Anti-Inflammatory Effects of Cycloheterophyllin on Dinitrochlorobenzene-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice
Abstract
:1. Introduction
2. Results
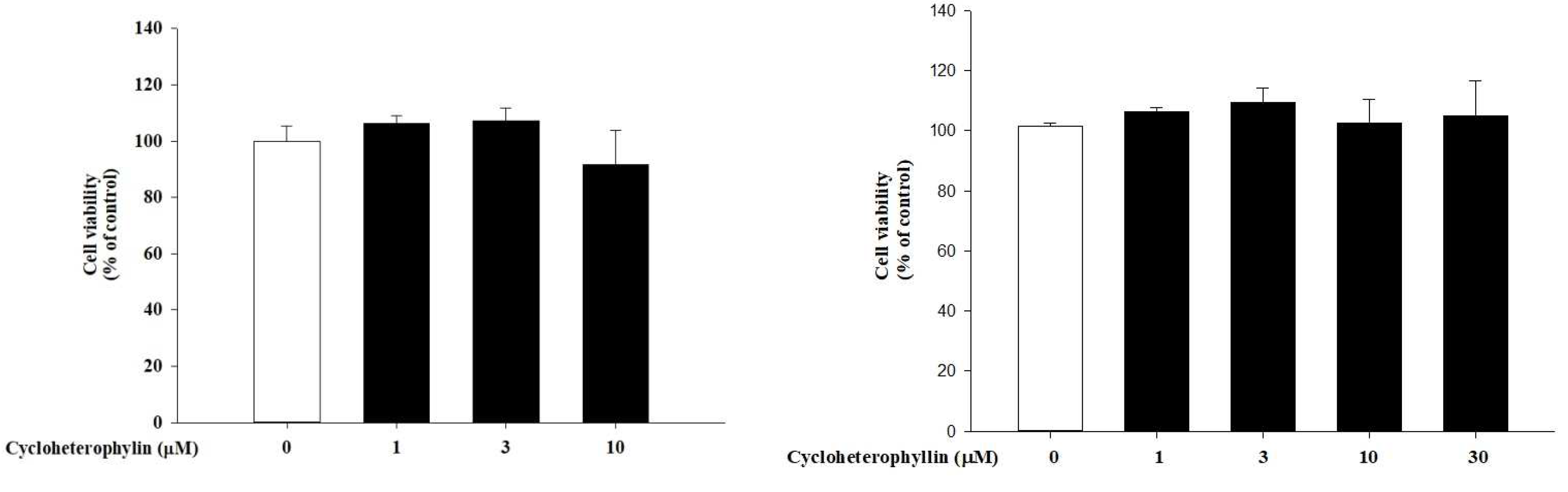
2.1. The Effect of Cycloheterophyllin on Skin Cell Viability
2.2. The Anti-Inflammatory Potential of Cycloheterophyllin in Tumor Necrosis Factor-α (TNF-α)/Interferon-γ (IFN-γ)-Induced Inflammatory Response in HaCaT Cells
2.3. The Effect of Cycloheterophyllin on the TNF-α/IFN-γ-Induced Activation of MAP Kinases in HaCaT Cells
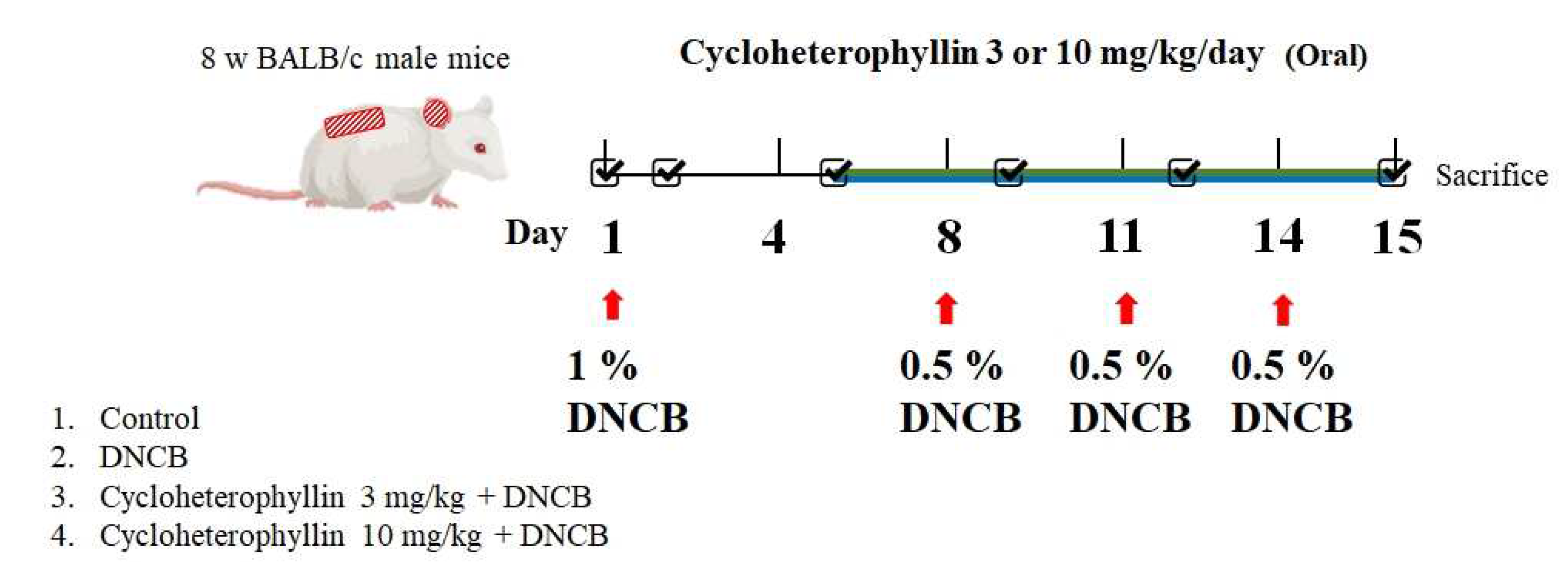
2.4. Effects of Cycloheterophyllin on Atopic Dermatitis (AD)-like Skin Lesion in BALB/c Mice
2.5. Effects of Cycloheterophyllin on Skin TEWL and Hydration in BALB/c Mice
2.6. Effects of Cycloheterophyllin on DNCB-Induced Scratching Behavior and Enlarged Spleen in BALB/c Mice
2.7. Effects of Cycloheterophyllin on Epidermal Thickness and Mast Cell infiltration in DNCB-Induced Atopic Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Cell Culture and MTT Assay
4.2.2. Quantitative Polymer Chain Reaction (PCR)
4.2.3. Western Blot Assay
4.2.4. DNCB-Induced Atopic-Dermatitis-Like Skin Inflammation in Mice
4.2.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Eichenfield, L.F.; Tom, W.L.; Berger, T.G.; Krol, A.; Paller, A.S.; Schwarzenberger, K.; Bergman, J.N.; Chamlin, S.L.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis. J. Am. Acad. Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef] [Green Version]
- Renert-Yuval, Y.; Guttman-Yassky, E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann. Allergy Asthma Immunol. 2020, 124, 28–35. [Google Scholar] [PubMed] [Green Version]
- Gu, S.; Pei, J. Innovating Chinese Herbal Medicine: From Traditional Health Practice to Scientific Drug Discovery. Front Pharm. 2017, 8, 381. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Wu, D.; Dong, N.; Ouyang, P.; Pu, J.; Hu, Q.; Wang, J.; Lu, W.; Huang, J. Moracin C, A Phenolic Compound Isolated from Artocarpus heterophyllus, Suppresses Lipopolysaccharide-Activated Inflammatory Responses in Murine Raw264.7 Macrophages. Int. J. Mol. Sci. 2016, 17, 1199. [Google Scholar] [CrossRef] [Green Version]
- Baliga, M.S.; Shivashankara, A.R.; Haniadka, R.; Dsouza, J.; Bhat, H.P. Phytochemistry, nutritional and pharmacological properties of Artocarpus heterophyllus Lam. (jackfruit): A review. Food Res. Int. 2011, 44, 1800–1811. [Google Scholar] [CrossRef]
