Properties of Rice-Based Beverages Fermented with Lactic Acid Bacteria and Propionibacterium
Abstract
:1. Introduction
2. Results and Discussion
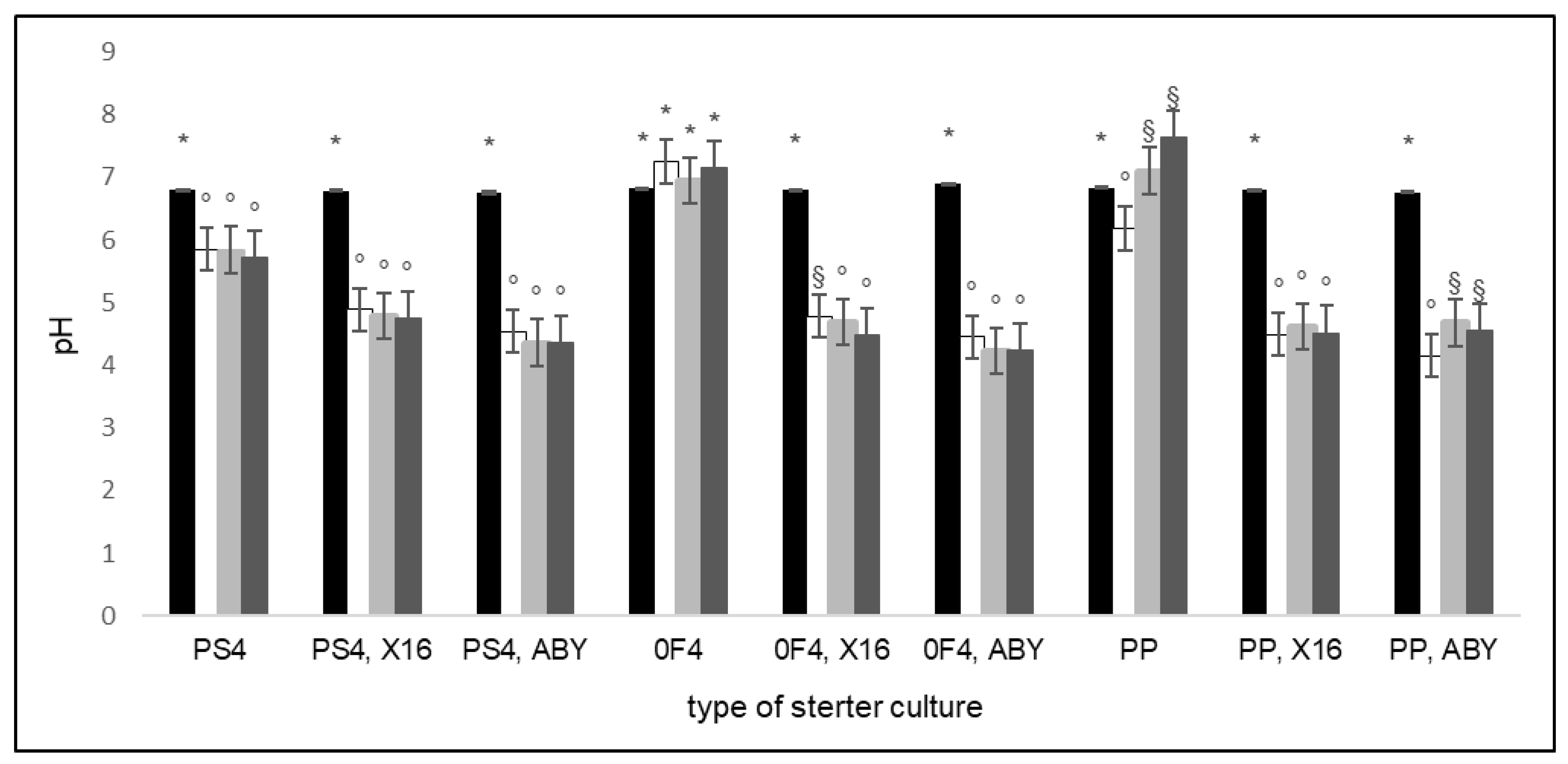
