Piperine Provides Neuroprotection against Kainic Acid-Induced Neurotoxicity via Maintaining NGF Signalling Pathway
Abstract
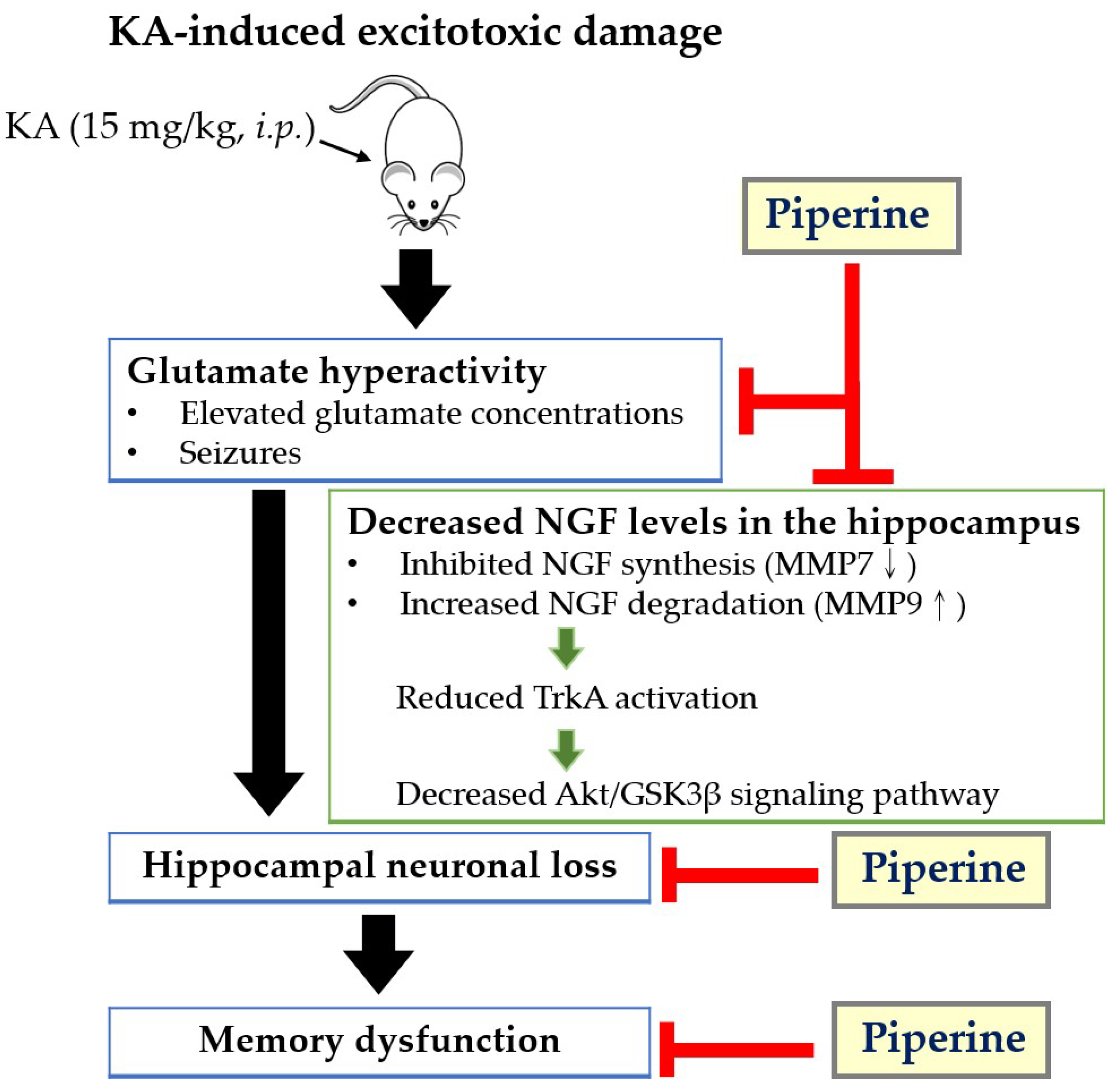
1. Introduction
2. Results
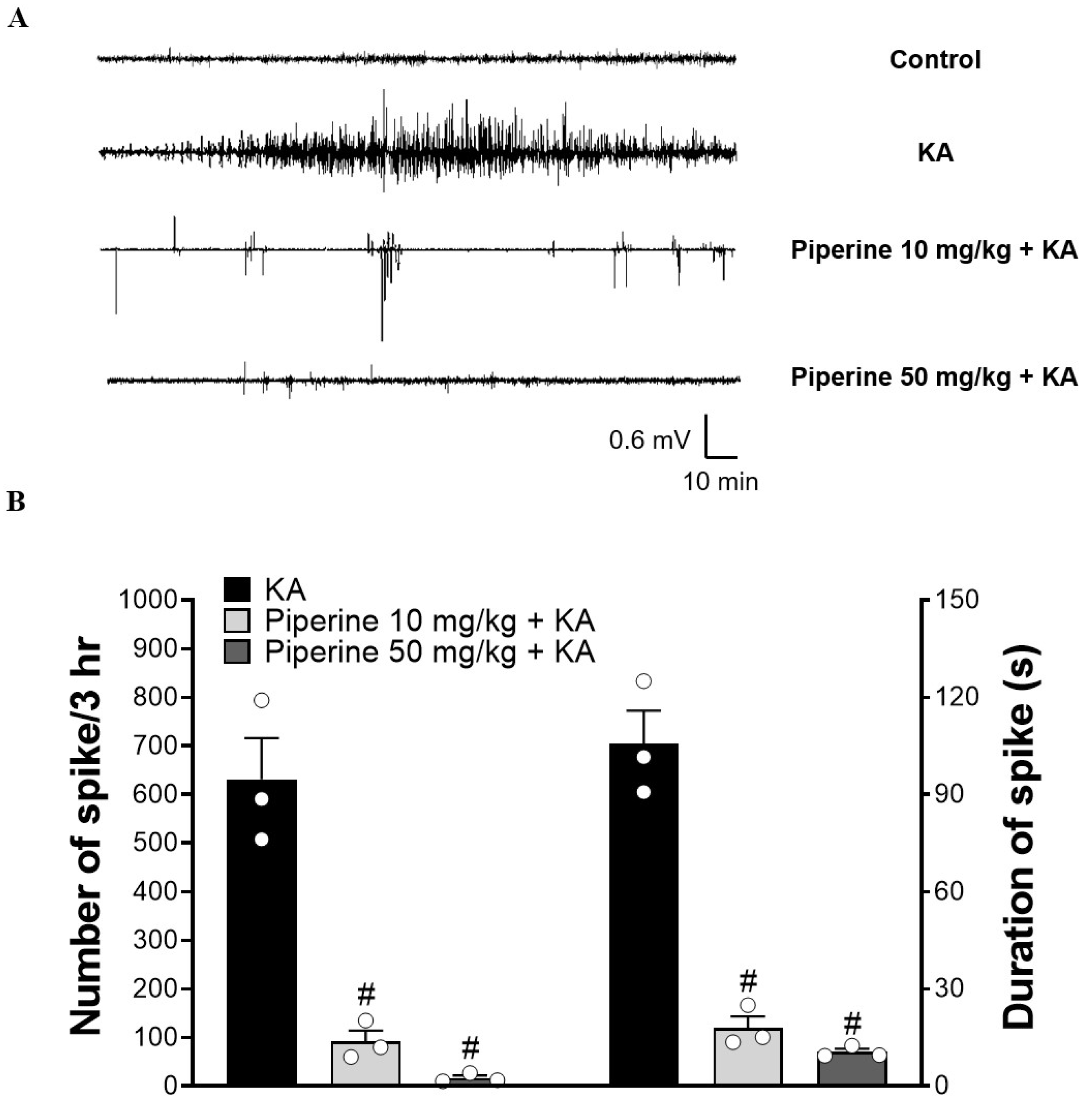
2.1. Piperine Pretreatment Attenuated Seizures and Hippocampal Glutamate Level Elevation in KA-Treated Rats
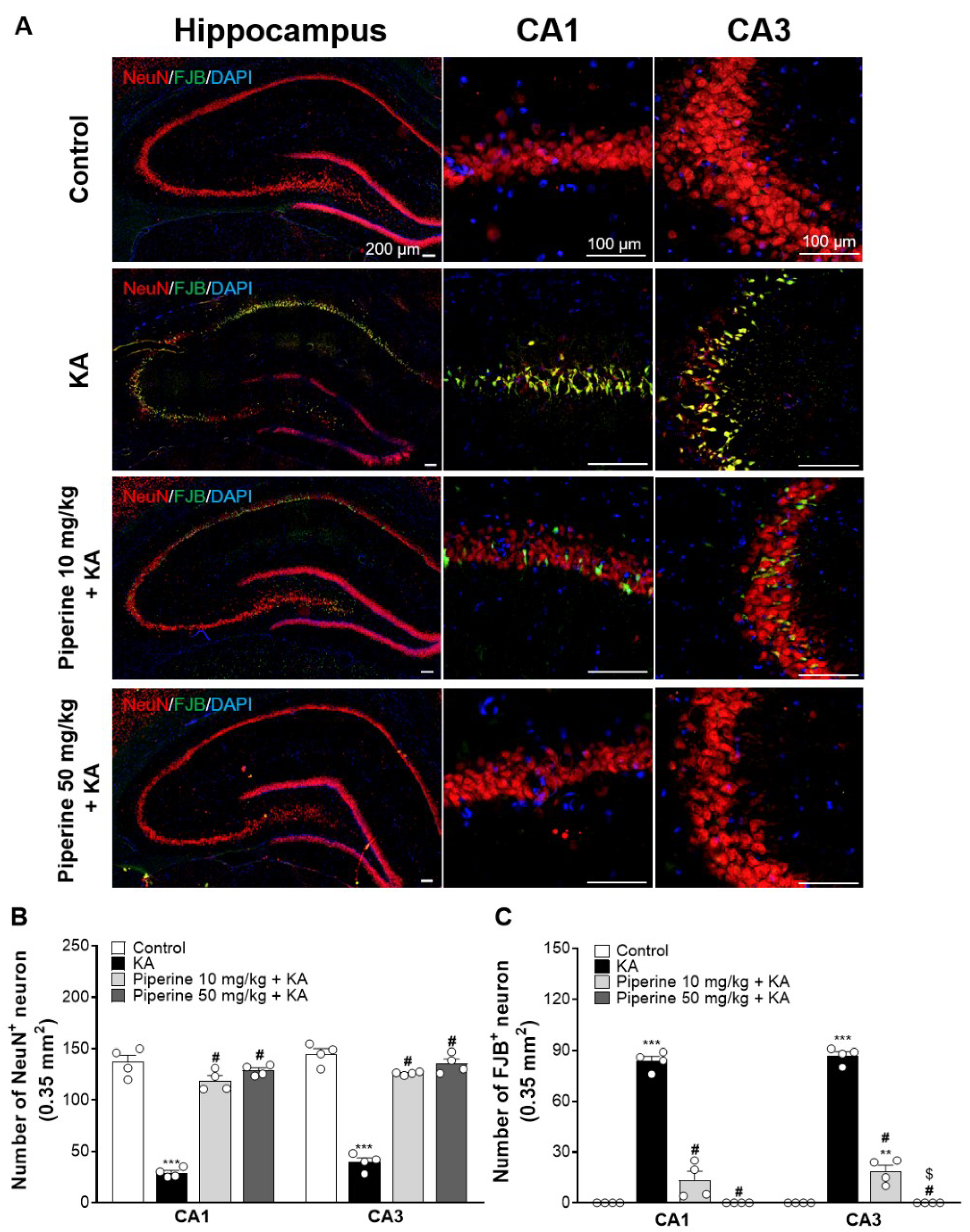
2.2. Piperine Pretreatment Attenuated KA-Induced Neuronal Cell Death in the Hippocampus of Rats
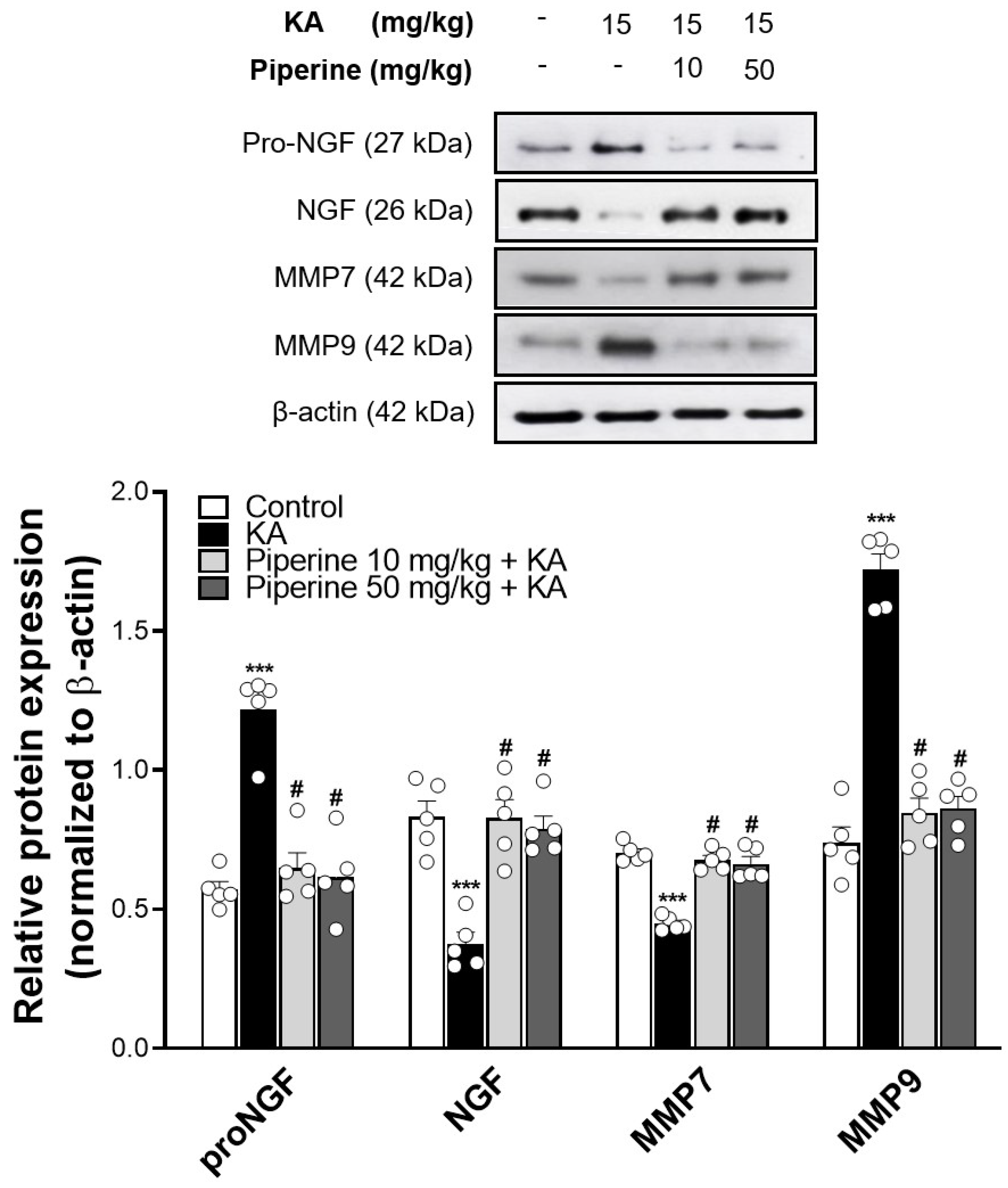
2.3. Piperine Pretreatment Maintained the Levels of NGF in the Hippocampus of Rats with KA Injection
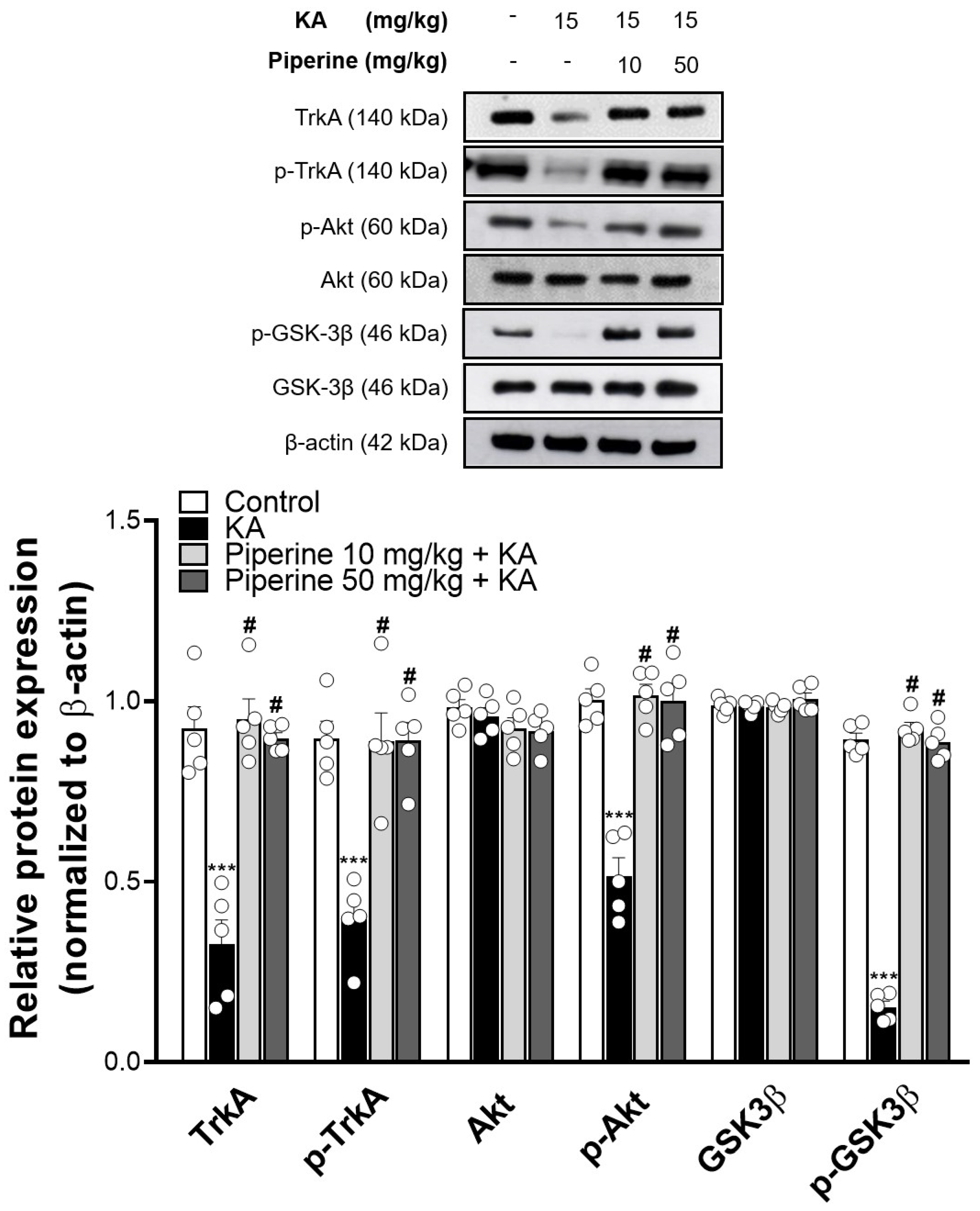
2.4. Piperine Pretreatment Increased the Phosphorylation Levels of TrkA, Akt, and GSK3β in the Hippocampus of KA-Treated Rats
2.5. Piperine Pretreatment Improved KA-Induced Spatial Memory Dysfunction in Rats
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Seizure Score
4.3. EEG Recording
4.4. Immunohistochemistry
4.5. Morris Water Maze
4.6. Glutamate Levels
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mehta, A.; Prabhakar, M.; Kumar, P.; Deshmukh, R.; Sharma, P.L. Excitotoxicity: Bridge to various triggers in neurodegenerative disorders. Eur. J. Pharmacol. 2013, 698, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Green, J.L.; Dos Santos, W.F.; Fontana, A.C.K. Role of glutamate excitotoxicity and glutamate transporter EAAT2 in epilepsy: Opportunities for novel therapeutics development. Biochem. Pharmacol. 2021, 193, 114786. [Google Scholar] [CrossRef] [PubMed]
- Olloquequi, J.; Cornejo-Córdova, E.; Verdaguer, E.; Soriano, F.X.; Binvignat, O.; Auladell, C.; Camins, A. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J. Psychopharmacol. 2018, 32, 265–275. [Google Scholar] [CrossRef]
- Rodriguez-Chavez, V.; Moran, J.; Molina-Salinas, G.; Ruiz, W.A.Z.; Rodriguez, M.C.; Picazo, O.; Cerbon, M. Participation of glutamatergic ionotropic receptors in excitotoxicity: The neuroprotective role of prolactin. Neuroscience 2021, 461, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Omae, F.; Furukawa, S. Reduction of NGF protein level in rat dorsal hippocampus following administration of kainic acid. Neurosci. Lett. 1992, 140, 203–205. [Google Scholar] [CrossRef]