- Abdullah, S.A.; Jamil, S.; Basar, N.; Abdul Lathiff, S.M.; Mohd Arriffin, N. Flavonoids from the leaves and heartwoods of Artocarpus lowii King and their bioactivities. Nat. Prod. Res. 2017, 31, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Rather, M.A.; Kumar Jha, A.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. Flowers: New Sources of Bioactive Compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef]
- Huang, C.H.; Li, H.J.; Wu, N.L.; Hsiao, C.Y.; Lin, C.N.; Chang, H.H.; Hung, C.F. Photoprotective Effects of Cycloheterophyllin against UVA-Induced Damage and Oxidative Stress in Human Dermal Fibroblasts. PLoS ONE 2016, 11, e0161767. [Google Scholar] [CrossRef]
- Shim, J.H. Inhibitory Effects of Cycloheterophyllin on Melanin Synthesis. Molecules 2021, 26, 2526. [Google Scholar] [CrossRef] [PubMed]
- Su, I.C.; Hung, C.F.; Lin, C.N.; Huang, S.K.; Wang, S.J. Cycloheterophyllin Inhibits the Release of Glutamate from Nerve Terminals of the Rat Hippocampus. Chem. Res. Toxicol. 2019, 32, 1591–1598. [Google Scholar] [CrossRef]
- Yang, C.-C.; Hung, Y.-L.; Ko, W.-C.; Tsai, Y.-J.; Chang, J.-F.; Liang, C.-W.; Chang, D.-C.; Hung, C.-F. Effect of Neferine on DNCB-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Int. J. Mol. Sci. 2021, 22, 8237. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Liu, J.; Wang, J.; Luo, Q.; Zhang, H.; Liu, B.; Xu, F.; Pang, Q.; Liu, Y.; Dong, J. Icariin inhibits TNF-α/IFN-γ induced inflammatory response via inhibition of the substance P and p38-MAPK signaling pathway in human keratinocytes. Int. Immunopharmacol. 2015, 29, 401–407. [Google Scholar] [CrossRef]
- Chiu, K.-M.; Hung, Y.-L.; Wang, S.-J.; Tsai, Y.-J.; Wu, N.-L.; Liang, C.-W.; Chang, D.-C.; Hung, C.-F. Anti-Allergic and Anti-Inflammatory Effects of Neferine on RBL-2H3 Cells. Int. J. Mol. Sci. 2021, 22, 10994. [Google Scholar] [CrossRef]
- Chu, T.; Wu, N.-L.; Hsiao, C.-Y.; Li, H.-J.; Lin, T.-Y.; Ku, C.-H.; Hung, C.-F. An isoflavone extract from soybean cake suppresses 2,4-dinitrochlorobenzene-induced contact dermatitis. J. Ethnopharmacol. 2020, 263, 113037. [Google Scholar] [CrossRef]
- Gröne, A. Keratinocytes and cytokines. Vet. Immunol. Immunopathol. 2002, 88, 1–12. [Google Scholar]
- Ansel, J.; Perry, P.; Brown, J.; Damm, D.; Phan, T.; Hart, C.; Luger, T.; Hefeneider, S. Cytokine Modulation of Keratinocyte Cytokines. J. Investig. Dermatol. 1990, 94 (Suppl. 6), s101–s107. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Saredy, J.; Zhang, R.; Shao, Y.; Sun, Y.; Yang, W.Y.; Wang, J.; Liu, L.; Drummer, C.; Johnson, C.; et al. Approaching Inflammation Paradoxes—Proinflammatory Cytokine Blockages Induce Inflammatory Regulators. Front. Immunol. 2020, 11, 101460. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Kim, Y.J.; Jegal, J.; Jo, B.-G.; Choi, H.-S.; Yang, M.H. Haplopine Ameliorates 2,4-Dinitrochlorobenzene-Induced Atopic Dermatitis-Like Skin Lesions in Mice and TNF-α/IFN-γ-Induced Inflammation in Human Keratinocyte. Antioxidants 2021, 10, 806. [Google Scholar] [CrossRef]
