The Potential Use of Herbal Fingerprints by Means of HPLC and TLC for Characterization and Identification of Herbal Extracts and the Distinction of Latvian Native Medicinal Plants
Abstract
1. Introduction
2. Results and Discussion
2.1. Advantages and Challenges of Thin Layer Chromatography
2.2. Method Validation of HPLC Fingerprint Analysis
2.3. Obtained Chemical Profiles and Identification of Chemical Compounds Using HPLC
2.4. The Efficacy of HPLC Data Alignment Optimization
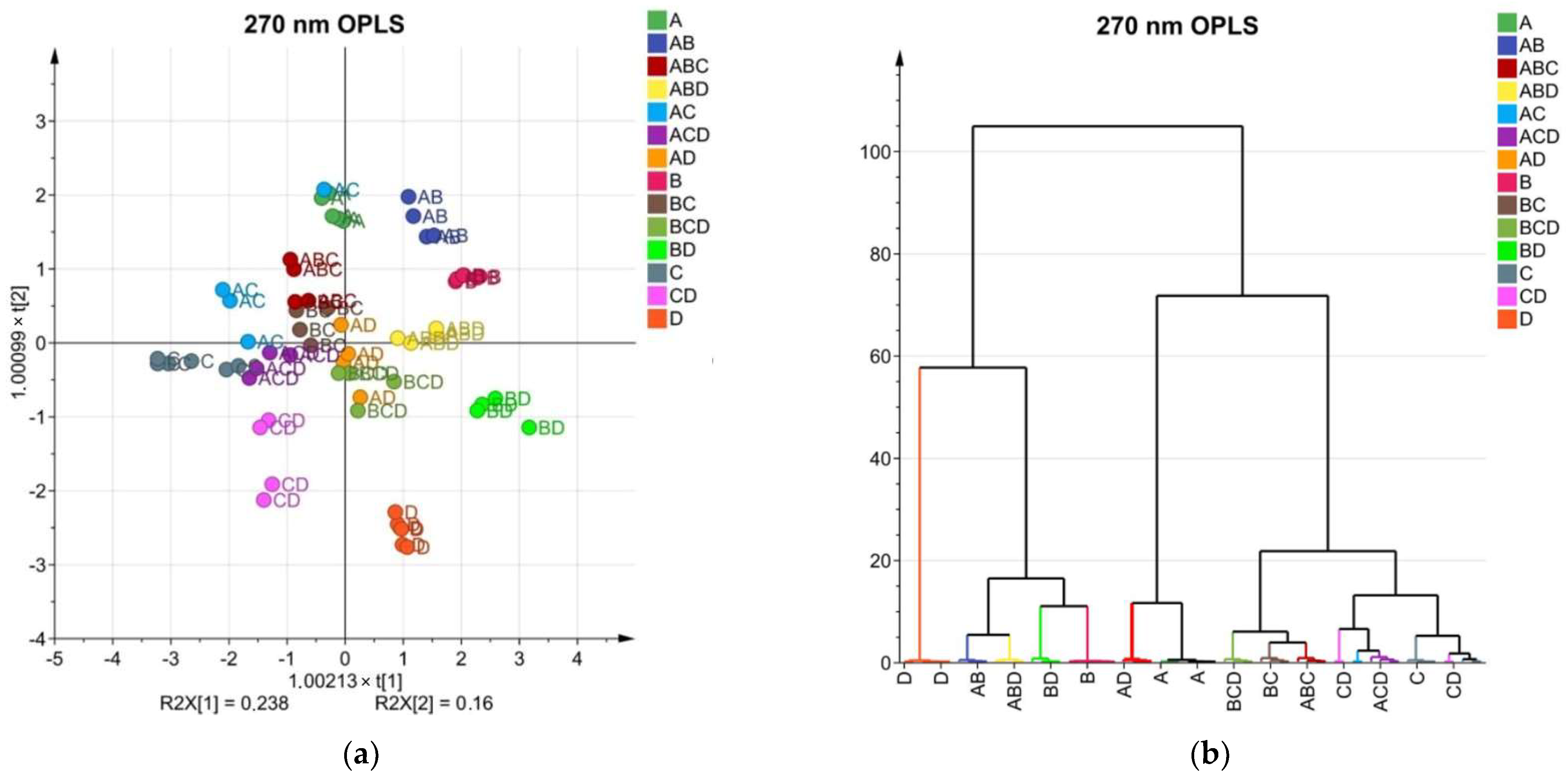
2.5. Multivariate Analysis and Phylogenetic Relationships
2.6. Application of the Herbal Fingerprinting Method
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Sample Preparation
3.4. High-Performance Liquid Chromatography (HPLC) Conditions, Sample Preparation and Data Processing
3.5. Thin Layer Chromatography (TLC) Conditions and Sample Preparation
3.6. Phylogenetic Tree
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Goodarzi, M.; Russell, P.J.; Vander Heyden, Y. Similarity analyses of chromatographic herbal fingerprints: A review. Anal. Chim. Acta 2013, 804, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Suroowan, S.; Mahomoodally, M.F. Herbal medicine of the 21st century: A focus on the chemistry, pharmacokinetics and toxicity of five widely advocated phytotherapies. Curr. Top. Med. Chem. 2019, 19, 2718–2738. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shen, Y.; Yao, C.L.; Guo, D.A. Quality assessment of herbal medicines based on chemical fingerprints combined with chemometrics approach: A review. J. Pharm. Biomed. Anal. 2020, 185, 113215. [Google Scholar] [CrossRef] [PubMed]
- Botanical Supplements Market Size, Share & Trends Analysis. Report by Source (Herbs, Leaves, Spices, Flowers), by Form (Tablets, Liquid), by Application, by Distribution Channel, by Region, and Segment Forecasts, 2020–2028. Available online: https://www.grandviewresearch.com/industry-analysis/botanical-supplements-market (accessed on 10 March 2022).
