containing polar lipids from seaweed Susabinori T (Pyropia yezoensis) alleviate hepatic steatosis in obese db/db mice. Arch. Biochem. Biophys. 2020, 691, 108486. [Google Scholar] [CrossRef] [PubMed]
- Hama, Y.; Yamagata, E.; Takahama, N.; Yoshimura, Y.; Yanagida, R.; Mitsutake, S. Liberation of eicosapentaenoic acid and degradation of the major cell wall polysaccharide porphyran by fermentation of nori, the dried thalli of Pyropia yezoensis, with koji. J. Appl. Phycol. 2021, 33, 4105–4111. [Google Scholar] [CrossRef]
- Watanabe, Y.; Tatsuno, I. Prevention of Cardiovascular Events with Omega-3 Polyunsaturated Fatty Acids and the Mechanism Involved. J. Atheroscler. Thromb. 2020, 27, 183–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curfman, G.; Shehada, E. Icosapent ethyl: Scientific and legal controversies. Open Heart 2021, 8, e001616. [Google Scholar] [CrossRef] [PubMed]
- Hehemann, J.H.; Correc, G.; Barbeyron, T.; Helbert, W.; Czjzek, M.; Michel, G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010, 464, 908–912. [Google Scholar] [CrossRef]
- Kusumoto, K.I.; Yamagata, Y.; Tazawa, R.; Kitagawa, M.; Kato, T.; Isobe, K.; Kashiwagi, Y. Japanese Traditional Miso and Koji Making. J. Fungi 2021, 7, 579. [Google Scholar] [CrossRef]
- Ito, K.; Matsuyama, A. Koji Molds for Japanese Soy Sauce Brewing: Characteristics and Key Enzymes. J. Fungi 2021, 7, 658. [Google Scholar] [CrossRef]
- Akaike, M.; Miyagawa, H.; Kimura, Y.; Terasaki, M.; Kusaba, Y.; Kitagaki, H.; Nishida, H. Chemical and Bacterial Components in Sake and Sake Production Process. Curr. Microbiol. 2020, 77, 632–637. [Google Scholar] [CrossRef]
- Nagao, K.; Yanagita, T. Bioactive lipids in met abolic syndrome. Prog. Lipid Res. 2008, 47, 127–146. [Google Scholar] [CrossRef]
- Takahashi, H.; Chi, H.Y.; Mohri, S.; Kamakari, K.; Nakata, K.; Ichijo, N.; Nakata, R.; Inoue, H.; Goto, T.; Kawada, T. Rice Koji Extract Enhances Lipid Metabolism through Proliferator-Activated Receptor Alpha (PPARalpha) Activation in Mouse Liver. J. Agric. Food Chem. 2016, 64, 8848–8856. [Google Scholar] [CrossRef] [PubMed]
- Son, H.K.; Shin, H.W.; Jang, E.S.; Moon, B.S.; Lee, C.H.; Lee, J.J. Comparison of Antiobesity Effects Between Gochujangs Produced Using Different Koji Products and Tabasco Hot Sauce in Rats Fed a High-Fat Diet. J. Med. Food 2018, 21, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Ogino, Y.; Nakamura, A.; Nakata, K.; Kitagawa, M.; Ito, S. Identification of Rice Koji Extract Components that Increase beta-Glucocerebrosidase Levels in Human Epidermal Keratinocytes. Foods 2018, 7, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Lee, S.; Kyung, S.; Ryu, J.; Kang, S.; Park, M.; Lee, C. Metabolite Profiling and Anti-Aging Activity of Rice Koji Fermented with Aspergillus oryzae and Aspergillus cristatus: A Comparative Study. Metabolites 2021, 11, 524. [Google Scholar] [CrossRef]
- Cheng, F.; Zhou, Y.; Wang, M.; Guo, C.; Cao, Z.; Zhang, R.; Peng, C. A review of pharmacological and pharmacokinetic properties of stachydrine. Pharmacol. Res. 2020, 155, 104755. [Google Scholar] [CrossRef]
- Meng, J.; Zhou, C.; Zhang, W.; Wang, W.; He, B.; Hu, B.; Jiang, G.; Wang, Y.; Hong, J.; Li, S.; et al. Stachydrine prevents LPS-induced bone loss by inhibiting osteoclastogenesis via NF-kappaB and Akt signalling. J. Cell Mol. Med. 2019, 23, 6730–6743. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.; Lu, J.; Zhang, C.; Zeng, G.; Chen, B.; Liang, H.; Wu, A.; Zhang, X.; Wang, X. Stachydrine ameliorates the progression of intervertebral disc degeneration via the PI3K/Akt/NF-kappaB signaling pathway: In vitro and in vivo studies. Food Funct. 2020, 11, 10864–10875. [Google Scholar] [CrossRef]
- Day, C.R.; Kempson, S.A. Betaine chemistry, roles, and potential use in liver disease. Biochim. Biophys. Acta 2016, 1860, 1098–1106. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ma, C.; Gong, L.; Dai, S.; Li, Y. Preventive and therapeutic role of betaine in liver disease: A review on molecular mechanisms. Eur. J. Pharmacol. 2021, 912, 174604. [Google Scholar] [CrossRef]