- Scartezzini, P.; Speroni, E. Review on some plants of Indian traditional medicine with antioxidant activity. J. Ethnopharmacol. 2000, 71, 23–43. [Google Scholar]
- Muthu, C.; Ayyanar, M.; Raja, N.; Ignacimuthu, S. Medicinal plants used by traditional healers in Kancheepuram district of Tamil Nadu, India. J. Ethnobiol. Ethnomed. 2006, 2, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, A.; Mishra, A.; Mahdi, A.A.; Ahmad, M.; Basit, A. Natural and herbal stress remedies: A review. Int. J. Pharmacogn. 2015, 2, 155–160. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Kong, K.W.; Ismail, A. Phytochemicals and Medicinal Properties of Indigenous Tropical Fruits with Potential for Commercial Development. Evid.-Based Complementary Altern. Med. 2016, 2016, 7591951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, H.-H.; Lu, Y.-H.; Yang, S.-Z.; Won, S.-J.; Lin, C.-N. Cytotoxic Prenylflavonoids from Artocarpus elasticus. J. Nat. Prod. 2005, 68, 1692–1695. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.-J.; Yuan, J.-B.; Peng, J.-B.; Ding, Y.-Q.; Zhu, J.-X.; Ren, G. Flavonoids from the roots of Artocarpus heterophyllus. Fitoterapia 2017, 117, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-P.; Xu, Y.; Qin, C.; Zhang, S.; Gu, X.; Lin, Y.; Xie, G.; Wang, M.; Chen, J. Characterization of Antiproliferative Activity Constituents from Artocarpus heterophyllus. J. Agric. Food Chem. 2014, 62, 5519–5527. [Google Scholar] [CrossRef]
- Ko, F.N.; Cheng, Z.J.; Lin, C.N.; Teng, C.M. Scavenger and Antioxidant Properties of Prenylflavones Isolated from Artocarpus Heterophyllus. Free Radic. Biol. Med. 1998, 25, 160–168. [Google Scholar] [CrossRef]
- Fang, S.-C.; Hsu, C.-L.; Yen, G.-C. Anti-inflammatory Effects of Phenolic Compounds Isolated from the Fruits of Artocarpus heterophyllus. J. Agric. Food Chem. 2008, 56, 4463–4468. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, M.H.K.; Nguyen, H.X.; Bui, N.K.N.; Nguyen, M.T.T. Tyrosinase Inhibitors from the Wood of Artocarpus heterophyllus. J. Nat. Prod. 2012, 75, 1951–1955. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Peng, J.; Liu, A.; Liang, J.; Yuan, W.; Wang, H.; He, J. Structure elucidation and NMR assignments of two new flavanones from the roots of Artocarpus heterophyllus. Magn. Reson. Chem. 2015, 53, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Okayama, Y. Oxidative Stress in Allergic and Inflammatory Skin Diseases. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 517–519. [Google Scholar] [CrossRef]