- Sendker, J.; Sheridan, H. Composition and quality control of herbal medicines. In Toxicology of Herbal Products; Pelkonen, O., Duez, P., Vuorela, P., Vuorela, H., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 29–65. [Google Scholar]
- Sile, I.; Romane, E.; Reinsone, S.; Maurina, B.; Tirzite, D.; Dambrova, M. Medicinal plants and their uses recorded in the Archives of Latvian Folklore from the 19th century. J. Ethnopharmacol. 2020, 249, 112378. [Google Scholar] [CrossRef] [PubMed]
- Plants and Parts of Plants, Incl. Seeds and Fruits, of a Kind Used Primarily in Perfumery, in Pharmacy or for Insecticidal, Fungicidal or Similar Purposes, Fresh, Chilled, Frozen or Dried, Whether or Not Cut, Crushed or Powdered. Available online: https://eksports.csb.gov.lv/en/years/products-selected/import/2020/TOTAL-II-12-1211/TOTAL (accessed on 25 February 2022).
- Srirama, R.; Santhosh Kumar, J.U.; Seethapathy, G.S.; Newmaster, S.G.; Ragupathy, S.; Ganeshaiah, K.N.; Uma Shaanker, R.; Ravikanth, G. Species adulteration in the herbal trade: Causes, consequences and mitigation. Drug Saf. 2017, 40, 651–661. [Google Scholar] [CrossRef]
- Newmaster, S.G.; Grguric, M.; Shanmughanandhan, D.; Ramalingam, S.; Ragupathy, S. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013, 11, 222. [Google Scholar] [CrossRef]
- Mishra, P.; Kumar, A.; Nagireddy, A.; Mani, D.N.; Shukla, A.K.; Tiwari, R.; Sundaresan, V. DNA barcoding: An efficient tool to overcome authentication challenges in the herbal market. Plant Biotechnol. J. 2016, 14, 8–21. [Google Scholar] [CrossRef]
- Torelli, A.; Marieschi, M.; Bruni, R. Authentication of saffron (Crocus sativus L.) in different processed, retail products by means of SCAR markers. Food Control 2014, 36, 126–131. [Google Scholar] [CrossRef]
- Urumarudappa, S.K.; Gogna, N.; Newmaster, S.G.; Venkatarangaiah, K.; Subramanyam, R.; Saroja, S.G.; Gudasalamani, R.; Dorai, K.; Ramanan, U.S. DNA barcoding and NMR spectroscopy-based assessment of species adulteration in the raw herbal trade of Saraca asoca (Roxb.) Willd, an important medicinal plant. Int. J. Legal Med. 2016, 130, 1457–1470. [Google Scholar] [CrossRef]
- Council of Europe-EDQM. European Pharmacopoeia 10th Edition. 10.0, Volume 1. Herbal Drugs and Herbal Drug Preparations. Available online: https://pheur.edqm.eu/app/arch/content/arch-0/2020_10th_Edition_10.0_Volume_1_E.pdf (accessed on 3 March 2022).
- Tistaert, C.; Dejaegher, B.; Vander Heyden, Y. Chromatographic separation techniques and data handling methods for herbal fingerprints: A review. Anal. Chim. Acta 2011, 690, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, J.; Gao, Z.; Zhang, Z.; Lyu, J. A review of quality assessment and grading for agarwood. Chin. Herb. Med. 2017, 9, 22–30. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, J.W.; Liu, X.G.; Wu, Q.W.; Li, X.S.; Gao, W.; Wang, H.Y.; Li, P.; Yang, H. Correlation between macroscopic characteristics and tissue-specific chemical profiling of the root of Salvia miltiorrhiza. Phytomedicine 2018, 51, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Guo, D. Quality marker concept inspires the quality research of traditional Chinese medicines. Chin. Herb. Med. 2017, 9, 1–2. [Google Scholar] [CrossRef]
- Li, P.; Qi, L.W.; Liu, E.H.; Zhou, J.L.; Wen, X.D. Analysis of Chinese herbal medicines with holistic approaches and integrated evaluation models. Trends Anal. Chem. 2008, 27, 66–77. [Google Scholar] [CrossRef]
- World Health Organization. Programme on Traditional Medicine. Guidelines for the Assessment of Herbal Medicines. 1991. Available online: https://apps.who.int/iris/bitstream/handle/10665/58865/WHO_TRM_91.4.pdf?sequence=1&isAllowed=y (accessed on 28 February 2022).