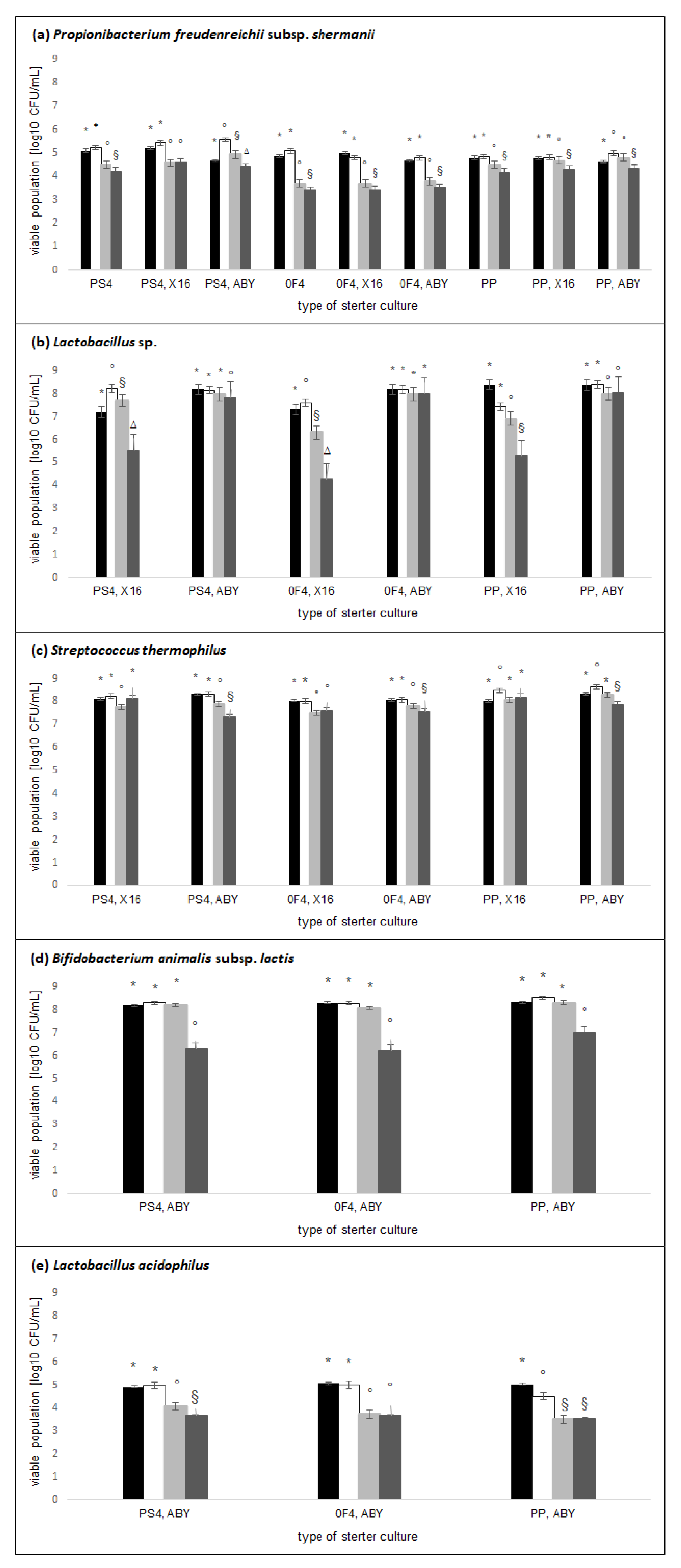
2.1. Active Acidity and Microflora Population
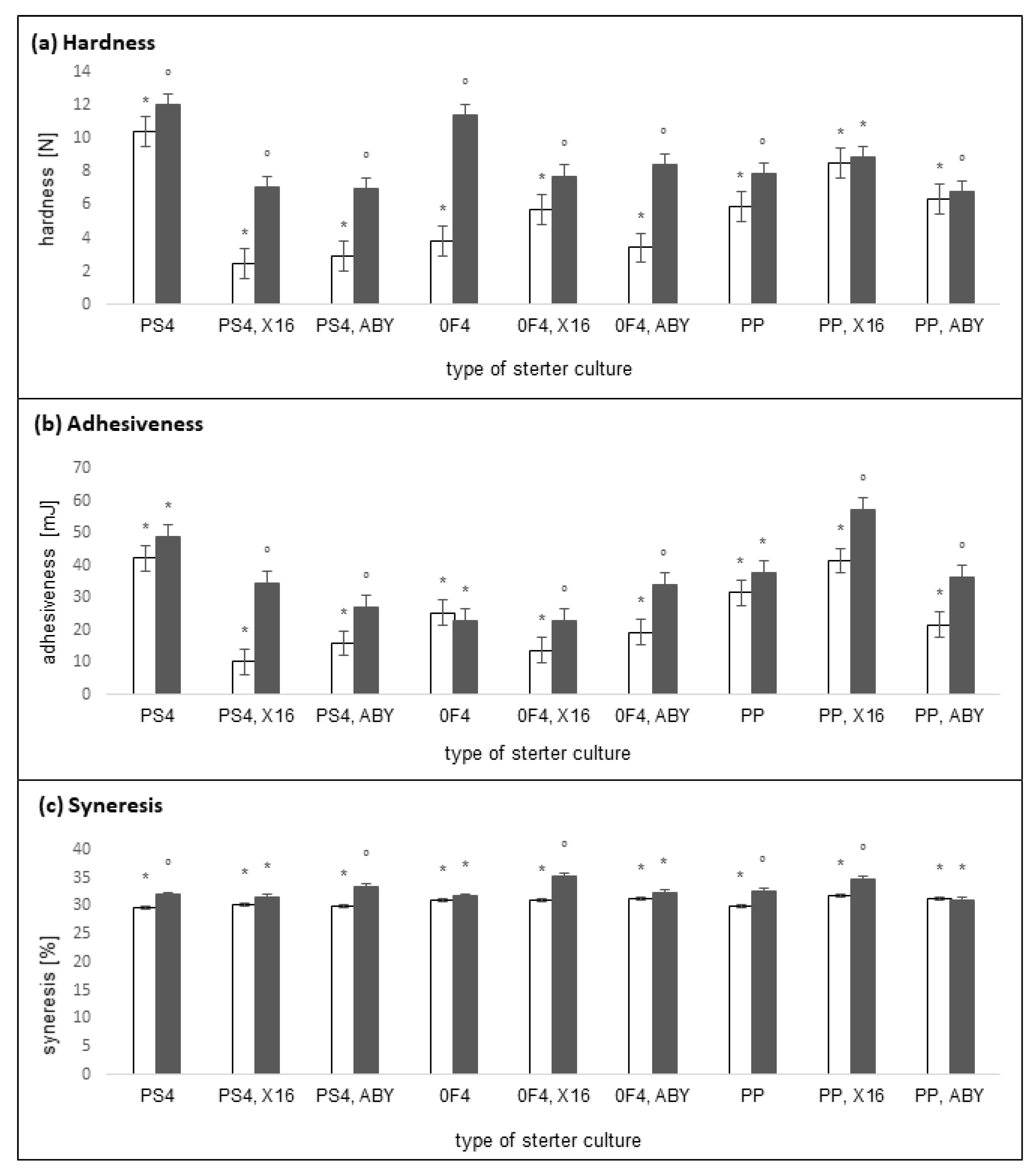
2.2. Physical Properties
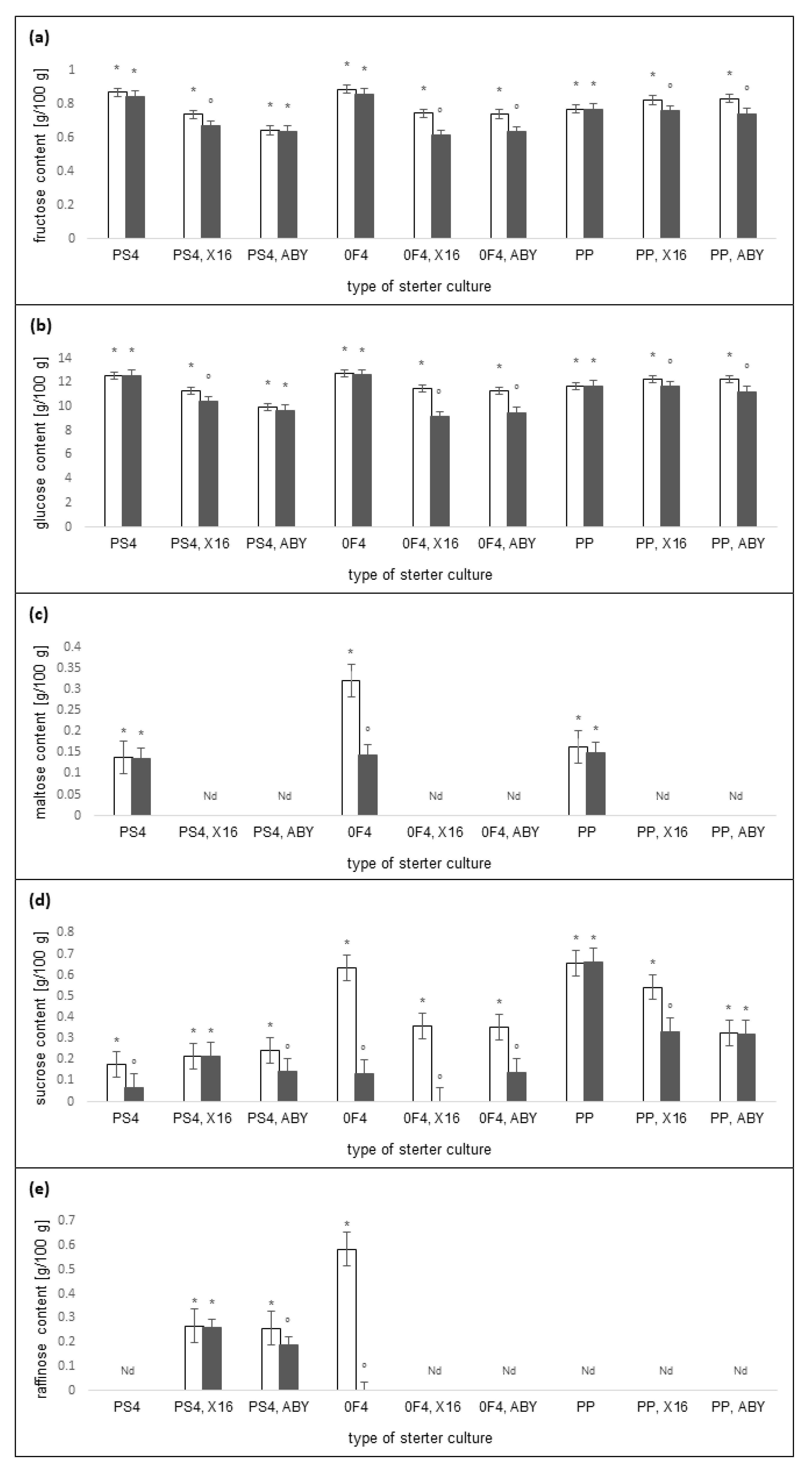
2.3. Carbohydrates Content
3. Materials and Methods
3.1. Preparation of Rice-Based Beverages
3.2. Bacterial Strains
3.3. Fermentation of Rice-Based Beverages
3.4. Active Acidity and Microflora Analysis
3.5. Texture Analysis
3.6. Syneresis Determination
- A—mass of the separated supernatant during centrifugation [g];
- B—mass of rice-based beverage before centrifugation [g].