- Nettis, E.; Distaso, M.; Saitta, S.; Casciaro, M.; Cristani, M.; Saija, A.; Vacca, A.; Gangemi, S.; Minciullo, P.L. Involvement of new oxidative stress markers in chronic spontaneous urticaria. Adv. Dermatol. Allergol./Postępy Dermatol. I Alergol. 2017, 34, 448–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [PubMed] [Green Version]
- Oyoshi, M.K.; He, R.; Kumar, L.; Yoon, J.; Geha, R.S. Cellular and molecular mechanisms in atopic dermatitis. Adv. Immunol. 2009, 102, 135–226. [Google Scholar]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Anti-inflammatory effect of quercetin and galangin in LPS-stimulated RAW264.7 macrophages and DNCB-induced atopic dermatitis animal models. Int. J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [CrossRef]











| Genes | Primers | Sequence (5′-3′) |
|---|---|---|
| IL-1β | Forward | CTC TCA CCT CTC CTA CTC ACT |
| Reverse | ATC AGA ATG TGG GAG CGA AT | |
| IL-6 | Forward | ATC AGA ATG TGG GAG CGA AT |
| Reverse | GGA CCG AAG GCG CTT GTG GAG | |
| IL-8 | Forward | ACT GAG AGT GAT TGA GAG TGG AC |
| Reverse | AAC CCT CTG CAC CCA GTT TTC | |
| GAPDH | Forward | CTG CTC CTG TTC GAC AGT |
| Reverse | CCG TTG ACT CCG ACC TTC AC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-C.; Hsiao, C.-Y.; Hsu, Y.-J.; Ko, H.-H.; Chang, D.-C.; Hung, C.-F. Anti-Inflammatory Effects of Cycloheterophyllin on Dinitrochlorobenzene-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Molecules 2022, 27, 2610. https://doi.org/10.3390/molecules27092610
Wang C-C, Hsiao C-Y, Hsu Y-J, Ko H-H, Chang D-C, Hung C-F. Anti-Inflammatory Effects of Cycloheterophyllin on Dinitrochlorobenzene-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Molecules. 2022; 27(9):2610. https://doi.org/10.3390/molecules27092610
Chicago/Turabian StyleWang, Chia-Chen, Chien-Yu Hsiao, Yu-Jou Hsu, Horng-Huey Ko, Der-Chen Chang, and Chi-Feng Hung. 2022. "Anti-Inflammatory Effects of Cycloheterophyllin on Dinitrochlorobenzene-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice" Molecules 27, no. 9: 2610. https://doi.org/10.3390/molecules27092610
APA StyleWang, C.-C., Hsiao, C.-Y., Hsu, Y.-J., Ko, H.-H., Chang, D.-C., & Hung, C.-F. (2022). Anti-Inflammatory Effects of Cycloheterophyllin on Dinitrochlorobenzene-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Molecules, 27(9), 2610. https://doi.org/10.3390/molecules27092610