- EMA. Guideline on Quality of Herbal Medicinal Products/Traditional Herbal Medicinal Products. 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-herbal-medicinal-products-traditional-herbal-medicinal-products-revision-2_en.pdf (accessed on 28 February 2022).
- Bansal, A.; Chhabra, V.; Rawal, R.K.; Sharma, S. Chemometrics: A new scenario in herbal drug standardization. J. Pharm. Anal. 2014, 4, 223–233. [Google Scholar] [CrossRef]
- Brangule, A.; Šukele, R.; Bandere, D. Herbal medicine characterization perspectives using advanced FTIR sample techniques–diffuse reflectance (DRIFT) and photoacoustic spectroscopy (PAS). Front. Plant Sci. 2020, 11, 356. [Google Scholar] [CrossRef]
- Brangule, A.; Bērtiņš, M.; Vīksna, A.; Bandere, D. Potential of multivariate analysis of X-ray fluorescence spectra for characterization of the microchemical composition of plant materials. Agron. Res. 2021, 19. [Google Scholar] [CrossRef]
- Jiang, Y.; David, B.; Tu, P.; Barbin, Y. Recent analytical approaches in quality control of traditional Chinese medicines-A review. Anal. Chim. Acta 2010, 657, 9–18. [Google Scholar] [CrossRef]
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Xie, P.; Chan, K. Quality control of herbal medicines. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 812, 53–70. [Google Scholar] [CrossRef]
- Council of Europe-EDQM. Council of Europe-EDQM. European Pharmacopoeia 9th Edition. 9.0, Volume 1. 2.2.27. Thin-Layer Chromatography, 01/2008:20227. Available online: https://pheur.edqm.eu/app/arch/content/arch-0/2017_9th_Edition_9.0_Volume_1_E.pdf (accessed on 3 March 2022).
- Cornejo-Báez, A.A.; Peña-Rodríguez, L.M.; Álvarez-Zapata, R.; Vázquez-Hernández, M.; Sánchez-Medina, A. Chemometrics: A complementary tool to guide the isolation of pharmacologically active natural products. Drug Discov. Today 2020, 25, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Z.; Su, R.; Ruan, G.; Du, F.; Li, G. Current application of chemometrics in traditional Chinese herbal medicine research. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1026, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gad, H.A.; El-Ahmady, S.H.; Abou-Shoer, M.I.; Al-Azizi, M.M. Application of chemometrics in authentication of herbal medicines: A review. Phytochem. Anal. 2013, 24, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Sima, I.A.; Andrási, M.; Sârbu, C. Chemometric assessment of chromatographic methods for herbal medicines authentication and fingerprinting. J. Chromatogr. Sci. 2018, 56, 49–55. [Google Scholar] [CrossRef]
- Xu, C.J.; Liang, Y.Z.; Chau, F.T.; Heyden, Y.V. Pretreatments of chromatographic fingerprints for quality control of herbal medicines. J. Chromatogr. A 2006, 1134, 253–259. [Google Scholar] [CrossRef]
- Blasco, H.; Błaszczyński, J.; Billaut, J.C.; Nadal-Desbarats, L.; Pradat, P.F.; Devos, D.; Moreau, C.; Andres, C.R.; Emond, P.; Corcia, P.; et al. Comparative analysis of targeted metabolomics: Dominance-based rough set approach versus orthogonal partial least square-discriminant analysis. J. Biomed. Inform. 2015, 53, 291–299. [Google Scholar] [CrossRef]
- Sun, M.; Wu, H.; He, M.; Jia, Y.; Wang, L.; Liu, T.; Hui, L.; Li, L.; Wei, S.; Van Wijk, E.; et al. Integrated assessment of medicinal rhubarb by combination of delayed luminescence and HPLC fingerprint with emphasized on bioactivities based quality control. Chin. Med. 2020, 15, 72. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, Q.; Chen, R.; Wang, J.; Jiang, S.; Hu, Y. A new method to evaluate the similarity of chromatographic fingerprints: Weighted Pearson product-moment correlation coefficient. J. Chromatogr. Sci. 2004, 42, 545–550. [Google Scholar] [CrossRef]
- Joshi, D.D. Herbal Drugs and Fingerprints; Springer: Dehli, India, 2012; pp. 29–48, 61–81. [Google Scholar]
- Gocan, S.; Cimpan, G. Review of the analysis of medicinal plants by TLC: Modern approaches. J. Liq. Chromatogr. Relat. Technol. 2003, 27, 1377–1411. [Google Scholar] [CrossRef]
- Council of Europe-EDQM. European Pharmacopoeia 7th Edition. 7.0, Volume 1. Monograph-Calendula Flower, 01/2011:1297. Available online: https://pheur.edqm.eu/app/arch/content/arch-0/2011_7th_Edition_7.0_Volume_1_E.pdf (accessed on 3 March 2022).