3.7. Carbohydrate Content Determination
3.8. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hayek, S.A.; Ibrahim, S.A. Current Limitations and Challenges with Lactic Acid Bacteria: A Review. Food Nutr. Sci. 2013, 4, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Tamang, J.P.; Waranabe, K.; Holzapfel, W.H. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemechu, T. Review on lactic acid bacteria function in milk fermentation and preservation. Afr. J. Food Sci. 2015, 9, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Khalid, K. An overview of lactic acid bacteria. Int. J. Biosci. 2011, 1, 1–13. [Google Scholar]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]
- Masood, M.I.; Qadir, M.I.; Shirazi, J.H.; Khan, I.U. Beneficial effects of lactic acid bacteria on human beings. Crit. Rev. Microbiol. 2011, 37, 91–98. [Google Scholar] [CrossRef]
- Widyastuti, Y.; Rohmatussolihat, R.; Febrisiantosa, A. The Role of Lactic Acid Bacteria in Milk Fermentation. Food Nutr. Sci. 2014, 5, 435–442. [Google Scholar] [CrossRef] [Green Version]
- Campaniello, D.; Bevilacqua, A.; Sinigaglia, M.; Altieri, C. Screening of Propionibacterium spp. for potential probiotic properties. Anaerobe 2015, 34, 169–173. [Google Scholar] [CrossRef]
- Borawska, J.; Warmińska-Radyko, I.; Darewicz, M. Role and application of the genus Propionibacterium in food and fodder production. Medycyna Wet. 2010, 66, 534–537. [Google Scholar]
- Chamlagain, B.; Sugito, T.A.; Deptula, P.; Edelmann, M.; Kariluoto, S.; Varmanen, P.; Piironen, V. In situ production of active vitamin B12 in cereal matrices using Propionibacterium freudenreichii. Food Sci. Nutr. 2017, 6, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Freitas, R.; Chuat, V.; Madec, M.N.; Nero, L.A.; Thierry, A.; Valence, F.; Carvalho, A.F. Biodiversity of dairy Propionibacterium isolated from dairy farms in Minas Gerais, Brazil. Int. J. Food Microbiol. 2015, 203, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Thierry, A.; Deutsch, S.M.; Falentin, H.; Dalmasso, M.; Cousin, F.J.; Jan, G. New insights into physiology and metabolism of Propionibacterium freudenreichii. Int. J. Food Microbiol. 2011, 149, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Le Maréchal, C.; Peton, V.; Ple, C.; Vroland, C.; Jardin, J.; Briard-Bion, V.; Durant, G.; Chuat, V.; Loux, V.; Foligné, B.; et al. Surface proteins of Propionibacterium freudenreichii are involved in its anti-inflammatory properties. J. Proteom. 2015, 113, 447–461. [Google Scholar] [CrossRef]
- Piwowarek, K.; Lipińska, E.; Hać-Szymańczuk, E.; Kieliszek, M.; Ścibisz, I. Propionibacterium spp.—Source of propionic acid, vitamin B12, and other metabolites important for the industry. Appl. Microbiol. Biotechnol. 2018, 102, 515–538. [Google Scholar] [CrossRef] [Green Version]
- Cousin, F.J.; Foligné, B.; Deutsch, S.M.; Massart, S.; Parayre, S.; Le Loir, Y.; Boudry, G.; Jan, G. Assessment of the Probiotic Potential of a Dairy Product Fermented by Propionibacterium freudenreichii in Piglets. J. Agric. Food Chem. 2012, 60, 7917–7927. [Google Scholar] [CrossRef]
- Ojala, T.; Laine, P.; Ahlroos, T.; Tanskanen, J.; Pitkänen, S.; Salusjärvi, T.; Kankainen, M.; Tynkkynen, S.; Paulin, L.; Auvinen, P. Functional genomics provides insights into the role of Propionibacterium freudenreichii ssp. shermanii JS in cheese ripening. Int. J. Food Microbiol. 2017, 241, 29–48. [Google Scholar] [CrossRef] [Green Version]