- Gleeson, L.C.; Ryan, K.J.; Griffin, E.W.; Connor, T.J.; Harkin, A. The β2-adrenoceptor agonist clenbuterol elicits neuroprotective, anti-inflammatory and neurotrophic actions in the kainic acid model of excitotoxicity. Brain Behav. Immun. 2010, 24, 1354–1361. [Google Scholar] [CrossRef]
- Tan, Z.; Kang, T.; Zhang, X.; Tong, Y.; Chen, S. Nerve growth factor prevents arsenic-induced toxicity in PC12 cells through the AKT/GSK-3β/NFAT pathway. J. Cell. Physiol. 2019, 234, 4726–4738. [Google Scholar] [CrossRef]
- Ding, X.-W.; Li, R.; Geetha, T.; Tao, Y.-X.; Babu, J.R. Nerve growth factor in metabolic complications and Alzheimer’s disease: Physiology and therapeutic potential. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165858. [Google Scholar] [CrossRef]
- Dos Santos Souza, C.; Grangeiro, M.S.; Lima Pereira, E.P.; Dos Santos, C.C.; da Silva, A.B.; Sampaio, G.P.; Ribeiro Figueiredo, D.D.; David, J.M.; David, J.P.; da Silva, V.D.A.; et al. Agathisflavone, a flavonoid derived from Poincianella pyramidalis (Tul.), enhances neuronal population and protects against glutamate excitotoxicity. Neurotoxicology 2018, 65, 85–97. [Google Scholar] [CrossRef]
- Colafrancesco, V.; Villoslada, P. Targeting NGF pathway for developing neuroprotective therapies for multiple sclerosis and other neurological diseases. Arch. Ital. Biol. 2011, 149, 183–192. [Google Scholar] [CrossRef]
- Lu, S.; Lu, C.; Han, Q.; Li, J.; Du, Z.; Liao, L.; Zhao, R.C. Adipose-derived mesenchymal stem cells protect PC12 cells from glutamate excitotoxicity-induced apoptosis by upregulation of XIAP through PI3-K/Akt activation. Toxicology 2011, 279, 189–195. [Google Scholar] [CrossRef]
- Li, H.; Dong, H.; Li, J.; Liu, H.; Liu, Z.; Li, Z. Neuroprotective effect of insulin-like growth factor-1: Effects on tyrosine kinase receptor (Trk) expression in dorsal root ganglion neurons with glutamate-induced excitotoxicity in vitro. Brain Res. Bull. 2013, 97, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Dalmagro, A.P.; Camargo, A.; Severo Rodrigues, A.L.; Zeni, A.L.B. Involvement of PI3K/Akt/GSK-3β signaling pathway in the antidepressant-like and neuroprotective effects of Morus nigra and its major phenolic, syringic acid. Chem. Biol. Interact. 2019, 314, 108843. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.S.; Dos Santos, N.A.G.; Bernardes, C.P.; Sisti, F.M.; Amaral, L.; Fontana, A.C.K.; Dos Santos, A.C. Caffeic acid phenethyl ester (CAPE) protects PC12 cells against cisplatin-induced neurotoxicity by activating the AMPK/SIRT1, MAPK/Erk, and PI3k/Akt signaling pathways. Neurotox. Res. 2019, 36, 175–192. [Google Scholar] [CrossRef]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Devel. Ther. 2016, 10, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Afshari, A.R.; Fanoudi, S.; Rajabian, A.; Sadeghnia, H.R.; Mollazadeh, H.; Hosseini, A. Potential protective roles of phytochemicals on glutamate-induced neurotoxicity: A review. Iran. J. Basic Med. Sci. 2020, 23, 1113–1123. [Google Scholar] [CrossRef]
- Ren, T.; Zuo, Z. Role of piperine in CNS diseases: Pharmacodynamics, pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2019, 15, 849–867. [Google Scholar] [CrossRef]
- Quijia, C.R.; Chorilli, M. Characteristics, biological properties and analytical methods of piperine: A review. Crit. Rev. Anal. Chem. 2020, 50, 62–77. [Google Scholar] [CrossRef]
- Fu, M.; Sun, Z.H.; Zuo, H.C. Neuroprotective effect of piperine on primarily cultured hippocampal neurons. Biol. Pharm. Bull. 2010, 33, 598–603. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Ren, T.; Wang, Q.; Li, C.; Yang, M.; Zuo, Z. Efficient brain uptake of piperine and its pharmacokinetics characterization after oral administration. Xenobiotica 2018, 48, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- D’Hooge, R.; Pei, Y.Q.; Raes, A.; Lebrun, P.; van Bogaert, P.P.; de Deyn, P.P. Anticonvulsant activity of piperine on seizures induced by excitatory amino acid receptor agonists. Arzneimittelforschung 1996, 46, 557–560. [Google Scholar] [PubMed]
- Song, Y.; Cao, C.; Xu, Q.; Gu, S.; Wang, F.; Huang, X.; Xu, S.; Wu, E.; Huang, J.H. Piperine attenuates TBI-induced seizures via inhibiting cytokine-activated reactive astrogliosis. Front. Neurol. 2020, 11, 431. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Ali, M.; Salman, M.; Tabassum, H.; Parvez, S. Harnessing the mitochondrial integrity for neuroprotection: Therapeutic role of piperine against experimental ischemic stroke. Neurochem. Int. 2021, 149, 105138. [Google Scholar] [CrossRef]