- Różyło, J.K.; Ościk-Mendyk, B. Influence of chamber saturation in thin-layer chromatography on theoretical and experimental parameters of mixture separations. J. High Resolut. Chromatogr. 1980, 3, 291–296. [Google Scholar] [CrossRef]
- Jesionek, W.; Majer-Dziedzic, B.; Choma, I. Separation, identification, and investigation of antioxidant ability of plant extract components using TLC, LC-MS, and TLC-DPPH. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1147–1153. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Loescher, C.M. Qualitative and quantitative high performance thin layer chromatography analysis of Calendula officinalis using high resolution plate imaging and artificial neural network data modelling. Anal. Chim. Acta 2013, 798, 103–108. [Google Scholar] [CrossRef]
- Shawky, E.; Abou El Kleir, R.M. Rapid discrimination of different Apiaceae species based on HPTLC fingerprints and targeted flavonoids determination using multivariate image analysis. Phytochem. Anal. 2018, 29, 452–462. [Google Scholar] [CrossRef]
- Milojković-Opsenica, D.; Ristivojević, P.; Andrić, F.; Trifković, J. Planar chromatographic systems in pattern recognition and fingerprint analysis. Chromatographia 2013, 76, 1239–1247. [Google Scholar] [CrossRef]
- Yang, X.; Yang, S.P.; Zhang, X.; Yu, X.D.; He, Q.Y.; Wang, B.C. Study on the multi-marker components quantitative HPLC fingerprint of the compound Chinese medicine Wuwei Changyanning granule. Iran J. Pharm. Res. 2014, 13, 1191–1201. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Frum, A. HPLC determination of polyphenols from Calendula officinalis L. flowers. Acta Univ. Cibiniensis. Ser. E Food Technol. 2017, 21, 97–101. [Google Scholar] [CrossRef][Green Version]
- Haghi, G.; Hatami, A.; Safaei, A.; Mehran, M. Analysis of phenolic compounds in Matricaria chamomilla and its extracts by UPLC-UV. Res. Pharm. Sci. 2014, 9, 31–37. [Google Scholar]