- Yerlikaya, O.; Akpinar, A.; Saygili, D.; Karagozlu, N. Incorporation of Propionibacterium shermanii subsp. freudenreichii in probiotic dairy drink production: Physicochemical, rheological, microbiological and sensorial properties. Int. J. Dairy Technol. 2019, 73, 392–402. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; da Silva, R.C.; Ibrahim, S.A. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Wanhalinna, V.; Zannini, E.; Arendt, E.K. Foods for special dietary needs: Non dairy plant based milk substitutes and fermented dairy type products. Crit. Rev. Food Sci. Nutr. 2016, 56, 339–349. [Google Scholar] [CrossRef]
- McClements, D.J. Development of Next-Generation Nutritionally Fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Aydar, E.F.; Tutuncu, S.; Ozcelik, B. Plant-based milk substitutes: Bioactive compounds, conventional and novel processes, bioavailability studies, and health effects. J. Funct. Foods 2020, 70, 103975. [Google Scholar] [CrossRef]
- Bernat, N.; Chafer, M.; Chiralt, A.; Gonzalez-Martinez, C. Vegetable milks and their fermented derivative products. Int. J. Food Stud. 2014, 3, 93–124. [Google Scholar] [CrossRef]
- Cichońska, P.; Ziarno, M. Legumes and Legume-Based Beverages Fermented with Lactic Acid Bacteria as a Potential Carrier of Probiotics and Prebiotics. Microorganisms 2022, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Tan, M.; Øiseth, S.; Buckow, R. An Emerging Segment of Functional Legume-Based Beverages: A Review. Food Rev. Int. 2020, 1–39. [Google Scholar] [CrossRef]
- Paul, A.A.; Kumar, S.; Kumar, V.; Sharma, R. Milk Analog: Plant based alternatives to conventional milk, production, potential and health concerns. Crit. Rev. Food Sci. Nutr. 2020, 60, 3005–3023. [Google Scholar] [CrossRef] [PubMed]
- Scholz-Ahrens, K.E.; Ahrens, F.; Barth, C.A. Nutritional and health attributes of milk and milk imitations. Eur. J. Nutr. 2020, 59, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Ziarno, M.; Cichońska, P. Lactic acid bacteria-fermentable cereal- and pseudocereal-based beverages. Microorganisms 2021, 9, 2532. [Google Scholar] [CrossRef] [PubMed]
- Salmeron, I. Fermented cereal beverages: From probiotic, prebiotic and synbiotic towards nanoscience designed healthy drinks. Lett. Appl. Microbiol. 2017, 65, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Vadusha, S.; Mishra, H.N. Non dairy probiotic beverages. Int. Food Res. J. 2013, 20, 7–15. [Google Scholar]
- Vanga, S.K.; Raghavan, V. How well do plant based alternatives fare nutritionally compared to cow’s milk? J. Food Sci. Technol. 2018, 55, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Jeske, S.; Zannini, E.; Arendt, E.K. Evaluation of Physicochemical and Glycaemic Properties of Commercial Plant-Based Milk Substitutes. Plant Foods Hum. Nutr. 2017, 72, 26–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojka, K. Characteristics of fermented milk drinks. Probl. Hig. Epidemiol. 2013, 94, 722–729. [Google Scholar]