- Khalili-Fomeshi, M.; Azizi, M.G.; Esmaeili, M.R.; Gol, M.; Kazemi, S.; Ashrafpour, M.; Moghadamnia, A.A.; Hosseinzadeh, S. Piperine restores streptozotocin-induced cognitive impairments: Insights into oxidative balance in cerebrospinal fluid and hippocampus. Behav. Brain Res. 2018, 337, 131–138. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Z.; Wang, W.; Wei, M.; Si, X.; Shang, Y.; Yang, Z.; Li, T.; Guo, H.; Li, S. Piperine regulates glycogen synthase kinase-3β-related signaling and attenuates cognitive decline in D-galactose-induced aging mouse model. J. Nutr. Biochem. 2020, 75, 108261. [Google Scholar] [CrossRef]
- Yang, X.; Zhi, J.; Leng, H.; Chen, Y.; Gao, H.; Ma, J.; Ji, J.; Hu, Q. The piperine derivative HJ105 inhibits Aβ(1-42)-induced neuroinflammation and oxidative damage via the Keap1-Nrf2-TXNIP axis. Phytomedicine 2021, 87, 153571. [Google Scholar] [CrossRef]
- Roshanbakhsh, H.; Salmani, M.E.; Dehghan, S.; Nazari, A.; Javan, M.; Pourabdolhossein, F. Piperine ameliorated memory impairment and myelin damage in lysolecethin induced hippocampal demyelination. Life Sci. 2020, 253, 117671. [Google Scholar] [CrossRef]
- Azam, S.; Park, J.Y.; Kim, I.S.; Choi, D.K. Piperine and its metabolite’s pharmacology in neurodegenerative and neurological diseases. Biomedicines 2022, 10, 154. [Google Scholar] [CrossRef]
- Rusina, E.; Bernard, C.; Williamson, A. The kainic acid models of temporal lobe epilepsy. eNeuro 2021, 8, ENEURO.0337-20.2021. [Google Scholar] [CrossRef]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.; Asari, M.A.; Muzaimi, M.; Mummedy, S.; Sulaiman, S.A. Kainic acid-induced excitotoxicity experimental model: Protective merits of natural products and plant extracts. Evid. Based Complement. Altern. Med. 2015, 2015, 972623. [Google Scholar] [CrossRef] [PubMed]
- Jakaria, M.; Park, S.Y.; Haque, M.E.; Karthivashan, G.; Kim, I.S.; Ganesan, P.; Choi, D.K. Neurotoxic agent-induced injury in neurodegenerative disease model: Focus on involvement of glutamate receptors. Front. Mol. Neurosci. 