- Miguel, F.G.; Cavalheiro, A.H.; Spinola, N.F.; Ribeiro, D.L.; Barcelos, G.R.; Antunes, L.M.; Hori, J.I.; Marquele-Oliveira, F.; Rocha, B.A.; Berretta, A.A. Validation of a RP-HPLC-DAD method for chamomile (Matricaria recutita) preparations and assessment of the marker, apigenin-7-glucoside, safety and anti-inflammatory effect. Evid. Based Complement Alternat. Med. 2015, 2015, 828437. [Google Scholar] [CrossRef]
- Viapiana, A.; Struck-Lewicka, W.; Konieczynski, P.; Wesolowski, M.; Kaliszan, R. An approach based on HPLC-fingerprint and chemometrics to quality consistency evaluation of Matricaria chamomilla L. Commercial samples. Front. Plant Sci. 2016, 7, 1561. [Google Scholar] [CrossRef]
- Benedec, D.; Popica, I.E.; Oniga, I.; Hanganu, D.; Duma, M.; Silaghi-Dumitrescu, R.; Bischin, C.; Vlase, L. Comparative HPLC-MS analysis of phenolics from achillea distans and achillea millefolium and their bioactivity. Stud. Univ. Babes-Bolyai Chem. 2015, 60, 257–266. [Google Scholar]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Iorizzi, M.; Fico, G. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 58, 203–209. [Google Scholar] [CrossRef]
- Benetis, R.; Radušiene, J.; Jakštas, V.; Janulis, V.; Malinauskas, F. Development of an RP-HPLC method for the analysis of phenolic compounds in Achillea millefolium L. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 596–610. [Google Scholar] [CrossRef]
- Mohd Ali, S.A.; Che Mohd, C.R.; Latip, J. Comparison of phenolic constituent in Hibiscus sabdariffa cv. UKMR-2 calyx at different harvesting times. Sains Malays. 2019, 48, 1417–1424. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Ferchaud, V.; Qi, Y.; Jiang, H.; Tang, F.; Yue, Y.; Chin, K.L. Variations in chemical fingerprints and major flavonoid contents from the leaves of thirty-one accessions of Hibiscus sabdariffa L. Biomed. Chromatogr. 2016, 30, 880–887. [Google Scholar] [CrossRef]
- Sarr, M.; Ngom, S.; Kane, M.O.; Wele, A.; Diop, D.; Sarr, B.; Gueye, L.; Andriantsitohaina, R.; Diallo, A.S. In vitro vasorelaxation mechanisms of bioactive compounds extracted from Hibiscus sabdariffa on rat thoracic aorta. Nutr. Metab. 2009, 6, 45. [Google Scholar] [CrossRef]
- Dolan, J.W. How much retention time variation Is normal? LC-GC N. Am. 2014, 32, 546–551. [Google Scholar]