- Smid, E.J.; Lacroix, C. Microbe–microbe interactions in mixed culture food fermentations. Curr. Opin. Biotechnol. 2013, 24, 148–154. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; González-Martínez, C.; Rodríguez-García, J.; Chiralt, A. Optimisation of oat milk formulation to obtain fermented derivatives by using probiotic Lactobacillus reuteri microorganisms. Food Sci. Technol. Int. 2015, 21, 145–157. [Google Scholar] [CrossRef]
- WHO; FAO. Codex Alimentarius: Codex Standards for Fermented Milks 243–2003. Pages 6–16 in Milk and Milk Products, 2nd ed.; World Health Organisation: Geneva, Switzerland; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Tamang, J.P.; Thapa, S. Fermentation Dynamics during Production of Bhaati Jaanr, a Traditional Fermented Rice Beverage of the Eastern Himalayas. Food Biotech. 2006, 20, 251–261. [Google Scholar] [CrossRef]
- Menezes, A.; Ramos, C.; Dias, D.; Schwan, R. Combination of probiotic yeast and lactic acid bacteria as starter culture to produce maize-based beverages. Food Res. Int. 2018, 111, 187–197. [Google Scholar] [CrossRef]
- Yuliana, N.; Rangga, A. Manufacture of fermented coco milk-drink containing lactic acid bacteria cultures. Afr. J. Food Sci. 2010, 4, 558–562. [Google Scholar]
- Mesquita, M.C.; Leandro, E.; Alencar, E.; Botelho, R. Fermentation of chickpea (Cicer arietinum L.) and coconut (Coccus nucifera L.) beverages by Lactobacillus paracasei subsp paracasei LBC 81: The influence of sugar content on growth and stability during storage. LWT 2020, 132, 109834. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Stathopoulos, C. Viability of Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus acidophilus and Lactobacillus casei in fermented milk supplemented with isomalto-oligosaccharides derived from banana flour. J. Food Nutr. Res. 2011, 50, 125–132. [Google Scholar]
- Dan, T.; Chen, H.; Li, T.; Tian, J.; Ren, W.; Zhang, H.; Sun, T. Influence of Lactobacillus plantarum P-8 on Fermented Milk Flavor and Storage Stability. Front. Microbiol. 2019, 9, 3133. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, S.; Evans, C.A.; Adams, M.; Baines, S. Co-culturing of probiotics influences the microbial and physico-chemical properties but not sensory quality of fermented dairy drink made from goats’ milk. Small Rumin. Res. 2016, 136, 104–108. [Google Scholar] [CrossRef]
- Zahed, O.; Khosravi-Darani, K.; Mortazavian, A.; Mohammadi, A. Bacterial conjugated linoleic acid bio-fortification of synbiotic yogurts using Propionibacterium freudenreichii as adjunct culture. Ital. J. Food Sci. 2021, 33, 1–11. [Google Scholar] [CrossRef]
- Gilbert, A.; Turgeon, S.L. Studying stirred yogurt microstructure and its correlation to physical properties: A review. Food Hydrocoll. 2021, 121, 106970. [Google Scholar] [CrossRef]
- Ziarno, M.; Zaręba, D.; Maciejak, M.; Veber, A.L. The impact of dairy starter cultures on selected qualitative properties of functional fermented beverage prepared from germinated White Kidney Beans. J. Food Nutr. Res. 2019, 2, 167–176. [Google Scholar]
- Mousavi, M.; Heshmati, A.; Garmakhany, A.D.; Vahidinia, A.; Taheri, M. Texture and sensory characterization of functional yogurt supplemented with flaxseed during cold storage. Food Sci. Nutr. 2019, 7, 907–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef] [Green Version]
- Dönmez, Ö.; Mogol, B.A.; Gökmen, V. Syneresis and rheological behaviors of set yogurt containing green tea and green coffee powders. J. Dairy Sci. 2017, 100, 901–907. [Google Scholar] [CrossRef]