2018, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Lei, D.; Zhang, H.; You, C. Anticonvulsant effect of piperine ameliorates memory impairment, inflammation and oxidative stress in a rat model of pilocarpine-induced epilepsy. Exp. Ther. Med. 2017, 13, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.Y.; Chang, Y.; Wang, S.J. Piperine-mediated suppression of voltage-dependent Ca2+ influx and glutamate release in rat hippocampal nerve terminals involves 5HT1A receptors and G protein βγ activation. Food Funct. 2019, 10, 2720–2728. [Google Scholar] [CrossRef] [PubMed]
- Sitges, M.; Guarneros, A.; Nekrassov, V. Effects of carbamazepine, phenytoin, valproic acid, oxcarbazepine, lamotrigine, topiramate and vinpocetine on the presynaptic Ca2+ channel-mediated release of [3H] glutamate: Comparison with the Na+ channel-mediated release. Neuropharmacology 2007, 53, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, M.; Brawek, B.; Freiman, T.M.; Jackisch, R.; Feuerstein, T.J. Effects of antiepileptic drugs on glutamate release from rat and human neocortical synaptosomes. Naunyn Schmiedeberg Arch. Pharmacol. 2011, 383, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Ciafrè, S.; Ferraguti, G.; Tirassa, P.; Iannitelli, A.; Ralli, M.; Greco, A.; Chaldakov, G.N.; Rosso, P.; Fico, E.; Messina, M.P.; et al. Nerve growth factor in the psychiatric brain. Riv. Psichiatr. 2020, 55, 4–15. [Google Scholar] [CrossRef]
- Allard, S.; Leon, W.C.; Pakavathkumar, P.; Bruno, M.A.; Ribeiro-Da-Silva, A.; Cuello, A.C. Impact of the NGF maturation and degradation pathway on the cortical cholinergic system phenotype. J. Neurosci. 2012, 32, 2002–2012. [Google Scholar] [CrossRef]
- Cuello, A.C.; Pentz, R.; Hall, H. The brain NGF metabolic pathway in health and in Alzheimer’s pathology. Front. Neurosci. 2019, 13, 62. [Google Scholar] [CrossRef]
- Vines, K.; Li, R.; Geetha, T.; Broderick, T.L.; Carroll, C.C.; Babu, J.R. Nerve growth factor receptor TrkA signaling in streptozotocin-induced type 1 diabetes rat brain. Biochem. Biophys. Res. Commun. 2019, 514, 1285–1289. [Google Scholar] [CrossRef]
- Bruno, M.A.; Cuello, A.C. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc. Natl. Acad. Sci. USA 2006, 103, 6735–6740. [Google Scholar] [CrossRef]
- Iulita, M.F.; Cuello, A.C. Nerve growth factor metabolic dysfunction in Alzheimer’s disease and Down syndrome. Trends Pharmacol. Sci. 2014, 35, 338–348. [Google Scholar] [CrossRef]