- Wong, J.W.; Cagney, G.; Cartwright, H.M. SpecAlign–processing and alignment of mass spectra datasets. Bioinformatics 2005, 21, 2088–2090. [Google Scholar] [CrossRef]
- Zheng, Q.X.; Fu, H.Y.; Li, H.D.; Wang, B.; Peng, C.H.; Wang, S.; Cai, J.L.; Liu, S.F.; Zhang, X.B.; Yu, Y.J. Automatic time-shift alignment method for chromatographic data analysis. Sci. Rep. 2017, 7, 256. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, X.; Bing, S.; Wang, L.; Li, H. The application of dynamic time warping to the quality evaluation of radix Puerariae thomsonii: Correcting retention time shifts in the chromatographic fingerprints. J. Chromatogr. Sci. 2015, 53, 968–973. [Google Scholar] [CrossRef]
- Nakhleh, L. Evolutionary Trees. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 549–550. [Google Scholar]
- Kharyuk, P.; Nazarenko, D.; Oseledets, I.; Rodin, I.; Shpigun, O.; Tsitsilin, A.; Lavrentyev, M. Employing fingerprinting of medicinal plants by means of LC-MS and machine learning for species identification task. Sci. Rep. 2018, 8, 17053. [Google Scholar] [CrossRef]
- Wang, X.; Ma, C.; Yang, P.; Wei, Y.; Jia, J.; Cao, G.; Li, J.; Xin, Y. Integrated HPLC fingerprinting and multivariate analysis differentiates between wild and cultivated Hedyotis diffusa Willd. Ind. Crops Prod. 2020, 148, 112223. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, G.; Hou, Z.; Yan, B.; Zhang, J. Evaluation of the quality consistency of powdered poppy capsule extractive by an averagely linear-quantified fingerprint method in combination with antioxidant activities and two compounds analyses. J. Sep. Sci. 2017, 40, 4511–4520. [Google Scholar] [CrossRef]
- Yang, L.; Sun, G.; Guo, Y.; Hou, Z.; Chen, S. Holistic evaluation of quality consistency of Ixeris sonchifolia (Bunge) hance injectables by quantitative fingerprinting in combination with antioxidant activity and chemometric methods. PLoS ONE 2016, 11, e0148878. [Google Scholar] [CrossRef]
- Maleš, Ž.; Medić-Šarić, M. Optimisation of thin-layer chromatographic analysis of flavanoids and phenolic acid of Salviae folium. Acta Pharm. 1998, 48, 85–92. [Google Scholar]