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M., Jr.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial Effects of Betaine: A Comprehensive Review. Biology 2021, 10, 456. [Google Scholar] [CrossRef]
- Savic, D.; Hodson, L.; Neubauer, S.; Pavlides, M. The Importance of the Fatty Acid Transporter L-Carnitine in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2020, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, H. Role of Carnitine in Non-alcoholic Fatty Liver Disease and Other Related Diseases: An Update. Front. Med. 2021, 8, 689042. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Hideshima, N.; Araki, T. Development of koji by culturing Aspergillus oryzae on nori (Pyropia yezoensis). J. Biosci. Bioeng. 2019, 127, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- American Institute of Nutrition. Report of the American Institute of Nutrition ad hoc committee on standards for nutritional studies. J. Nutr. 1977, 107, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Fletcher, M.J. A colorimetric method for estimating serum triglycerides. Clin. Chim. Acta 1968, 22, 393–397. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Kelley, D.S.; Nelson, G.J.; Hunt, J.E. Effect of prior nutritional status on the activity of lipogenic enzymes in primary monolayer cultures of rat hepatocytes. Biochem. J. 1986, 235, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Kelley, D.S.; Kletzien, R.F. Ethanol modulation of the hormonal and nutritional regulation of glucose 6-phosphate dehydrogenase activity in primary cultures of rat hepatocytes. Biochem. J. 1984, 217, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, S. Malic enzyme. In Merhods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1955; Volume 1, pp. 739–753. [Google Scholar]
- Markwell, M.A.; McGroarty, E.J.; Bieber, L.L.; Tolbert, N.E. The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. A new peroxisomal enzyme. J. Biol. Chem. 1973, 248, 3426–3432. [Google Scholar] [CrossRef]
- Oikawa, A.; Matsuda, F.; Kikuyama, M.; Mimura, T.; Saito, K. Metabolomics of a single vacuole reveals metabolic dynamism in an alga chara australis. Plant Physiol. 2011, 157, 544–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Co | Np | Fp | |
|---|---|---|---|
| Initial body weight (g) | 34.8 ± 0.4 | 34.9 ± 0.4 | 34.9 ± 0.3 |
| Final body weight (g) | 42.0 ± 1.0 | 40.6 ± 1.4 | 40.4 ± 1.1 |
| Body weight gain (g) | 7.16 ± 0.64 | 5.77 ± 1.13 | 5.55 ± 0.81 |
| Food intake (g) | 142 ± 3 | 141 ± 1 | 141 ± 4 |
| Liver weight (g/100 g body weight) | 6.87 ± 0.32 a | 4.87 ± 0.13 b | 5.15 ± 0.37 b |
| White adipose tissue weight (g/100 g body weight) | |||
| Total | 8.00 ± 0.36 | 8.20 ± 0.19 | 8.06 ± 0.34 |
| Epididymal | 4.60 ± 0.10 | 4.61 ± 0.13 | 4.87 ± 0.25 |
| Perirenal | 3.39 ± 0.29 | 3.59 ± 0.18 | 3.19 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagao, K.; Inoue, N.; Tsuge, K.; Oikawa, A.; Kayashima, T.; Yanagita, T. Dried and Fermented Powders of Edible Algae (Neopyropia yezoensis) Attenuate Hepatic Steatosis in Obese Mice. Molecules 2022, 27, 2640. https://doi.org/10.3390/molecules27092640
Nagao K, Inoue N, Tsuge K, Oikawa A, Kayashima T, Yanagita T. Dried and Fermented Powders of Edible Algae (Neopyropia yezoensis) Attenuate Hepatic Steatosis in Obese Mice. Molecules. 2022; 27(9):2640. https://doi.org/10.3390/molecules27092640
Chicago/Turabian StyleNagao, Koji, Nao Inoue, Keisuke Tsuge, Akira Oikawa, Tomoko Kayashima, and Teruyoshi Yanagita. 2022. "Dried and Fermented Powders of Edible Algae (Neopyropia yezoensis) Attenuate Hepatic Steatosis in Obese Mice" Molecules 27, no. 9: 2640. https://doi.org/10.3390/molecules27092640
APA StyleNagao, K., Inoue, N., Tsuge, K., Oikawa, A., Kayashima, T., & Yanagita, T. (2022). Dried and Fermented Powders of Edible Algae (Neopyropia yezoensis) Attenuate Hepatic Steatosis in Obese Mice. Molecules, 27(9), 2640. https://doi.org/10.3390/molecules27092640