- Sherif, I.O.; Al-Gayyar, M.M.H. Oleuropein potentiates anti-tumor activity of cisplatin against HepG2 through affecting proNGF/NGF balance. Life Sci. 2018, 198, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Le, A.P.; Friedman, W.J. Matrix metalloproteinase-7 regulates cleavage of pro-nerve growth factor and is neuroprotective following kainic acid-induced seizures. J. Neurosci. 2012, 32, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.W.; Leiner, B.; Blechschmitt, C.; Arevalo, J.C.; Lee, R.; Mörl, K.; Meyer, M.; Hempstead, B.L.; Yoon, S.O.; Giehl, K.M. Secreted proNGF is a pathophysiological death-inducing ligand after adult CNS injury. Proc. Natl. Acad. Sci. USA 2004, 101, 6226–6230. [Google Scholar] [CrossRef]
- Volosin, M.; Trotter, C.; Cragnolini, A.; Kenchappa, R.S.; Light, M.; Hempstead, B.L.; Carter, B.D.; Friedman, W.J. Induction of proneurotrophins and activation of p75NTR-mediated apoptosis via neurotrophin receptor-interacting factor in hippocampal neurons after seizures. J. Neurosci. 2008, 28, 9870–9879. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Dilnashin, H.; Birla, H.; Singh, S.S.; Zahra, W.; Rathore, A.S.; Singh, B.K.; Singh, S.P. The role of PI3K/Akt and ERK in neurodegenerative disorders. Neurotox. Res. 2019, 35, 775–795. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wu, Y.; Zou, S.; Wang, X.; Li, Y.; Xu, K.; Gong, F.; Liu, Y.; Wang, J.; Liao, Y.; et al. NGF attenuates high glucose-induced ER stress, preventing schwann cell apoptosis by activating the PI3K/Akt/GSK3β and ERK1/2 pathways. Neurochem. Res. 2017, 42, 3005–3018. [Google Scholar] [CrossRef] [PubMed]
- Smith-Swintosky, V.L.; Kraemer, P.J.; Bruce, A.J.; McCants, N.; Maki, A.; Brown, R.W.; Alcala, M.; Goodman, Y.; Slevin, J.T.; Mattson, M.P. Bacterial alkaloids mitigate seizure-induced hippocampal damage and spatial memory deficits. Exp. Neurol. 1996, 141, 287–296. [Google Scholar] [CrossRef]
- Choucry, A.M.; Al-Shorbagy, M.Y.; Attia, A.S.; El-Abhar, H.S. Pharmacological manipulation of Trk, p75NTR, and NGF balance restores memory deficit in global ischemia/reperfusion model in rats. J. Mol. Neurosci. 2019, 68, 78–90. [Google Scholar] [CrossRef]