- Olah, N.K.; Mureşan, L.; Cîmpan, G.; Gocan, S. Normal-phase high-performance thin-layer chromatography and automated multiple development of hydroalcoholic extracts of Artemisia abrotanum, Artemisia absinthium, Artemisia vulgaris, and Artemisia cina. J. Planar. Chcromat. 1998, 11, 361–364. [Google Scholar]
- phyloT v2. A Phylogenetic Tree Generator, Based on NCBI or GTD Taxonomy. Available online: https://phylot.biobyte.de/ (accessed on 3 March 2022).
- iTOL. Interactive Tree of Life. Available online: https://itol.embl.de/ (accessed on 3 March 2022).
- Menges, F. SpectraGryph–Optical Spectroscopy Software, Version 1.2.15. Available online: https://www.effemm2.de/spectragryph/ (accessed on 9 March 2022).
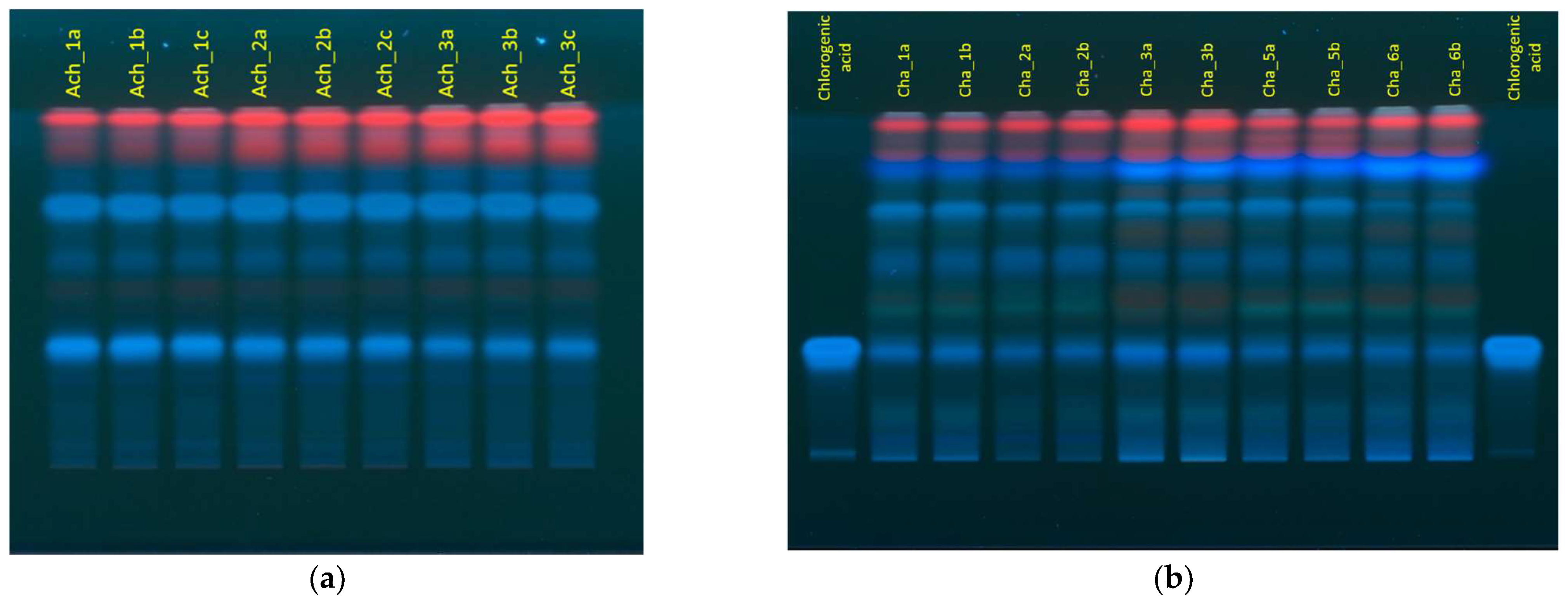
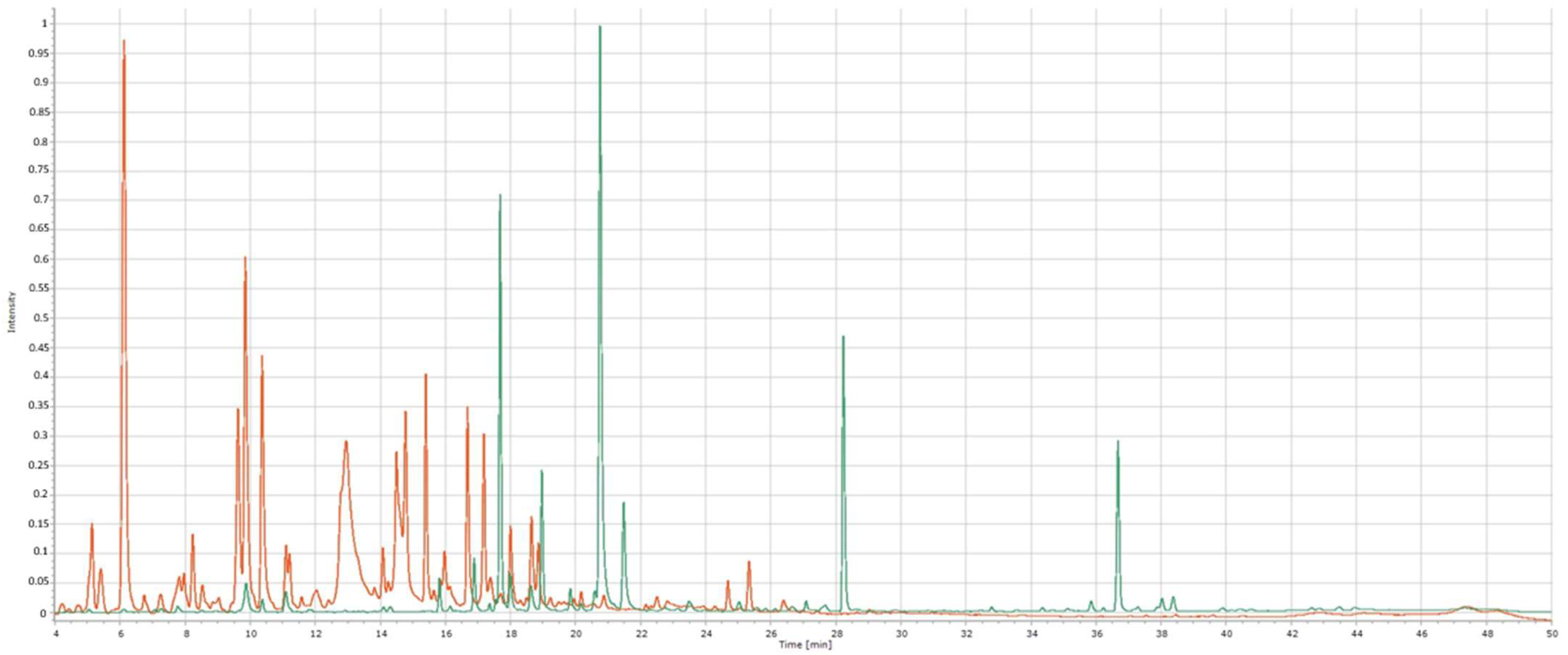


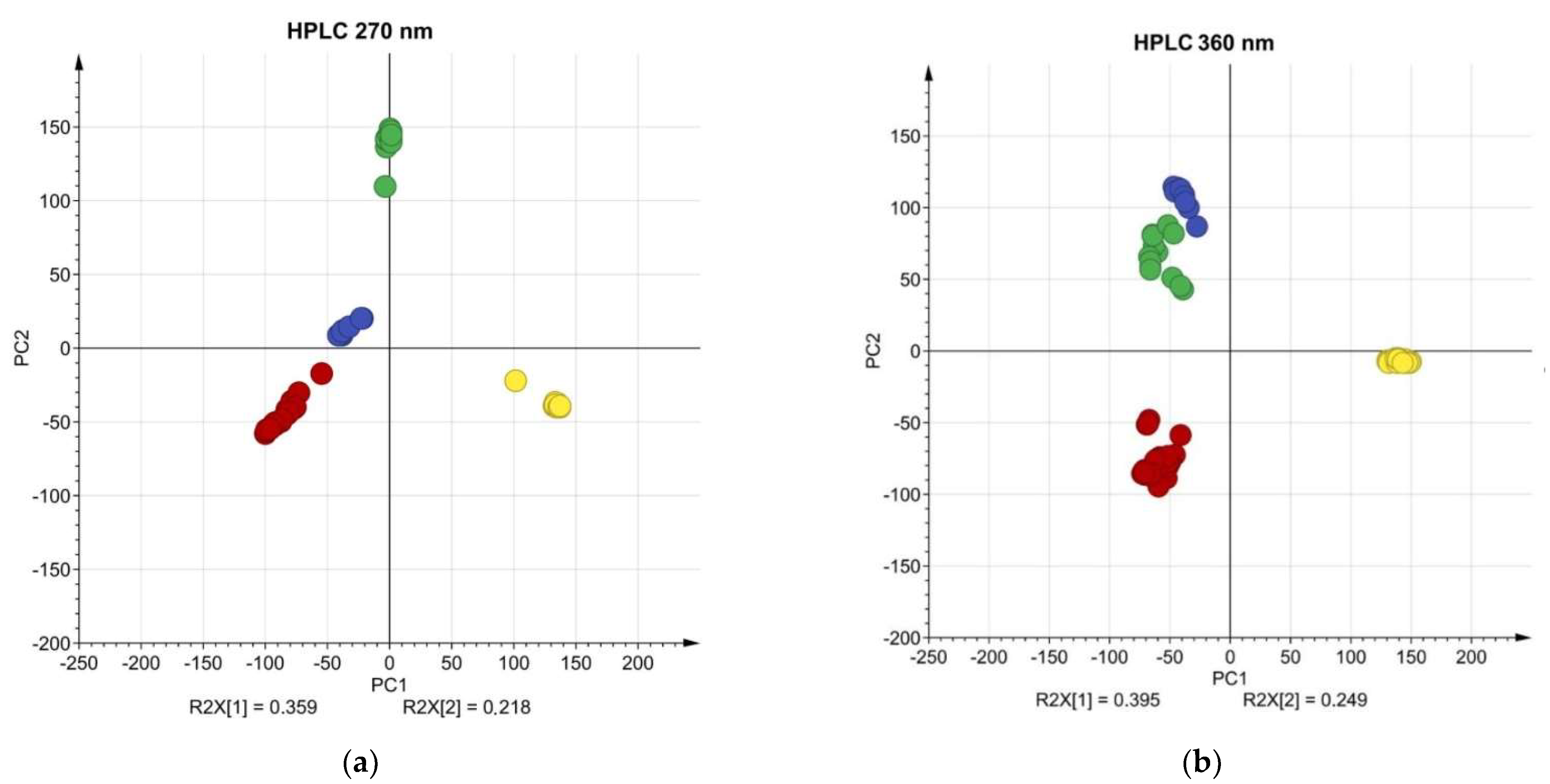

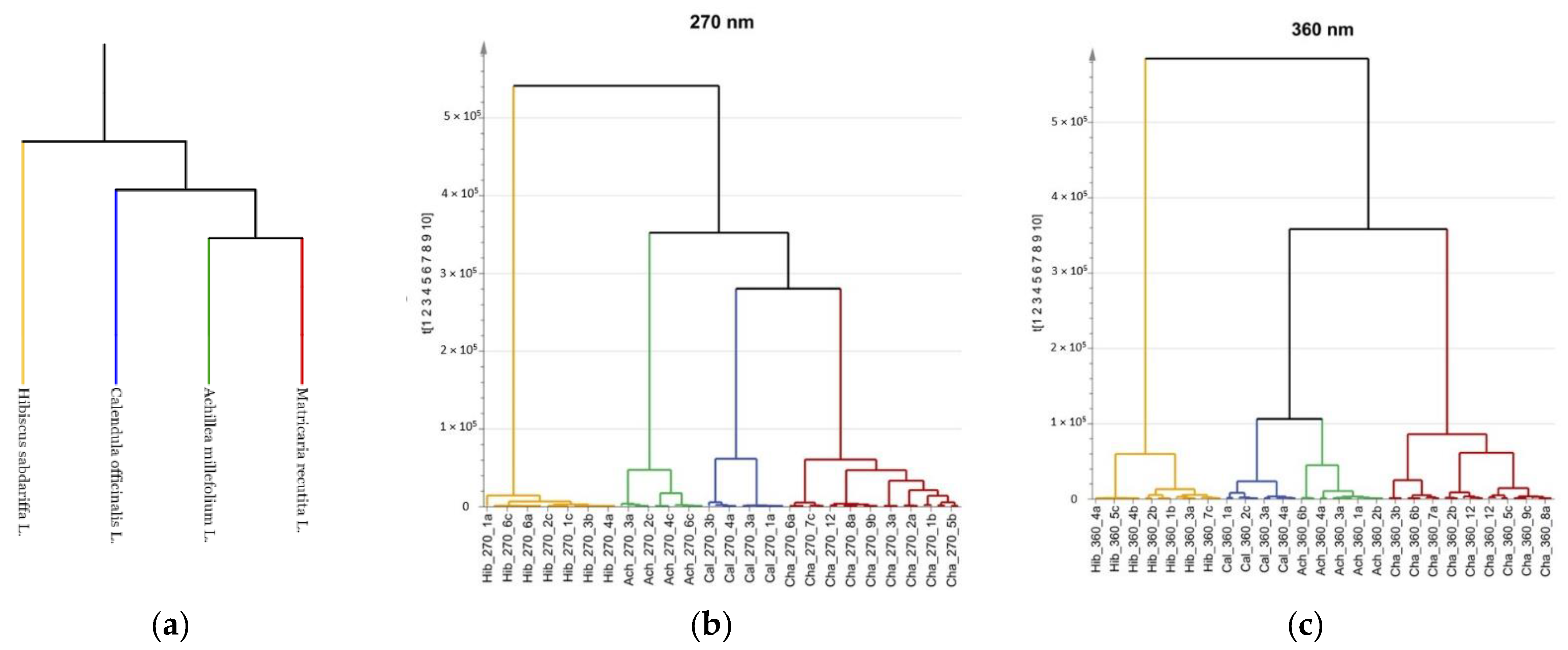

| Wavelength | Chemical Compound | Standard RT (min) | Sample RT 1 (min) | Yarrow | Chamomile | Calendula | Roselle |
|---|---|---|---|---|---|---|---|
| 270 nm | Chlorogenic acid | 9.86 | 9.85 ± 0.04 | + | + | + | + |
| Caffeic acid | 10.37 | 10.37 ± 0.01 | + | + | + | ||
| Rutin | 17.99 | 17.99 ± 0.01 | + | + | + | ||
| Apigenin-7-glucoside | 21.41 | 21.42 ± 0.01 | + | + | |||
| Apigenin | 32.34 | 32.36 ± 0.05 | + | + | |||
| 360 nm | Chlorogenic acid | 9.87 | 9.88 ± 0.02 | + | + | + | + |
| Caffeic acid | 10.37 | 10.39 ± 0.05 | + | + | + | ||
| Rutin | 17.99 | 17.99 ± 0.01 | + | + | + | ||
| Apigenin-7-glucoside | 21.42 | 21.42 ± 0.02 | + | + | |||
| Apigenin | 32.37 | 32.37 ± 0.05 | + | + |
| Medicinal Plant | Average Pearson’s Correlation Coefficient Value (270 nm) | Average Pearson’s Correlation Coefficient Value (360 nm) | ||
|---|---|---|---|---|
| Raw Data | Data with Adjusted Retention Times | Raw Data | Data with Adjusted Retention Times | |
| Roselle | 0.9628 | 0.9764 | 0.8519 | 0.9648 |
| Chamomile | 0.7122 | 0.7995 | 0.7220 | 0.8116 |
| Calendula | 0.7997 | 0.9457 | 0.8909 | 0.9762 |
| Yarrow | 0.6805 | 0.9305 | 0.8191 | 0.9305 |