- Li, N.; Zhang, B.; Zhao, S.; Niu, M.; Jia, C.; Huang, Q.; Liu, Y.; Lin, Q. Influence of Lactobacillus/Candida fermentation on the starch structure of rice and the related noodle features. Int. J. Biol. Macromol. 2019, 121, 882–888. [Google Scholar] [CrossRef]
- Geng, D.; Liang, T.; Yang, M.; Wnag, L.; Zhou, X.; Sun, X.; Liu, L.; Zhou, S.; Tong, L. Effects of Lactobacillus combined with semidry flour milling on the quality and flavor of fermented rice noodles. Food Res. Int. 2019, 126, 108612. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.; Oliveira, E.; Ribeiro, A.; Oliveira, A.; Vinhal, G.; de Oliveira, T.; Caliari, M.; Soares, M. Texture profile of fermented rice extracts with probiotic strains and different contents of waxy maize starch, and sensory acceptance of flavoured selected extract. Int. J. Food Sci. Technol. 2019, 55, 490–499. [Google Scholar] [CrossRef]
- Ziarno, M.; Zaręba, D. The use of propionic acid bacteria in dairy production. Przemysł Spożywczy 2019, 73, 13–15. [Google Scholar]
- Obadina, A.O.; Akinola, O.J.; Shittu, T.A.; Bakare, H.A. Effect of Natural Fermentation on the Chemical and Nutritional Composition of Fermented Soymilk Nono. Niger. Food J. 2013, 31, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Jood, S.; Khetarpaul, N. Effect of germination and probiotic fermentation on nutrient composition of barley based food mixtures. Food Chem. 2010, 119, 779–784. [Google Scholar] [CrossRef]
- Süle, J.; Kõrösi, T.; Hucker, A.; Varga, L. Evaluation of culture media for selective enumeration of bifidobacteria and lactic acid bacteria. Braz. J. Microbiol. 2014, 45, 1023–1030. [Google Scholar] [CrossRef] [Green Version]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; ASM Press: Washington, DC, USA, 2010; p. 57. [Google Scholar]
| Starter Cultures | Codes |
|---|---|
| Propionibacterium FD-DVS PS-4 FlavorControlTM | PS4 |
| Propionibacterium FD-DVS PS-4 FlavorControlTM + YC-X16 | PS4, X16 |
| Propionibacterium FD-DVS PS-4 FlavorControlTM + ABY-1 | PS4, ABY |
| Propionibacterium Propionici 000F0004 | 0F4 |
| Propionibacterium Propionici 000F0004 + YC-X16 | 0F4, X16 |
| Propionibacterium Propionici 000F0004 + ABY-1 | 0F4, ABY |
| Propionibacterium PP | PP |
| Propionibacterium PP + YC-X16 | PP, X16 |
| Propionibacterium PP + ABY-1 | PP, ABY |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichońska, P.; Ziębicka, A.; Ziarno, M. Properties of Rice-Based Beverages Fermented with Lactic Acid Bacteria and Propionibacterium. Molecules 2022, 27, 2558. https://doi.org/10.3390/molecules27082558
Cichońska P, Ziębicka A, Ziarno M. Properties of Rice-Based Beverages Fermented with Lactic Acid Bacteria and Propionibacterium. Molecules. 2022; 27(8):2558. https://doi.org/10.3390/molecules27082558
Chicago/Turabian StyleCichońska, Patrycja, Anna Ziębicka, and Małgorzata Ziarno. 2022. "Properties of Rice-Based Beverages Fermented with Lactic Acid Bacteria and Propionibacterium" Molecules 27, no. 8: 2558. https://doi.org/10.3390/molecules27082558
APA StyleCichońska, P., Ziębicka, A., & Ziarno, M. (2022). Properties of Rice-Based Beverages Fermented with Lactic Acid Bacteria and Propionibacterium. Molecules, 27(8), 2558. https://doi.org/10.3390/molecules27082558