- Wang, M.Y.; Meng, M.; Yang, C.C.; Zhang, L.; Li, Y.L.; Zhang, L.; Li, L. Cornel iridoid glycoside improves cognitive impairment induced by chronic cerebral hypoperfusion via activating PI3K/Akt/GSK-3β/CREB pathway in rats. Behav. Brain Res. 2020, 379, 112319. [Google Scholar] [CrossRef] [PubMed]
- Pragnya, B.; Kameshwari, J.S.; Veeresh, B. Ameliorating effect of piperine on behavioral abnormalities and oxidative markers in sodium valproate induced autism in BALB/C mice. Behav. Brain Res. 2014, 270, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, Y.H.; Liu, H.; Qu, H.D. Neuroprotective effects of piperine on the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease mouse model. Int. J. Mol. Med. 2015, 36, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Nazifi, M.; Oryan, S.; Esfahani, D.E.; Ashrafpoor, M. The functional effects of piperine and piperine plus donepezil on hippocampal synaptic plasticity impairment in rat model of Alzheimer’s disease. Life Sci. 2021, 265, 118802. [Google Scholar] [CrossRef]
- Mori, A.; Kabuto, H.; Pei, Y.Q. Effects of piperine on convulsions and on brain serotonin and catecholamine levels in E1 mice. Neurochem. Res. 1985, 10, 1269–1275. [Google Scholar] [CrossRef]
- Bhat, B.G.; Chandrasekhara, N. Studies on the metabolism of piperine: Absorption, tissue distribution and excretion of urinary conjugates in rats. Toxicology 1986, 40, 83–92. [Google Scholar] [CrossRef]
- Haq, I.U.; Imran, M.; Nadeem, M.; Tufail, T.; Gondal, T.A.; Mubarak, M.S. Piperine: A review of its biological effects. Phytother. Res. 2021, 35, 680–700. [Google Scholar] [CrossRef]
- Bastaki, M.; Aubanel, M.; Bauter, M.; Cachet, T.; Demyttenaere, J.; Diop, M.M.; Harman, C.L.; Hayashi, S.-M.; Krammer, G.; Li, X.; et al. Absence of adverse effects following administration of piperine in the diet of Sprague-Dawley rats for 90 days. Food Chem. Toxicol. 2018, 120, 213–221. [Google Scholar] [CrossRef]
- O’Connor, A.; Corbin, K.D.; Nieman, D.C.; Swick, A.G. A randomized, controlled trial to assess short-term black pepper consumption on 24-hour energy expenditure and substrate utilization. Funct. Foods Health Dis. 2013, 3, 377. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Yu, S.; Simonyi, A.; Sun, G.Y.; Sun, A.Y. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol. Neurobiol. 