| Medicinal Plant | Number of Samples | Sample Origin Country/Region | Code in Figures |
|---|---|---|---|
| Roselle (Hibiscus sabdariffa L.) | 7 | Africa (5) | Hib or D |
| Uzbekistan (1) | |||
| Jamaica (1) | |||
| Chamomile (Matricaria recutita L.) | 9 | Latvia (7) | Cha or C |
| Poland (2) | |||
| Calendula (Calendula officinalis L.) | 4 | Latvia (4) | Cal or B |
| Yarrow (Achillea millefolium L.) | 5 | Latvia (5) | Ach or A |
| Medicinal Plants | Code in Figures |
|---|---|
| Yarrow and calendula | AB |
| Yarrow and chamomile | AC |
| Yarrow and roselle | AD |
| Calendula and chamomile | BC |
| Calendula and roselle | BD |
| Chamomile and roselle | CD |
| Yarrow, calendula, chamomile | ABC |
| Yarrow, calendula, roselle | ABD |
| Yarrow, chamomile, roselle | ACD |
| Calendula, chamomile, roselle | BCD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bārzdiņa, A.; Paulausks, A.; Bandere, D.; Brangule, A. The Potential Use of Herbal Fingerprints by Means of HPLC and TLC for Characterization and Identification of Herbal Extracts and the Distinction of Latvian Native Medicinal Plants. Molecules 2022, 27, 2555. https://doi.org/10.3390/molecules27082555
Bārzdiņa A, Paulausks A, Bandere D, Brangule A. The Potential Use of Herbal Fingerprints by Means of HPLC and TLC for Characterization and Identification of Herbal Extracts and the Distinction of Latvian Native Medicinal Plants. Molecules. 2022; 27(8):2555. https://doi.org/10.3390/molecules27082555
Chicago/Turabian StyleBārzdiņa, Ance, Artūrs Paulausks, Dace Bandere, and Agnese Brangule. 2022. "The Potential Use of Herbal Fingerprints by Means of HPLC and TLC for Characterization and Identification of Herbal Extracts and the Distinction of Latvian Native Medicinal Plants" Molecules 27, no. 8: 2555. https://doi.org/10.3390/molecules27082555
APA StyleBārzdiņa, A., Paulausks, A., Bandere, D., & Brangule, A. (2022). The Potential Use of Herbal Fingerprints by Means of HPLC and TLC for Characterization and Identification of Herbal Extracts and the Distinction of Latvian Native Medicinal Plants. Molecules, 27(8), 2555. https://doi.org/10.3390/molecules27082555