2005, 31, 3–16. [Google Scholar] [CrossRef]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Huo, Q.W.; Tabassum, S.; Misrani, A.A.; Long, C. Recording EEG in freely moving neonatal rats using a novel method. J. Vis. Exp. 2017, 123, e55489. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.-C.; Kuo, Y.-H.; Hsieh, P.-W.; Hsieh, T.-Y.; Kuo, J.-R.; Wang, S.-J. Chlorogenic acid decreases glutamate release from rat cortical nerve terminals by P/Q-Type Ca2+ channel suppression: A possible neuroprotective mechanism. Int. J. Mol. Sci. 2021, 22, 11447. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Lin, T.-Y.; Pan, T.-L.; Wang, P.-W.; Chiu, K.-M.; Lee, M.-Y.; Wang, S.-J. Asiatic acid prevents cognitive deficits by inhibiting calpain activation and preserving synaptic and mitochondrial function in rats with kainic acid-induced seizure. Biomedicines 2021, 9, 284. [Google Scholar] [CrossRef] [PubMed]
- Pegg, C.C.; He, C.; Stroink, A.R.; Kattner, K.A.; Wang, C.X. Technique for collection of cerebrospinal fluid from the cisterna magna in rat. J. Neurosci. Methods 2010, 187, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-W.; Huang, Y.-C.; Chiu, K.-M.; Lee, M.-Y.; Lin, T.-Y.; Wang, S.-J. Enmein decreases synaptic glutamate release and protects against kainic acid-induced brain injury in rats. Int. J. Mol. Sci. 2021, 22, 12966. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, T.-Y.; Chang, Y.; Wang, S.-J. Piperine Provides Neuroprotection against Kainic Acid-Induced Neurotoxicity via Maintaining NGF Signalling Pathway. Molecules 2022, 27, 2638. https://doi.org/10.3390/molecules27092638
Hsieh T-Y, Chang Y, Wang S-J. Piperine Provides Neuroprotection against Kainic Acid-Induced Neurotoxicity via Maintaining NGF Signalling Pathway. Molecules. 2022; 27(9):2638. https://doi.org/10.3390/molecules27092638
Chicago/Turabian StyleHsieh, Ting-Yang, Yi Chang, and Su-Jane Wang. 2022. "Piperine Provides Neuroprotection against Kainic Acid-Induced Neurotoxicity via Maintaining NGF Signalling Pathway" Molecules 27, no. 9: 2638. https://doi.org/10.3390/molecules27092638
APA StyleHsieh, T.-Y., Chang, Y., & Wang, S.-J. (2022). Piperine Provides Neuroprotection against Kainic Acid-Induced Neurotoxicity via Maintaining NGF Signalling Pathway. Molecules, 27(9), 2638. https://doi.org/10.3390/molecules27092638






